Calcium signaling in lymphocytes (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 1.
Published in final edited form as: Curr Opin Immunol. 2008 Jun;20(3):250–258. doi: 10.1016/j.coi.2008.04.004
Abstract
In cells of the immune system, calcium signals are essential for diverse cellular functions including differentiation, effector function and gene transcription. After engagement of immunoreceptors such as T-cell and B-cell antigen receptors and the Fc receptors on mast cells and NK cells, the intracellular concentration of calcium ions is increased through the sequential operation of two interdependent processes: depletion of endoplasmic reticulum Ca2+ stores as a result of binding of inositol trisphosphate (IP3) to IP3 receptors, followed by “store-operated” Ca2+ entry through plasma membrane Ca2+ channels. In lymphocytes, mast cells and other immune cell types, store-operated Ca2+ entry through specialised Ca2+ release-activated calcium (CRAC) channels constitutes the major pathway of intracellular Ca2+ increase. A recent breakthrough in our understanding of CRAC channel function is the identification of STIM and ORAI, two essential regulators of CRAC channel function. This review focuses on the signaling pathways upstream and downstream of Ca2+ influx (the STIM/ ORAI and calcineurin/ NFAT pathways respectively).
Introduction
Calcium (Ca2+) is a universal second messenger with a pivotal role in almost all cell types[1,••2,••3]. In cells of the immune system, including T cells, B cells, mast cells and many other cell types, Ca2+ signals control proliferation, differentiation, apoptosis and a variety of transcriptional programmes[4,••5,••6]. The consequences of Ca2+ signals can be distinguished by whether short- or long-term functions are affected. Short-term functions are generally influenced within minutes and are independent of new gene expression. They include the regulation of lymphocyte motility and the degranulation of allergen-sensitized mast cells or cytolytic CD8+ T cells[7–10]. The interaction of T cells with antigen-presenting cells (APC) bearing antigenic peptides induces a quick increase of cytoplasmic Ca2+ concentration, which stops the movement of T cells and allows them to form stable immunological synapses, a process that is crucial for long-term function. Under conditions where high-affinity antigenic peptides and costimulatory signals are absent, T cells make only brief engagements with APC and display weak and infrequent Ca2+ spikes[11]. The long-term functions downstream of Ca2+ signalling include lymphocyte proliferation, expression of activation-associated genes, effector functions such as the production of cytokines and chemokines, the differentiation of naïve T cells into various effector or memory T cells, and the establishment – in the absence of costimulation -- of an antigen-unresponsive state known as anergy[4]. These events all need sustained Ca2+ influx to keep cytoplasmic Ca2+ concentrations at higher than basal levels for several hours.
Integration and crosstalk between Ca2+ and other signalling pathways in lymphocytes
In lymphocytes and mast cells, the main mechanism for entry of extracellular Ca2+ across the plasma membrane is store-operated Ca2+ entry (SOCE) through Ca2+ release-activated calcium (CRAC) channels[1]. Opening of CRAC channels leads directly to the sustained increase of intracellular Ca2+ concentrations and the long-term functional consequences described above. The importance of Ca2+ influx through store-operated CRAC channels is highlighted by the existence of at least three families of patients with severe combined immunodeficiency (SCID) associated with severe defects in store-operated Ca2+ entry and CRAC channel function, resulting in severely compromised cytokine expression and lymphocyte function[12–14]. Besides CRAC channels, other channels found in lymphocytes include L-type voltage-gated Ca2+ channel subunits, the P2X receptor, TRPC channels, TRPV channels and some TRPM channels[••2,••3], but their physiological importance in lymphocyte function remains unclear.
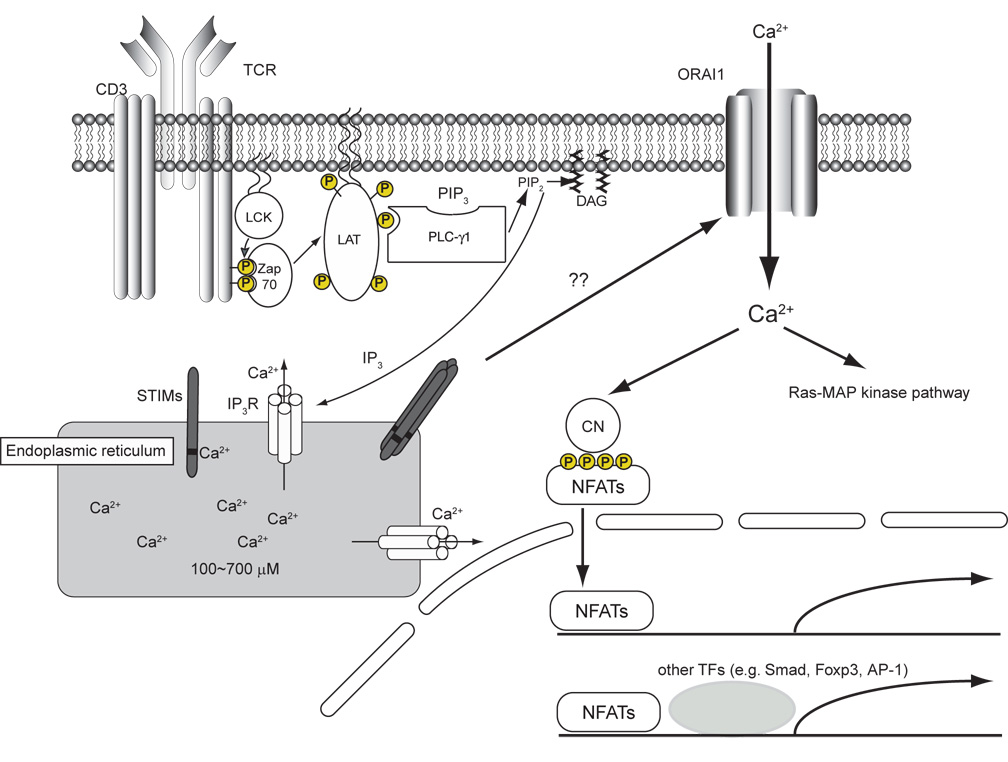
CRAC channels open in response to a lengthy signaling cascade, initiated when immunoreceptors, such as T and B cell antigen receptors (TCR, BCR) as well as mast cell and NK cell Fc receptors, are bound by antigenic complexes (MHC-peptide and antigen-Ig, respectively). Immunoreceptor activation leads to recruitment and activation of protein tyrosine kinases and formation of large protein complexes scaffolded by adapter proteins, ultimately resulting in tyrosine phosphorylation and activation of phospholipase C (PLC)-γ (PLC-γ1 in T cells and mast cells and PLC-γ2 in B cells)[••2,••3,9]. PLC-γ hydrolyzes phosphatidylinositol-3,4-bisphosphate (PIP2) to the two second messengers inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to IP3 receptors in the ER membrane and causes the release of ER Ca2+ stores; in turn, ER store depletion opens store-operated CRAC channels, which permit sustained Ca2+ influx into the cell (Figure 1). As a result, several Ca2+-dependent signaling proteins and their target transcription factors are activated, including the phosphatase calcineurin and its target NFAT(nuclear factor of activated T cells), CaMK(Ca2+- calmodulin-dependent kinase) and its target CREB(cyclic-AMP-responsive-element-binding protein) MEF2(myocyte enhancer factor 2) which is acted upon by both the calcineurin and CaMK pathways, and NFκB(nuclear factor κB). Simultaneously, DAG production activates the Ras-mitogen activated protein kinase (MAPK) and protein kinase C (PKC) pathways, which in turn lead to activation of the transcription factors AP-1 (a transcriptional complex formed by c-Jun and c-Fos) and NFκB[15,16]. Thus Ca2+ signaling is integrated with other signaling pathways and the integration occurs at the level of the binding of transcription factors to DNA response elements, resulting in cell proliferation and cytokine gene expression. A notable example of such integration involves the formation of a cooperative NFAT:Fox:Jun complex on DNA, which drives the transcription of a large number of activation-associated genes[4,17].
Figure 1.
Store-operated Ca2+ entry in T cells. The binding of antigen/MHC complexes to T cell receptor (TCR) triggers the activation of protein tyrosine kinases, such as LCK and ZAP70, which eventually results in tyrosine phosphorylation and activation of PLC-γ1. PLC-γ1 hydrolyzes the membrane phospholipid PIP2 to IP3 and DAG. IP3 opens IP3 receptors (IP3R), which permits Ca2+ efflux from ER Ca2+ stores. The ER Ca2+ sensors STIM1 and STIM2 sense the resulting reduction of ER Ca2+ stores via their paired N-terminal EF-hands located in the ER lumen. After Ca2+ dissociates from the EF-hands, STIM proteins aggregate into small clusters (“puncta”) in the ER membrane and trigger store-operated Ca2+ entry via the CRAC channel, ORAI1. Ca2+ influx elevates intracellular Ca concentration and activates the calcineurin-NFAT pathway as well as regulates the Ras-MAPK pathway.
In addition, Ca2+ signaling directly regulates the Ras-MAPK signalling pathway. Ras is activated by Ras guanine nucleotide exchange factor (RasGEF) and inactivated by Ca2+-activated RasGAPs, including CAPRI and GAP1[18]. Major RasGEFs in T cells are Sos (son of sevenless) and RasGRP1 (Ras guanine nucleotide releasing protein 1)[19,20]. RasGRP1 has a DAG-binding C1 domain and a pair of EF hand motifs that bind to Ca2+ directly[21]. RasGRP1 localizes in the cytoplasm of resting T cells and translocates to the Golgi apparatus in response to weak stimulation, such as with soluble anti-CD3 antibodies or with low-affinity peptides that induce positive selection of thymocytes[22,••23]. The RasGEF activity of RasGRP1 in the Golgi depends both on DAG and on increased intracellular Ca2+ concentration[22]. RasGRP1 is barely expressed in thymocytes at the double-negative (CD4− CD8−, DN) to double-positive (CD4+CD8+, DP) stages[24]; instead, the calcineurin/ NFAT pathway was shown to modulate Ras activation in CD4+CD8+CD69− DP thymocytes that have not yet undergone positive selection, altering the threshold for ERK activity and allowing weak TCR signals to induce positive selection[25]. B-Raf, MEK and ERK activation are all impaired in DP thymocytes from mice lacking the calcineurin regulatory subunit CnB1, suggesting that calcineurin/ NFAT-dependent transcription regulates the Raf/ Ras/ MAP kinase pathway at several levels, both upstream and downstream of Raf.
In T cells, a major pathway downstream of Ca2+ influx is the calcineurin-NFAT pathway, which is well characterized in its molecular aspects. Calcineurin is a calmodulin-dependent serine/threonine phosphatase, consists of the catalytic subunit calcineurin A and a regulatory subunit, calcneurin B. In mammals, there are three known isoforms of calcineurin A, CnAα, CnAβ and CnAγ, and two isoforms of calcineurin B, CnB1 and CnB2. NFAT consists of a family of four transcription factors (NFAT1-4, also known as NFATc1-c4)[4,17]. All NFATs except NFAT3 are expressed in peripheral lymphocytes, however, NFAT1 is preferentially expressed in SP thymocytes, NFAT2 in DN thymocytes and B cells, NFAT4 in DP thymocytes[26,27]. An increase in [Ca2+]i results in binding of Ca2+-calmodulin to calcineurin and activation of its phosphatase activity. Dephosphorylation of cytoplasmic NFAT proteins by calcineurin unmasks the nuclear localization sequence and then NFAT proteins translocate into nucleus[4]. NFAT-driven gene expression is highly dependent on sustained Ca2+ influx and calcineurin activity, because a decrease in intracellular Ca2+ levels or treatment with the calcineurin inhibitor cyclosporin A results in the immediate export of NFAT from nucleus by NFAT kinases, which include GSK3 (glycogen synthase kinase 3), CK1 (casein kinase 1) and DYRK1A (dual-specificity tyrosine-phosphorylation regulated kinase 1A)[28–30].
The physiological roles of the calcineurin-NFAT pathway have been extensively studied using gene-disrupted mice. The most severe phenotypes are observed in _Cnb1_-deficient mice, in which conventional TCRαβ T cell development is blocked at the double-positive (DP) to single-positive (SP) transition and thymocyte positive selection is impaired[31]. CnB1 is also crucial for B cell responses in vivo: mice lacking Cnb1 specifically in B cells show reduced plasma cell differentiation and decreased antigen-specific antibody production during T cell-dependent immune responses [32]. In contrast, the impact on conventional T cell development observed in mice singly disrupted for individual Nfat genes or combinations of two Nfat genes is less than what is observed in _Cnb1_-deficient mice[33]. Probably, residual Nfat gene products can compensate for the loss of other isoforms.
Crosstalk between Ca2+ and Notch signaling appears to be important in both keratinocytes and T cells. In keratinocytes, Notch signaling increases calcineurin/NFAT activity by downregulating calcipressin, an endogenous calcineurin inhibitor[34]. In turn, calcineurin/NFAT signaling affects the expression of Notch-responsive genes. Notch signaling is crucial for early T cell development[35]. Thus Notch signaling potentially cooperates with the calcineurin/NFAT pathway in T cells as well as in keratinocytes.
Store-operated Ca2+ entry through CRAC channels: STIM and ORAI
As a result of recent technological advances that have made it possible to perform large-scale RNA interference (RNAi) screens, two key regulators of store-operated Ca2+ entry, STIM (an ER Ca2+ sensor)[36,37] and ORAI (a pore subunit of the CRAC channel)[••38,•39,•40], have been identified by several groups. These proteins have been extensively investigated over the last two years, and their analysis has greatly advanced our understanding of the mechanisms of SOCE. Stim (stromal cell interaction molecule[41]) was identified in two limited RNAi screens, performed in Drosophila and HeLa cells respectively[36,37]. Flies and worms express only one STIM protein[36], whereas mammals express two STIM proteins, STIM1 and STIM2, with 47% amino acid identity[42]. STIM1 and STIM2 are both single-pass transmembrane proteins with paired N-terminal EF-hands located in the ER lumen and protein interaction domains located both in the ER lumen and in the cytoplasm. The first EF-hand of each pair binds Ca2+ with low affinity matched to the high concentration of Ca2+ in the ER (~100–700 µM) [••6,36,37].
STIM1 is an acknowledged activator of store-operated Ca2+ entry and CRAC channel function as discussed below, but the role of STIM2 was initially controversial, with conflicting results reported by different groups[36,37,43]. An important clue as to the possible distinction between the two proteins came when STIM2 showed up as a major hit in an RNAi screen designed to find regulators of basal Ca2+ concentration, which is kept within very narrow limits in cells[••44]. Both STIM1 and STIM2 were able to sense depletion of ER Ca2+ stores and trigger store-operated Ca2+ entry via ORAI1, but RNAi-mediated knockdown of STIM2 selectively decreased basal cytosolic and ER Ca2+ levels in several cell lines. These findings are supported by studies of STIM-deficient cells. T cells and fibroblasts from mice with a conditional deletion of STIM1 showed a pronounced decrease in store-operated Ca2+ entry and CRAC channel function, which could be rescued efficiently by STIM1 and less efficiently by STIM2[••45]. T cells lacking STIM1 showed only a transient blip of NFAT nuclear localisation due to ER store depletion, whereas T cells lacking STIM2 showed a normal initial phase of NFAT nuclear localisation which however was not sustained[••45]. Together, these results confirm that both STIM1 and STIM2 are functional ER Ca2+ sensors that can trigger store-operated Ca2+ entry through CRAC channels in activated (store-depleted) cells. However, STIM1 is activated primarily during the early phase of the response when ER Ca2+ stores are strongly depleted, whereas STIM2 operates both in resting cells with Ca2+-replete stores where it is already partially active and controls basal Ca2+ influx, and also during the late stages of a response when ER stores are becoming refilled. At these late time, STIM1 is inactivated by rebinding of Ca2+ whereas STIM2 remains active[••44,••45].
Drosophila Orai (olf186-F) was discovered as an essential regulator of store-operated Ca2+ influx in genome-wide Drosophila RNAi screens performed by three different groups[••38,•39,•40]. Drosophila Orai and its three human homologues, ORAI1-3, are small proteins with four transmembrane domains, whose N and C-termini are both located in the cytoplasm[••38,46, •47]. In humans, a single missense mutation in the Orai1 gene caused severe combined immunodeficiency disease characterized by the absence of SOCE and ICRAC[••38]. Mutational and electrophysiological analyses have identified Drosophila Orai and human ORAI1 as pore subunits of the CRAC channel[•47,•48,•49]. Consistent with this hypothesis, ORAI1 exists in the plasma membrane as at least a dimer, and its active form is likely to be a homotetramer[46,50]. Moreover, combined overexpression of ORAI1 and STIM1 leads to marked increases in CRAC current in cells[•49,51,52]. ORAI2 and ORAI3 have similar but not identical electrophysiological characteristics to ORAI1[53,54].
Gating of CRAC channels by local aggregation of STIM and ORAI
Following store depletion, STIM1 aggregates into small clusters (“puncta”) in the ER membrane[37,55]; STIM1 with an EF-hand mutation that impairs Ca2+ binding is constitutively present in puncta even in resting cells with replete Ca2+ stores[37]. The propensity of STIM1 to aggregate when its EF-hands are not bound by Ca2+ can be demonstrated using just a short recombinant N-terminal fragment of STIM1, containing only the EF-hands and the adjacent SAM (sterile α-motif) domain[56,57]. Once formed, the STIM1 clusters preferentially localize to sites of ER-plasma membrane apposition and colocalize partially with clusters of ORAI1[55,58,••59]. The regions of STIM1 clustering coincide with sites of Ca2+ entry[••60].
STIM1 and ORAI1 are the primary regulators of store-operated Ca2+ entry. Combined overexpression of STIM1 and ORAI1 leads to massive (up to 100-fold) increase in CRAC currents in many cell types, whereas expression of the individual proteins has less effect or may even be inhibitory[•49,51,52], implying that STIM1 is the major limiting component needed to open ORAI1-containing CRAC channels in response to store depletion. Whether there is a direct, physiologically relevant physical interaction between STIM1 and ORAI1 in cells remains controversial, however: no interaction, constitutive interaction and increased interaction after store depletion have all been reported [46, •47, •49]. Two reports suggest that overexpression of the cytoplasmic region of STIM1 in cells suffices to induce CRAC currents[61,62]. A coiled-coil domain in the cytoplasmic region of STIM1 is crucial for puncta formation and store-operated Ca2+ entry[63,64], and this region has been suggested to interact directly with a coiled-coil domain at the C-terminus of ORAI1, both in cell-free systems and by FRET after store depletion in cells[62]. Wu et al. estimated a minimum distance of 17 ± 10 nM between the ER and plasma membranes for ER-plasma membrane appositions at which ORAI1 and STIM1 come together[••59]; this distance could potentially be spanned by the cytoplasmic region of STIM1 in an extended conformation, but does not rule out the existence of a larger protein complex bridging STIM1 and ORAI1. Using chemical dimerisers to artificially bring together the ER and plasma membranes, Varnai et al. demonstrated that before store depletion, STIM1 was capable of diffusing freely within these regions even when the membranes were estimated to be only 4–6 nm apart, whereas ORAI1 was excluded unless the membranes were between 8 and 14 nm apart[•65]. After store depletion, an estimated distance of 12–14 nm between the ER and plasma membranes was required for STIM1 and ORAI1 to colocalise within the area of the apposed membranes instead of being confined to their periphery[•65]. This estimated distance is almost the same as the maximal distance for an efficient FRET signal (usually within 10 nm), suggesting that the possibility of indirect rather than direct interaction between STIM1 and ORAI1 still cannot be ruled out.
In T cells, STIM1 and ORAI1 both translocate to the region of the immunological synapse that forms between T cells and antigen-presenting dendritic cells, colocalising with the T cell receptor (TCR) and co-stimulatory molecules[•66]. This interface coincides with local Ca2+ influx from the extracellular space. Interestingly, tyrosine-phosphorylated IP3R1 also colocalizes with the TCR after stimulation; this modification seems to increase the IP3 sensitivity of the receptor while decreasing its inactivation by high concentrations of Ca2+[67]. Presumably, co-localisation of these molecules at the immunological synapse is essential for augmentation and sustenance of local Ca2+ influx at the contact zone between T cells and antigen-presenting cells, thus facilitating long-term activation of antigen-stimulated T cells.
Physiological consequences of individual deficiency of STIM1, STIM2 or ORAI1 in mice
_Stim1_-deficient mice have been generated by our laboratory[••45] and by one other group[•68] Stim1 deficiency results in perinatal lethality, leading us to investigate mice in which STIM1 was deleted only in peripheral T cells. _Stim1_-deficient T cells completely lack store-operated Ca2+ entry, ICRAC and Ca2+-dependent cytokine expression[••45]. In contrast, _Stim2_-deficient naïve T cells showed normal store-operated Ca2+ entry and cytokine production; this was not due to compensatory upregulation of STIM1, but rather reflects the fact that STIM2 constitutes only a very small proportion of total STIM protein in T cells. Mast cells from _Stim1_-deficient mice were defective in degranulation owing to a severe decrease of store-operated Ca2+ entry[•68]; this was also true of mast cells from mice with a gene-trap insertion in the Orai1 gene[•69]. Passive cutaneous anaphylaxis, a reaction which measures mast degranulation in vivo, was substantially impaired in the Orai1 gene-trap mutant mice, and in fact was decreased even in heterozygous (haploinsufficient) Stim1+/− mice[•68,•69]. These data emphasise that mast cell function is strongly dependent on store-operated Ca2+ entry through STIM1 and ORAI1.
In contrast to NFAT, NFκB is selectively activated by a large transient increase of Ca2+ concentration and activates target gene expression[1,4]. Activation of NFAT and NFκB is also sensitive to the frequency of [Ca2+]i oscillations[1,4]. NFκB is preferentially activated by low-frequency Ca2+ oscillations, whereas both NFAT and NFκB are activated by high-frequency oscillations. Consistent with these observations, nuclear translocation of p65 RelA is significantly decreased in STIM1-deficient mast cells and T cells[•68](M Oh-hora et al., unpublished).
T cell development was found to be normal in Orai1 gene-trap mutant mice[•69], as well as in mice with a null mutation in the Orai1 gene generated in our laboratory (Y Gwack, S Srikanth, M Oh-hora et al., unpublished). However, a major difference between these two mouse lines lies in the degree of impairment of T cell function. T cells from the ORAI1 gene-trap mice showed decreased cytokine expression in response to stimulation but no obvious impairment in store-operated Ca2+ entry or CRAC channel function[•69], suggesting that these mice are hypomorphs rather than true nulls. In contrast, T cells from ORAI1-null mice showed a clear impairment in all three functions, that was especially pronounced in previously activated and differentiated cells (Y Gwack, S Srikanth, M Oh-hora et al., unpublished). This result probably reflects compensation from ORAI2 or ORAI3; in particular, ORAI2 is expressed at relatively high levels in naïve T cells but is downregulated upon activation. Surprisingly, however, acute expression of ORAI2 in Orai1-null T cells did not reconstitute function, implying that other factors are needed.
Unexpected requirement for STIM proteins in development and function of regulatory T cells
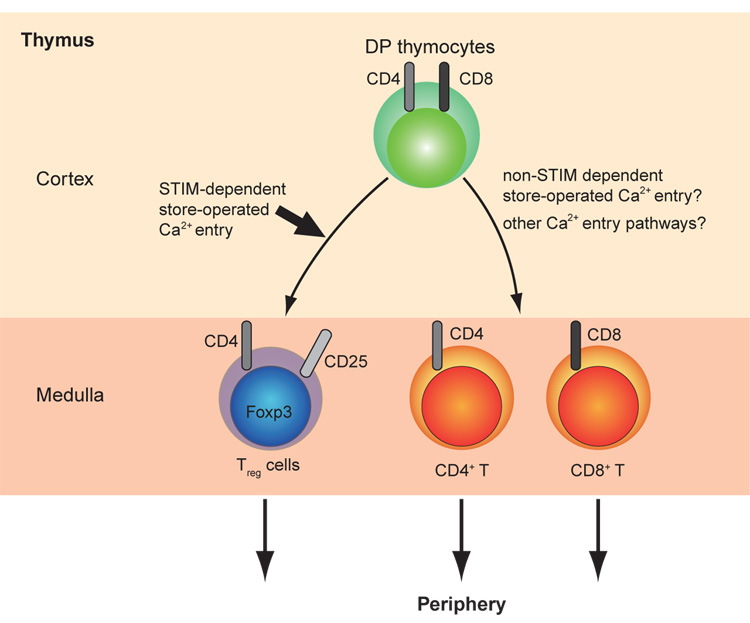
Unexpectedly, mice in which both Stim1 and Stim2 had been deleted in peripheral T cells by expression of Cre under control of the CD4 promoter showed a striking decrease of regulatory T cell (Treg) population in all immune organs and developed autoimmune symptoms[••45]. The effect was selective for the Treg lineage, since the mice showed normal thymic cellularity and normal development of thymocytes and peripheral T cells. Thus, Treg development appears to be especially sensitive to the absence of STIM-mediated Ca2+ influx (Figure 2). These data are consistent with other studies showing involvement of the calcineurin-NFAT pathway in Treg development and function[70–•72].
Figure 2.
T cell development in thymus. CD4+ CD8+ double positive (DP) thymocytes differentiate into conventional CD4+ or CD8+ single positive (SP) αβTCR+ thymocytes, or CD4+ CD25+ Foxp3+ regulatory T cells (Treg cells). STIM-dependent store-operated Ca2+ entry appears to be crucial for the development of Treg cells but dispensable for the development of conventional αβTCR+ thymocytes[••45]. We speculate that other Ca2+ entry pathways might compensate for loss of STIM-dependent store-operated Ca2+ entry in the differentiation of DP thymocytes into conventional αβTCR+ SP thymocytes.
How does the calcineurin-NFAT pathway impinge on Treg development and function? In “natural” (i.e. thymic-derived) regulatory T cells and “induced” regulatory T cells (i.e. induced by TGFβ treatment in culture), NFAT regulates Foxp3 expression in combination with Smad3[•73]. Smad3 binds to a Foxp3 distal enhancer early during TGFβ treatment, whereas NFAT binding to the 5’ enhancer occurs at later times, which suggests that NFAT is responsible for maintaining Foxp3 expression. Indeed, Foxp3 expression in activated, TGFβ-treated T cells is blocked by treatment with either cyclosporin A or the Smad3 inhibitor SIS3[•73,74]. Foxp3 can also physically interact with NFAT, and this complex regulates the suppressive function of Foxp3-expressing T cells in a mouse model of autoimmune diabetes[•72]. Together, these data show that STIM proteins, NFAT and Foxp3 are all involved in a positive feedback pathway that enhances Treg development and function.
In contrast to the clear effect of combined Stim1, Stim2 deletion, the role of the downstream proteins calcineurin and NFAT in Treg development and function is not yet fully resolved. Mice with a double deletion of Nfat1 and Nfat4 also show lymphoproliferative autoimmune phenotypes[75]. In these mice, Treg development and function appeared normal, and hyperproliferation was attributed to the fact that their T cells were not effectively inhibited by wild-type Tregs[76]. Furthermore, _Cnb1_-deficient mice have not so far been reported to show any dysfunction or impaired development of Tregs[31]. Further studies are needed to elucidate the complex effects of mutations in the Ca2+/ calcineurin/ NFAT signalling pathway on T cell and Treg development.
Conclusions
The field of Ca2+ signalling has been reinvigorated in the last two years by the discovery of two key players in store-operated Ca2+ entry, STIM and ORAI. It is now possible to investigate the function of this pathway in molecular detail. Outstanding questions remain: What is the molecular composition of CRAC channels? How are CRAC channels gated through co-aggregation of STIM and ORAI? What are the unique biological functions of STIM and ORAI proteins and other components of the Ca2+ signalling pathway, including downstream effectors such as NFAT proteins, in vivo? The availability of conditional mouse genetic models will greatly facilitate the study of these questions in a physiological context in different cell types. Both store-operated Ca2+ entry and the Ca2+-calcineurin-NFAT pathway are validated targets for the development of immunosuppressive drugs, the first because of the existence of a SCID patient with an Orai1 R186W mutation who shows only mild extra-immunological symptoms[12, ••38] and the second because of the well-established clinical efficacy of the immunosuppressive drugs cyclosporin A and FK506[4,17]. A further understanding of these pathways will not only provide further insights into lymphocyte and mast cells activation, but may also contribute to the development of new therapeutic modalities.
ACKNOWLEDGMENTS
This work was supported by NIH and JDRF grants (to A.R.). M.O. was supported by a research fellowship from the Uehara Memorial Foundation.
Footnotes
Conflict of interest. A.R. is a scientific advisor to CalciMedica, a company whose research on immune therapies includes a focus on inhibitors of STIM-ORAI pathway.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- ••2.Feske S.Calcium signalling in lymphocyte activation and disease Nat Rev Immunol 20077690–702.A good recent review on the role of calcium signalling in lymphocytes from basic mechanism of calcium influx to clinical aspects. [DOI] [PubMed] [Google Scholar]
- ••3.Scharenberg AM, Humphries LA, Rawlings DJ.Calcium signalling and cell-fate choice in B cells Nat Rev Immunol 20077778–789.A good recent review on the role of calcium signalling in B cell differentiation and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- ••5.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- ••6.Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32:235–245. doi: 10.1016/j.tibs.2007.03.009.••5, ••6These two recent reviews provide comprehensive accounts of CRAC channels:history, electrophysiology, gating, and function.
- 7.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 8.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 9.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 10.Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr Opin Immunol. 2007;19:301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei SH, Safrina O, Yu Y, Garrod KR, Cahalan MD, Parker I. Ca2+ signals in CD4+ T cells during early contacts with antigen-bearing dendritic cells in lymph node. J Immunol. 2007;179:1586–1594. doi: 10.4049/jimmunol.179.3.1586. [DOI] [PubMed] [Google Scholar]
- 12.Feske S, Muller JM, Graf D, Kroczek RA, Drager R, Niemeyer C, Baeuerle PA, Peter HH, Schlesier M. Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur J Immunol. 1996;26:2119–2126. doi: 10.1002/eji.1830260924. [DOI] [PubMed] [Google Scholar]
- 13.Le Deist F, Hivroz C, Partiseti M, Thomas C, Buc HA, Oleastro M, Belohradsky B, Choquet D, Fischer A. A primary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood. 1995;85:1053–1062. [PubMed] [Google Scholar]
- 14.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 15.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 16.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 18.Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- 19.Gong Q, Cheng AM, Akk AM, Alberola-Ila J, Gong G, Pawson T, Chan AC. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 20.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 21.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-inding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 22.Bivona TG, Perez De Castro I, Ahearn IM, Grana TM, Chiu VK, Lockyer PJ, Cullen PJ, Pellicer A, Cox AD, Philips MR. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- ••23.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269.Using elegant technique, the authors show that RasGRP1 translocates to the Golgi apparatus in response to stimulation of thymocytes with low-affinity peptide/MHC complexes, confirming the results in [22].
- 24.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 25.Gallo EM, Winslow MM, Cante-Barrett K, Radermacher AN, Ho L, McGinnis L, Iritani B, Neilson JR, Crabtree GR. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature. 2007;450:731–735. doi: 10.1038/nature06305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oukka M, Ho IC, de la Brousse FC, Hoey T, Grusby MJ, Glimcher LH. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 1998;9:295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- 27.Amasaki Y, Masuda ES, Imamura R, Arai K, Arai N. Distinct NFAT family proteins are involved in the nuclear NFAT-DNA binding complexes from human thymocyte subsets. J Immunol. 1998;160:2324–2333. [PubMed] [Google Scholar]
- 28.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 29.Okamura H, Garcia-Rodriguez C, Martinson H, Qin J, Virshup DM, Rao A. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol. 2004;24:4184–4195. doi: 10.1128/MCB.24.10.4184-4195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 31.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 32.Winslow MM, Gallo EM, Neilson JR, Crabtree GR. The calcineurin phosphatase complex modulates immunogenic B cell responses. Immunity. 2006;24:141–152. doi: 10.1016/j.immuni.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Cante-Barrett K, Winslow MM, Crabtree GR. Selective role of NFATc3 in positive selection of thymocytes. J Immunol. 2007;179:103–110. doi: 10.4049/jimmunol.179.1.103. [DOI] [PubMed] [Google Scholar]
- 34.Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, Botchkarev VA, Crabtree GR, Dotto GP. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005;8:665–676. doi: 10.1016/j.devcel.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 36.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and consestore-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••38.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- •39.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •40.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl Acad. Sci.U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103.••38, •39, •40. In these articles, three separate groups describe how they independently identified Drosophila ORAI as an essential regulator of store-operated Ca entry. Feske et al [••52] additionally showed that a single missense mutation in human Orai1 is the underlying molecular defect in a family with hereditary severe combined immunodeficiency disease.
- 41.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J. Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem. J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr. Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- ••44.Brandman O, Liou J, Park WS, Meyer T.STIM2 Is a Feedback Regulator that Stabilizes Basal Cytosolic and Endoplasmic Reticulum Ca(2+) Levels Cell 20071311327–1339.The authors showed in an elegant RNAi screen that STIM2 is one of the primary regulators of basal Ca2+ concentration in several cell types. They also showed that the Ca2+ sensitivity of STIM2 is two-fold lower than that of STIM1, such that STIM2 remained active even after partial refilling of ER Ca2+ stores when STIM1 was no longer active.18160041 [Google Scholar]
- ••45.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574.Through analysis of T cells and fibroblasts from mice lacking STIM1 or STIM2, the authors show -- confirming the results in [••44] -- that both STIM proteins are positive regulators of store-operated Ca2+ entry. They further find that regulatory T cell development and function are strikingly impaired in mice with combined T-cell-specific deletion of both STIM1 and STIM2.
- 46.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- •47.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •48.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- •49.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108.•47–49. These three articles show that mutation of conserved acidic residues in and near predicted transmembrane segments of ORAI1 change the ion selectivity of the CRAC current, proving that ORAI1 is a pore subunit of the CRAC channel.
- 50.Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586:419–425. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 52.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 54.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 57.Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca(2+) sensory region of STIM1 and STIM2. Biochem Biophys Res Commun. 2007 doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 58.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- ••59.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••60.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015.••59,60. These two papers show that the formation of STIM1 clusters is induced before the beginning of store-operated Ca2+ entry and that STIM1 preferentially localizes to sites of ER-plasma membrane apposition. The regions of STIM1 clusters coincide with ORAI1 clusters and sites of local Ca2+entry.
- 61.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nature Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 62.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, et al. Dynamic Coupling of the Putative Coiled-coil Domain of ORAI1 with STIM1 Mediates ORAI1 Channel Activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 63.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl Acad. Sci. U S A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- •65.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T.Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex J Biol Chem 200728229678–29690.The authors show that ORAI1 needs a larger intermembrane space between the ER and plasma membranes than STIM1 for free diffusion in resting cells. Moreover a similar large distance is required for ORAI1-STIM1 colocalisation after store-depletion. Both results suggest that ORAI1 is associated with a large macromolecular complex. [DOI] [PubMed] [Google Scholar]
- •66.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD.Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation Proc Natl Acad Sci U S A 20081052011–2016.The first report of the behaviour of STIM1 and ORAI in antigen-stimulated T cells. The authors show that both molecules localise to the immunological synapse, which is also the site of Ca2+ influx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.deSouza N, Cui J, Dura M, McDonald TV, Marks AR. A function for tyrosine phosphorylation of type 1 inositol 1,4,5-trisphosphate receptor in lymphocyte activation. J Cell Biol. 2007;179:923–934. doi: 10.1083/jcb.200708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •68.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546.These authors established a different line of STIM1-deficient mice from those described in [••45], and showed that STIM1-deficient mast cells and mice displayed impaired degranulation and an impaired passive cutaneous anaphylaxis response respectively.
- •69.Vig M, Dehaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550.The authors generated ORAI1-deficient mice from ES cells with a gene-trap insertion in the Orai1 gene. The phenotypes of mast cells from these mice are similar to those observed in STIM1-deficient mice [•68].
- 70.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity. Transplantation of the thymus from cyclosporin A-treated mice causes organ-specific autoimmune disease in athymic nude mice. J Exp Med. 1988;167:1479–1485. doi: 10.1084/jem.167.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem. 2006;281:36828–36834. doi: 10.1074/jbc.M608848200. [DOI] [PubMed] [Google Scholar]
- •72.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042.This article illustrates that NFAT can physically interact with Foxp3, and this complex regulates Treg suppressive function. These results support the phenotypes of STIM1 and STIM2 double deficient mice [••45].
- •73.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M.Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer Nat Immunol 20089194–202.NFAT regulates Foxp3 expression in combination with Smad3. [DOI] [PubMed] [Google Scholar]
- 74.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 75.Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9:627–635. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- 76.Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C, Klein M, Schild H, Schmitt E, Stassen M. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201:181–187. doi: 10.1084/jem.20041538. [DOI] [PMC free article] [PubMed] [Google Scholar]