The vitamin D receptor is required for iNKT cell development (original) (raw)
Abstract
CD1d-reactive natural killer T (NKT) cells with an invariant T cell receptor Vα14 rearrangement are a unique subset of lymphocytes, which play important roles in immune regulation, tumor surveillance, and host defense against pathogens. Vitamin D is a nutrient/hormone that has been shown to regulate conventional T cell responses but not T cell development. The data show that expression of the vitamin D receptor (VDR) is required for normal development and function of iNKT cells. The iNKT cells from VDR KO mice are intrinsically defective and lack T-bet expression. VDR KO iNKT cells fail to express NK1.1, although they express normal levels of CD122. Extrinsic factors that impact iNKT cell development and function in VDR KO mice include a failure of the liver to support homeostatic proliferation and reduced thymic expression of CD1d and other factors important for optimal antigen presentation in the thymus. In addition, VDR KO iNKT cells were intrinsically defective even when WT antigen-presenting cells were used to stimulate them.
Natural killer T (NKT) cells are a subset of T lymphocytes that play an important regulatory role in several models of autoimmunity, infection and cancer (1, 2). NKT cells participate in the innate immune system and have been shown to be very early producers of high amounts of cytokines including IL-4 and IFN-γ (3). In vivo, NKT cell activation has been shown to alter the outcome of autoimmunity either reducing or exacerbating disease symptoms depending on when in the disease process they are stimulated (4). Therefore, a role for NKT cells in the development of autoimmune disease has been proposed.
NKT cells are thymically derived lymphocytes that express the TCR and receptors of the NK lineage including NK1.1, NKG2D, and members of the Ly-49 family (5–7). The majority of mouse NKT cells express a semiinvariant TCR composed of the Vα14-Jα18 rearrangement and are selected in the thymus through the interaction with CD1d expressed on CD4CD8 double-positive (DP) thymocytes (8–10). These Vα14 invariant NKT cells (iNKT) are present in the thymus, bone marrow (BM), spleen, and liver and are reactive with the glycolipid α-galactosylceramide (αGalCer) presented in the context of CD1d (11). iNKT cells were shown to recognize a lysosomal sphingolipid as an endogenous ligand (12), and it was recently shown that iNKT cells can recognize exogenous ligands from Sphingomonas and Borelia burgdorferi (13, 14). iNKT cells develop from DP Vα14-Jα18 TCR thymocyte precursors that lack expression of NK markers and then undergo robust proliferation and up-regulation of CD44 before terminal maturation that is accompanied by the expression of NK1.1, Ly-49, and CD122 (9, 15). The final steps of iNKT cell development require the transcription factor T-bet and cytokine signaling initiated by IL-15 (16).
CD1d is a nonclassical MHC class I-like molecule that associates with β2-microglobulin (17). CD1d molecules can present exogenous and endogenous ligand to iNKT cells. CD1d trafficking through endosomal compartments is necessary for presentation of ligands to iNKT cells (18, 19). In the thymus CD1d expression of endogenous antigens by DP thymocytes is critical for normal development of iNKT cells (9). CD1d null mice lack iNKT cells, which illustrates the importance of CD1d in iNKT cell development (20).
Vitamin D has been well characterized as a regulator of bone and mineral metabolism. 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) is the active form of vitamin D and functions through binding to the vitamin D receptor (VDR) (21). The VDR is a member of the steroid thyroid super family of nuclear receptors. Various cells of the immune system express the VDR and 1,25(OH)2D3 has been shown to be an important regulator of T cell function (21). 1,25(OH)2D3 treatment has been shown to suppress animal models of autoimmune diseases, whereas VDR KO mice are more susceptible to inflammatory bowel disease (21). I_n vitro_ studies showed that 1,25(OH)2D3 treatment increased the production of IL-4 by Th2 cells and decreased the production of IFN-γ by Th1 cells (22). Other CD4+ T cell populations including Th2 and Treg cells have been shown to express the VDR and to be 1,25(OH)2D3 targets (23).
Here, we show that iNKT cells fail to develop in the absence of the VDR. A blockade in iNKT cell development results in few iNKT cells in the periphery of VDR KO mice. In addition, homeostatic proliferation of iNKT cells is impaired in the liver. 1,25(OH)2D3 treatment increased the function of WT iNKT cells but not development. CD1d expression in the VDR KO thymus was reduced and as a result VDR KO thymocytes showed decreased stimulation of an iNKT cell hybridoma that could be partially rescued by retroviral CD1d expression. The VDR is required for normal expression of CD1d in the thymus, and the data suggest that the VDR KO thymus is not optimal for antigen presentation and selection of iNKT cells. In addition, the iNKT cells from VDR KO mice are intrinsically defective and lack T-bet expression. The data show that development of iNKT cells requires the expression of the VDR both intrinsically and extrinsically.
Results
Diminished Responses of VDR KO Mice to αGalCer.
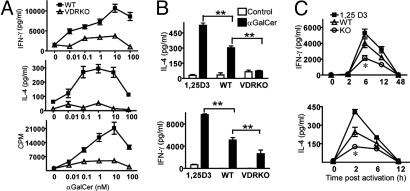
WT NKT cells express the VDR and activation of iNKT cells with αGalCer caused a significant increase in mRNA for the VDR [Fig. 1A and supporting information (SI) Fig. S1_A_]. Splenocytes from WT and VDR KO mice were isolated and stimulated in vitro with increasing concentrations of αGalCer (Fig. 1A). Proliferation and production of IFN-γ was maximal for WT iNKT cells at 10 nM αGalCer. Peak levels of IL-4 were produced between 0.1 and 10 nM αGalCer (Fig. 1A). Stimulation of VDR KO splenocytes with equivalent amounts of αGalCer resulted in significantly less proliferation and IL-4 and IFN-γ production (Fig. 1A). Feeding WT mice 1,25(OH)2D3 (1,25D3) for 1 week increased the production of both IL-4 and IFN-γ by splenocytes cultured ex vivo with αGalCer (Fig. 1B). Furthermore, addition of 100 nM 1,25(OH)2D3 in vitro to WT cultures stimulated with αGalCer significantly increased IFN-γ secretion over control (Fig. S1_B_). To investigate whether vitamin D affects in vivo iNKT cell function, serum cytokine secretion was measured after injection with αGalCer. IFN-γ peaked 6 h after activation and then decreased and disappeared by 48 h (Fig. 1C). IL-4 peaked early after αGalCer injection (2 h) and disappeared by 12 h after injection. Feeding mice 1,25D3 for 1 week before αGalCer injection increased IFN-γ and IL-4 production but did not alter the kinetics of the response (Fig. 1C). VDR KO mice produced significantly less IFN-γ and IL-4 than both the WT and 1,25D3 fed WT mice (Fig. 1C).
Fig. 1.
iNKT cells from VDR KO mice are hyporesponsive. (A) Splenocytes from WT and VDR KO mice were stimulated with different concentrations of αGalCer in vitro. IFN-γ, IL-4, and proliferation were measured. Graphs represent mean ± SD from triplicate samples. Data are one representative experiment of three. (B) IL-4 and IFN-γ production by splenocytes stimulated with 10 nM αGalCer. The splenocytes were from 1,25(OH)2D3-fed WT (1,25D3), WT, and VDR KO mice. Data are from the mean ± SEM of three experiments. **, P < 0.001. (C ) Serum cytokine production in WT and VDR KO mice induced by systemic administration of αGalCer. Levels of IFN-γ and IL-4 in the serum were determined at different times after injection (n = 15 per group, *, P < 0.05).
Reduced Numbers of NKT Cells in VDR KO Mice.
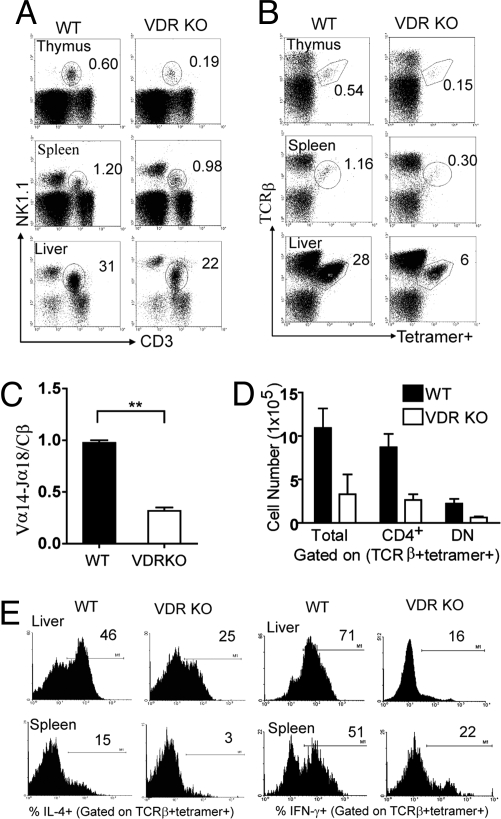
The numbers of NKT cells in the thymus, BM, spleen, and liver of WT and VDR KO mice were determined by using NK1.1 and CD3 staining. The percentage of NK1.1/CD3-positive NKT cells were significantly less in the thymus and liver of VDR KO mice compared with WT mice, whereas the BM and spleen of WT and VDR KO mice showed similar percentages of NK1.1/CD3 NKT cells (Fig. 2A and Fig. S2_A_). 1,25(OH)2D3 treatment had no effect on NKT cell numbers in any tissue (Fig. S2_A_). The percentages of iNKT cells (defined as TCRβ and CD1d-αGalCer tetramer-positive) were significantly lower in VDR KO thymus, and liver compared with WT mice (Fig. 2B). In addition, there were significantly fewer iNKT cells in the spleen of VDR KO mice compared with WT spleen (Fig. 2B). The VDR KO spleen and liver contained a high proportion of NKT cells that were not reactive with αGalCer (Fig. S2_B_). Expression of the TCR Vα14-Jα18 chain was significantly less in VDR KO than WT thymocytes (Fig. 2C).
Fig. 2.
Reduced NKT cell development in VDR KO mice. (A) Dot plots showing percentage of NK1.1 and CD3-positive NKT cells in VDR KO and WT controls. Data show one representative of 25 mice per group. Numbers indicate percentage of NK1.1 and CD3 double-positive cells. Fig. S2_A_ shows the means ± SEM for NK1.1 and CD3 double staining. (B) Dot plot showing iNKT cells (TCRβ and CD1d-αGalCer double-positive). Fig. S3_D_ shows the TCRβ empty CD1d control staining. Data show one representative of 20 mice per group. Numbers indicate percentage of iNKT cells in the circled gate. (C ) Real-time PCR analysis of the TCR Vα14-Jα18 expression in WT and VDR KO thymocytes. Expression of the TCR constant β chain was used as an amplification control. Data are the mean of three independent experiments ± SEM. **, P < 0.001. (D) The absolute number of total iNKT, CD4+ iNKT, and DN iNKT (TCRβ and tetramer double-positive) cells in the spleen were compared between WT and VDR KO mice (n = 15). Fig. S3_B_ shows the isotype control for CD4 staining. (E) Frequency of cytokine-producing iNKT cells in WT and VDR KO mice. Mice were injected with αGalCer in vivo followed by intracellular cytokine staining ex vivo as described in Materials and Methods. Histograms show production of IFN-γ and IL-4 by iNKT cells. Data from one representative of 10 mice. Fig. S1_C_ shows the means ± SEM for the IL-4 and IFN-γ responses. Fig. S3_A_ shows the isotype control staining for both IL-4 and IFN-γ.
iNKT cells consist of two defined populations: a CD4+ and a CD4−CD8− double negative (DN) population. When the absolute numbers of iNKT cells were examined, there was a decrease in both the CD4+ and DN iNKT cells in the periphery of VDR KO mice (Fig. 2D). It should be noted that the percentage and absolute numbers of conventional T cells and NK cells were not different in WT and VDR KO mice (Fig. 2A and data not shown).
VDR KO iNKT Cells Are Hyporesponsive.
To determine whether VDR KO iNKT cells are functionally different from WT iNKT cells; cytokine production from iNKT cells was assessed at the single-cell level by intracellular staining. WT and VDR KO mice were injected with αGalCer and animals were then killed after 2 h. iNKT cells were gated by flow cytometry and then analyzed for intracellular IL-4 and IFN-γ. Three percent of the iNKT cells from the spleen of VDR KO mice made IL-4 and 22% made IFN-γ (Fig. 2E). Conversely, 15% of the spleen derived WT iNKT cells produced IL-4 and 51% produced IFN-γ (Fig. 2E). A similar result was found when iNKT cells were analyzed from the liver in that at least twice as many WT iNKT cells produced IL-4 and IFN-γ than VDR KO iNKT cells (Fig. 2E and Fig. S1_C_). This was a reproducible result and significantly more iNKT cells from WT mice made both IFN-γ and IL-4 compared with the iNKT cells from VDR KO mice (Fig. S1_C_).
Defective iNKT Cell Maturation in the Absence of the VDR.
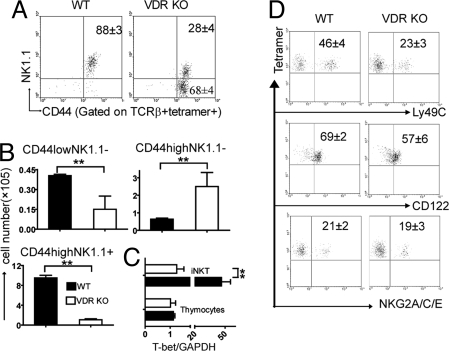
Immature iNKT cells have rearranged the Vα14-Jα18 TCR and are therefore tetramer and TCRβ-positive. The steps in iNKT cell development are (i) CD44low/NK1.1− followed by (ii) CD44high/NK1.1− and leading to (iii) CD44high/NK1.1+ (9, 15). Analysis of thymic iNKT cells showed that VDR KO iNKT cells did up-regulate CD44 expression but that the majority of the iNKT cells failed to express NK1.1 (Fig. 3A). The difference between mature CD44high/NK1.1+ cells in WT and VDR KO is more obvious when the cell numbers of the different iNKT cell subpopulations were compared (Fig. 3B). The majority of iNKT cells in the VDR KO mouse were CD44high/NK1.1− NKT cells, whereas the majority of iNKT cells in WT mice were CD44high/NK1.1+NKT cells (Fig. 3B). In addition, the data show that there are fewer of the iNKT cell early thymic precursors or CD44low/NK1.1− in the VDR KO mice (Fig. 3B). By contrast, VDR KO mice had higher than normal numbers of CD44high/NK1.1− iNKT cells in the thymus (Fig. 3B). The absence of mature CD44high/NK1.1+ NKT cells in the VDR KO mouse also occurred in the periphery (data not shown) and is consistent with the complete absence of T-bet expression in VDR KO iNKT cells but normal levels in the other VDR KO thymocytes (Fig. 3C). The predominately CD44high/NK1.1− iNKT cells from VDR KO mice had decreased expression of Ly49C but normal levels of CD122 and NKG2A/C/E compared with WT iNKT cells (Fig. 3D).
Fig. 3.
VDR KO iNKT cells fail to mature fully. (A) Dot plots showing expression of CD44 and NK1.1 on TCRβ and CD1d-αGalCer tetramer double-positive thymocytes. Data from one representative of 10 mice is shown. (B) Absolute numbers of CD44lowNK1.1−, CD44highNK1.1−, and CD44highNK1.1+ iNKT cells in the thymus of WT and VDR KO mice (n = 10, **, P < 0.001). (C ) Real-time PCR analysis of T-bet expression in thymocytes and thymic iNKT cells from WT and VDR KO mice. Expression of GAPDH was used as an amplification control. Data are the mean of two independent experiments ± SEM. **, P < 0.001. (D) Tetramer-positive thymocytes were analyzed for Ly49C, CD122, and NKG2A/C/E expression. Data from one representative of six mice is shown.
Reduced Homeostatic Proliferation in the Liver of VDR KO Mice.
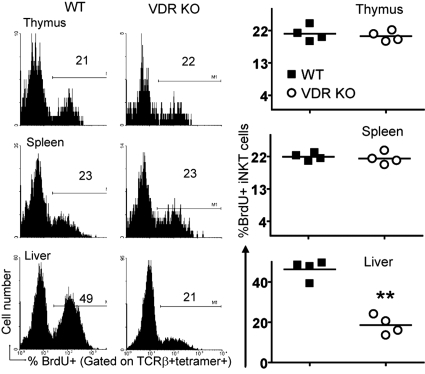
iNKT cell apoptosis was measured in thymocytes from WT and VDR KO mice. VDR KO and WT iNKT cells had similar percentages of annexin V+ cells, suggesting that iNKT cells from VDR KO mice were not dying because of increased apoptosis (data not shown). Homeostatic proliferation of iNKT cells from VDR KO mice was also examined by using a BrdU incorporation assay. A similar percentage of WT and VDR KO iNKT cells in the thymus and spleen incorporated BrdU (Fig. 4). Conversely, a significantly smaller percentage of iNKT cells in the liver of VDR KO mice incorporated BrdU compared with the values from WT mice (Fig. 4). The VDR KO liver showed reduced homeostatic proliferation of iNKT cells.
Fig. 4.
Reduced iNKT cell homeostatic proliferation in the liver of VDR KO mice. BrdU incorporation by iNKT cells from WT and VDR KO mice. Fig. S3_C_ shows the BrDU isotype control staining. Histograms of one representative of four mice. (Right) Individual values and the mean (line) of the data. **, P < 0.001.
VDR Expression in the Thymus Is Required for Normal iNKT Cell Development.
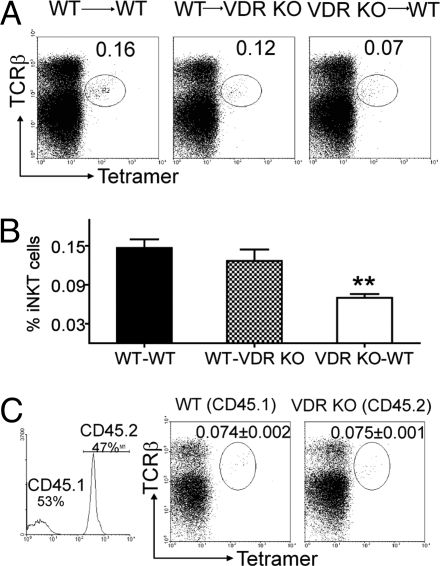
Reciprocal BM transplants were successful as judged by the complete reconstitution of the thymus of recipients with the donor BM (WT-WT, 98% donor, WT-VDR KO 99% donor, and VDR KO-WT 99% donor). A small (0.16%) and consistent number of iNKT cells were recovered from WT recipients of WT BM (WT-WT, Fig. 5 A and B). Others have shown similar levels of iNKT cell reconstitution in the thymus after BM reconstitution (24, 25). The same numbers and percentage of iNKT cells were recovered from VDR KO recipients of WT BM (WT-VDR KO, Fig. 5 A and B). Conversely, WT recipients of VDR KO BM had significantly reduced iNKT cell reconstitution of the thymus (0.07%), compared with either recipient of WT BM (Fig. 5 A and B). WT BM transplantation into either WT or VDR KO recipients resulted in similar percentages of donor derived iNKT cells in the spleen of recipients (Fig. S4_A_). VDR KO BM into WT recipients resulted in half as many donor derived iNKT cells in the liver and a small but significant decrease in donor derived iNKT cells in the spleen compared with the WT-WT combination (Fig. S4_A_). The liver of the WT-VDR KO mice showed very low reconstitution with donor derived iNKT cells and the result resembled that from the VDR KO-WT instead of the WT-WT combination (Fig. S4_A_).
Fig. 5.
iNKT cell development requires VDR expression in the hematopoetic compartment. (A) Reciprocal BM transplants were done by using WT (CD45.1) and VDR KO (CD45.2) mice (donor BM → recipient). The success of the transplant was determined by staining for donor cells in the recipient mice. Percentage of donor iNKT cells in the thymus is shown. Data shown is one representative of four mice per group and two independent experiments. Fig. S4_A_ shows the percentage of donor iNKT cells in liver and spleen of the recipient mice (mean ± SEM of eight mice). (B) Pooled data showing the % of donor iNKT cells recovered in the thymus of reciprocal BM transplantation experiments. Data are the mean ± SEM of eight mice. **, P < 0.01. (C ) Competitive BM chimeras were generated by using a 1:1 ratio of WT CD45.1 and VDR KO CD45.2 BM and WT CD45.1 recipients. Thymocyte chimerism was determined by flow cytometry (Left) and shown to be 53% of WT CD45.1 origin and 47% of VDR KO CD45.2 origin. Fig. S4_B_ shows the percentage of CD45.1 and CD45.2 iNKT cells in the liver and spleen of recipient mice. The data shown is from one representative of five mice (mean ± SEM) and one of two experiments.
Liver mononuclear cells from the recipient mice were stimulated in vitro with αGalCer for intracellular cytokine staining. The liver mononuclear cells were 82–92% of donor origin. The staining was done by gating on donor iNKT cells and quantitating cytokine-producing cells. Thirteen percent of the iNKT cells from WT-WT liver were IL-4-positive, and 43% were IFN-γ-positive (Fig. S5). In the WT-VDR KO or VDR KO-WT combination, the percentage of IL-4- and IFN-γ-producing donor derived iNKT cells was 50% or less of the WT-WT response (Fig. S5).
Expression of CD1d on DP Thymocytes Is Required for iNKT Cell Development.
Reconstitution efficiency in the reciprocal BM transplants above showed that after BM transplantation 98–99% of the thymocytes were of donor origin. To investigate whether reduced iNKT cell precursor frequency or altered antigen presentation caused the reduced iNKT cell numbers in VDR KO mice, mixed BM chimeras were performed. CD45.1 WT mice were reconstituted with 1:1 mixture of CD45.1 WT BM and CD45.2 VDR KO BM. Approximately 50% of the thymocytes were from the CD45.2 VDR KO BM (Fig. 5C). The frequency of iNKT cells derived from VDR KO BM (CD45.2, 0.075%) was comparable to that from WT BM (CD45.1, 0.074%), and the total of the CD45.1 and CD45.2 iNKT cells (0.15%) was the same as shown when WT BM was transplanted into WT or VDR KO mice (Fig. 5A). Analysis of the spleen and liver showed a 50/50 split in each of the donor populations in the peripheral tissues as well (Fig. S4_B_). The equal representation of VDR KO and WT iNKT cells in the BM chimeras suggest that the VDR KO mice have normal numbers of iNKT cell precursors.
VDR Mediated CD1d Expression in the Thymus.
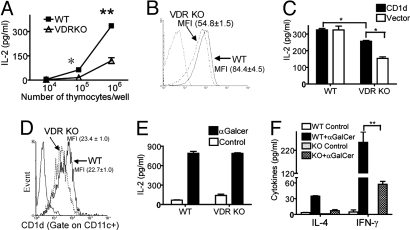
Thymoctyes from VDR KO and WT mice were used as stimulators of the CD1d restricted NKT cell hybridoma. As expected WT thymocytes stimulated the NKT cell hybridoma to produce IL-2 (Fig. 6A). The use of VDR KO thymocytes as stimulators of the NKT cell hybridoma resulted in significantly less IL-2 production (Fig. 6A). Notably, the frequency of CD1d-positive and the mean fluorescence intensity of CD1d expression was significantly lower on VDR KO thymocytes compared with WT thymocytes (Fig. 6B). The intracellular distribution of CD1d in WT and VDR KO thymocytes was determined by using lysosome-associated membrane protein (LAMP)-1 as a marker. The degree of CD1d colocalization with LAMP-1 was comparable in VDR KO and WT thymocytes (Fig. S6). To determine whether increasing CD1d expression in VDR KO thymocytes would have the cells act like WT thymocytes, a retroviral vector that expresses CD1d was used to infect WT and VDR KO thymocytes and produce additional CD1d. Retroviral expression of CD1d had no effect (compared with infection with the empty vector) on the stimulatory ability of WT thymocytes for the NKT cell hybridoma (Fig. 6C). Conversely, retroviral expression of CD1d in the VDR KO thymocytes up-regulated CD1d expression and induced higher IL-2 production from the NKT cell hybridoma (Fig. 6C). However, the CD1d transfected VDR KO thymocytes were still not as good as WT thymocytes at stimulating the NKT cell hybridoma.
Fig. 6.
Low CD1d expression and reduced antigen presentation in the thymus of VDR KO mice. (A) Equal numbers of the CD1d-restricted NKT hybridoma cell were incubated in the presence of different concentrations of thymocytes from WT and VDR KO mice and IL-2 production by the NKT cell hybridoma was measured. Results shown are from one representative of four independent experiments. *, P < 0.05; **, P < 0.001. (B) Thymocytes isolated from WT and VDR KO mice were analyzed by flow cytometry for CD1d expression. Mean fluorescent intensity (MFI) ± SEM. Data shown are from one representative of 15 mice. (C) Thymocytes from WT and VDR KO mice were infected with a CD1d-expressing retrovirus or empty vector and used to stimulate the NKT cell hybridoma for IL-2 production. Graph represents the mean ± SD from triplicate samples. Data shown are one individual experiment of two. *, P < 0.05. (D) CD1d expression on purified VDR KO and WT DC (MFI ± SEM). (E) Purified DC from WT and VDR KO spleens were pulsed with αGalCer or media (Control) and cocultured with CD1d-restricted hybridoma cells for IL-2 production. Graph represents the mean ± SD from triplicate samples. Data shown are one representative experiment of two. (F) iNKT cells from WT and VDR KO (KO) mice were purified and cultured with Rag KO splenocytes in media only (Control) or with αGalCer. Graph represents the mean ± SD from triplicate samples. **, P < 0.001.
Dendritic cells (DC) were purified from the spleens of WT and VDR KO mice, and CD1d expression was measured and shown to be similar on the WT and VDR KO DC (Fig. 6D). CD1d expression levels on hepatocytes from WT and VDR KO mice were also similar (data not shown). αGalCer or control pulsed DC were used as antigen-presenting cells (APCs) for the NKT cell hybridoma. Media pulsed (control) DC did not induce IL-2 production, whereas both the WT and VDR KO DC pulsed with αGalCer stimulated the NKT cell hybridoma to secrete similar levels of IL-2 (Fig. 6E). Last, iNKT cells were purified from VDR KO and WT mice and cultured with Rag KO splenocytes as APCs. Rag KO mice do not have NKT cells, and, therefore, αGalCer treatment of Rag KO splenocytes did not induce cytokine production (data not shown). iNKT cells from WT mice were induced to produce IL-4 and IFN-γ after culture with Rag KO splenocytes and αGalCer (Fig. 6F). Equal numbers of VDR KO iNKT cells produced significantly less IFN-γ than the WT iNKT cells and produced undetectable levels of IL-4 (Fig. 6F).
Discussion
The data demonstrate a critical role for the VDR in directing maturation of iNKT cells. In the absence of the VDR, both thymic and peripheral numbers of iNKT cells are significantly diminished because the iNKT cells are halted at a stage before the final step in maturation. VDR deficiency results in a specific defect in the numbers of iNKT cells and not other T cells or NK cell subsets. iNKT cell development largely occurs in the thymus with the final step in maturation (NK1.1 expression) occurring in both the thymus and the periphery (26). This process has been shown to depend on the transcription factor T-bet and T-bet KO mice have iNKT cells that are blocked at the CD44high/NK1.1− stage (27). The iNKT cells in the VDR KO mouse fail to express T-bet. Other factors are important in iNKT cell development, including IL-15 and relB; however, IL-15 KO and relB KO mice show blocks at earlier stages of iNKT cell development (16, 25). CD122 expression is normal on the VDR KO iNKT cells, suggesting that like the T bet KO iNKT cells, VDR KO iNKT cells are responsive to IL-15 but NK1.1 negative. Expression of the VDR in the hematopoietic compartment is required for T bet expression in iNKT cells and conversion to the NK1.1 expressing mature cell type.
iNKT cells differ from conventional T cells in that resting iNKT cells have transcriptionally active IL-4 and IFN-γ gene loci (28), whereas conventional T cells do not. Even the precursors of iNKT cells, NK1.1− thymic iNKT cells, transcribe and translate their IL-4 loci (15, 28, 29). It has been suggested that iNKT cells acquire this effector property during thymic development at the stage when they diverge into a distinct lineage from conventional T lymphocytes (30). Not only do VDR KO mice have fewer iNKT cells, but the remaining iNKT cells produce less IL-4 and IFN-γ. Therefore, there may be additional defects at the iNKT cell commitment stage that result in the diminished cytokine production by VDR KO iNKT cells.
The decreased numbers of iNKT cells in the VDR KO thymus are not due to increased cell death or reduced basal proliferation rates. WT BM transplantation into the VDR KO mouse resulted in normal numbers of iNKT cells in the thymus and spleen but significantly fewer in the liver (compared with WT recipients). The slower homeostatic proliferation in the VDR KO liver was not due to reduced CD1d expression in that organ, because hepatocytes from VDR KO and WT mice expressed the same level of CD1d. The failure of WT iNKT cells to repopulate the liver of VDR KO mice is likely because of decreased homeostatic proliferation and suggests that an important signal in this process is missing in the VDR KO liver.
The results of reciprocal BM transfers suggest that the VDR must be expressed in BM for normal positive selection of iNKT cells in the thymus. However, the precursor frequency for iNKT cells is not different in the VDR KO thymus as determined by competitive BM transplantation; because the percentage of VDR KO and WT iNKT cells in the repopulated WT thymus are the same. In addition, VDR expression did not affect Vα14-Jα18 rearrangement, because the Vα14-J18α transcript levels are comparable between VDR KO and WT TCRlow/−CD1d-αGalCer-DP thymoctyes (data not shown). We therefore conclude that the iNKT cell precursor frequency is not different in the VDR KO and WT mice.
Positive selection of iNKT cells is mediated by CD1d-expression on DP thymocytes (5). Analysis of CD1d expression and function showed lower surface CD1d expression and impaired antigen-presentation by VDR KO thymocytes. CD1d transfected VDR KO thymocytes were better than VDR KO thymocytes but still not as good as WT thymocytes at stimulating the NKT cell hybridoma. CD1d endosomal trafficking was not altered in the VDR KO mice. Instead, it seems likely that other factors, such as the level of the endogenous ligand or the level of a factor like Niemann-Pick type C2 protein needed to load endogenous ligand onto CD1d, might be defective in the VDR KO thymus (31). Reduced surface expression and function of CD1d in the VDR KO thymus is not a global result of the VDR affecting CD1d expression, because the peripheral DC from VDR KO mice had normal CD1d expression and functioned normally as antigen-presenting cells. In addition, iNKT cells develop normally in the CD1d heterozygous mice despite reduced CD1d expression in the thymus and the periphery (32, 33).
The VDR is a member of the steroid hormone superfamily of nuclear receptors that act as ligand inducible transcription factors. The ligand for the VDR is the active form of vitamin D, which is a nutrient/hormone with profound effects on the immune system. The absence of the VDR leads to diminished iNKT cell numbers in both the thymus and the periphery. The remaining iNKT cells fail to mature or express NK1.1 and T-bet. VDR deficiency results in the reduced thymic expression of CD1d that impairs the ability of thymocytes but not peripheral DC to act as APCs. The defects in VDR KO iNKT cells stem from both intrinsic and extrinsic requirements for VDR expression in the developmental pathway of iNKT cells.
Materials and Methods
Mice.
Age- and sex-matched C57BL/6 VDR KO and WT mice were produced at Pennsylvania State University. For some experiments, mice were fed synthetic diets that either included 50 ng of 1,25(OH) 2D3 per day (1,25D3) or did not include 1,25(OH) 2D3. The diets were fed beginning 1 week before and continuing throughout the experiment. Experimental procedures received approval from the Office of Research Protection Institutional Animal Care and Use Committee at the Pennsylvania State University.
αGalCer Stimulation.
αGalCer (Axxora) was dissolved in PBS containing 0.5% Tween 20, heated to 80°C for 10 min, and sonicated for 5 min on ice. Mice were given an i.p. injection of 2 μg of αGalCer or vehicle. Blood was collected from the retro-orbital plexus for serum isolation.
Flow Cytometry.
Single-cell suspensions of thymus, spleen, and liver were prepared. The liver was perfused with PBS via the portal vein and pressed through nylon screens (PGC Scientific). Total liver cells were layered onto a 40/80% Percoll solution (Sigma). Cells were stained with PE-labeled CD1d- αGalCer tetramers (gift from the National Institutes of Health Tetramer Facility, Atlanta, GA). mAbs used included PE-labeled anti-NK1.1 clone PK136, PE-Cy5-labeled anti-TCRβ clone H57–597, PE-labeled anti-CD1d clone 1B1, PE-Cy5-labeled anti-CD44 clone IM-7, FITC-labeled anti-CD3 clone 17A2, FITC-labeled anti-CD4 clone L3T4, FITC-labeled Annexin V, FITC BrdU flow kit, and FITC-labeled anti-CD45.1 clone A20 (BD Pharmigen). For intracellular staining, mice were injected i.p. with 2 μg of αGalcer. Two hours later, cells were isolated and cultured in the presence of brefeldin A (Sigma) for 6 h. Surface markers were stained and the cells were fixed, permeabilized, and stained with Alexa Fluor 488-labeled anti-IL-4 clone 11B11 or FITC-labeled anti-IFNγ clone B27 (BD PharMingen). The isotype controls were FITC-labeled or Alexa Fluor 488-labeled mouse IgG1 clone MOPC21 (BD PharMingen).
Cell Culture.
Splenocytes (5 × 105) were stimulated for 72 h with various concentrations of αGalCer or medium alone. For some cultures, 100 nM 1,25(OH) 2D3 dissolved in ethanol or the equivalent amount of ethanol was added to in vitro cultures. Supernatants were collected 48 h later for cytokine ELISA (BD PharMingen). Proliferation was measured by using [3H]thymidine incorporation and a Microbeta Trilux scintillation counter (LKB Wallac).
APCs, including purified splenic dendritic cells (DC, 98% CD11c+), thymocytes, and Rag KO splenocytes, were incubated alone or with 100 ng/ml of αGalCer and 5 × 104 of the NKT cell hybridoma DN32D3 (gift from A. Bendelac, University of Chicago, Chicago) or purified liver iNKT cells for 24 h. IL-2 content of the supernatants were measured by ELISA (BD PharMingen).
Retroviral CD1d Expression.
Thymocytes from WT and VDR KO mice were infected with the control retrovirus or CD1d-expressing retrovirus (gift from M. Kronenberg, La Jolla Institute for Allergy and Immunology, San Diego, CA) generated as described in ref. 34. Twenty-four hours later, infected thymocytes were incubated with 5 × 104 DN32D3 for 48 h. IL-2 content of the supernatants was measured by ELISA.
Quantitative mRNA Analysis.
Thymocytes and purified thymic iNKT cells were used to isolate mRNA, using the RNeasy mini kit (Qiagen) and reverse-transcribed (Promega). Real-time quantitative PCR was performed with DNA engine OpticonII (Bio-Rad), using iQ SYBR Green (Bio-Rad).
BrdU Incorporation Assay.
Mice were injected i.p. with 100 μl of 10 mg/ml BrdU dissolved in PBS at the onset of experiments. At the same time, mice were fed water that contained 0.8 mg/ml BrdU/5% glucose mixture, and the water was changed daily. Nine days later, thymocytes, splenocytes, and mononuclear cells from the liver were analyzed for BrdU incorporation, using a BrdU flow kit (BD PharMingen).
BM Transplantation.
BM cells were harvested from WT (CD45.1) and VDR KO (CD45.2) mice and transferred into lethally irradiated WT or VDR KO mice. For competitive BM transplantation, 1:1 mixtures of WT (CD45.1) BM cells and VDR KO (CD45.2) BM cells were transferred into WT (CD45.1) mice.
Confocal Microscopy.
Fresh thymocytes were adhered to poly-l-lysine treated coverslips. After fixation/permeabilization, cells were stained with FITC rat anti-mouse Lamp-1 20H2-biotin anti-CD1d revealed by PE Texas red Streptavidin (BD PharMingen). Coverslips were mounted onto slides, using the SlowFade kit (Molecular Probes). Samples were visualized under an Olympus IX70 inverted system fluorescence microscope (Hitech Instruments). Images were captured by using a SPOT camera (Diagnostic Instruments).
Statistical Analysis.
Statistical analyses were performed by using PRISM software (GraphPad). Cell percentage and numbers were compared by ANOVA. P values ≤ 0.05 were considered significant.
Acknowledgments.
We thank the National Institutes of Health tetramer facility and Dr. Bendelac and Dr. Kronenberg for the kind gifts of crucial reagents. This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant 1R01 NS38888.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: Facts, functions and fallacies. Immunol Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: Innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 3.Gumperz J, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 6.Ho I, Glimcher LH. Transcription: Tantalizing times for T cells. Cell. 2002;109:S109–S120. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 7.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: Prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 8.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 10.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 11.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 12.Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 13.Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 14.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 15.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 18.Roberts TJ, et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168:5409–5414. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- 19.Sugita M, et al. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity. 2002;16:697–706. doi: 10.1016/s1074-7613(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y-H, Chiu NM, Mandal M, Wang N, Wang C. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 21.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med. 2004;229:1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 22.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 23.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 27.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 28.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellicci DG, et al. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bezbradica J, et al. Granulocyte-macrophage colony-stimulating factor regulates effector differentiation of invariant natural killer T cells during thymic ontogeny. Immunity. 2006;25:487–497. doi: 10.1016/j.immuni.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Schrantz N, et al. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J Exp Med. 2007;204:841–852. doi: 10.1084/jem.20061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forestier C, et al. T cell development in mice expressing CD1d directed by a classical MHC class II promoter. J Immunol. 2003;171:4096–4104. doi: 10.4049/jimmunol.171.8.4096. [DOI] [PubMed] [Google Scholar]
- 33.Schumann J, et al. Targeted expression of human CD1d in transgenic mice reveals independent roles for thymocytes and thymic APCs in positive and negative selection of Valpha14i NKT cells. J Immunol. 2005;175:7303–7310. doi: 10.4049/jimmunol.175.11.7303. [DOI] [PubMed] [Google Scholar]
- 34.Elewaut D, et al. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Valpha14i NKT cells. J Exp Med. 2003;198:1133–1146. doi: 10.1084/jem.20030143. [DOI] [PMC free article] [PubMed] [Google Scholar]