Smad Proteins and Hepatocyte Growth Factor Control Parallel Regulatory Pathways That Converge on β1-Integrin To Promote Normal Liver Development (original) (raw)
Abstract
Smads serve as intracellular mediators of transforming growth factor β (TGF-β) signaling. After phosphorylation by activated type I TGF-β receptors, Smad proteins translocate to the nucleus, where they serve as transcription factors and increase or decrease expression of TGF-β target genes. Mice lacking one copy each of Smad2 and Smad3 suffered midgestation lethality due to liver hypoplasia and anemia, suggesting essential dosage requirements of TGF-β signal components. This is likely due to abnormal adhesive properties of the mutant hepatocytes, which may result from a decrease in the level of the β1-integrin and abnormal processing and localization of E-cadherin. Culture of mutant livers in vitro revealed the existence of a parallel developmental pathway mediated by hepatocyte growth factor (HGF), which could rescue the mutant phenotype independent of Smad activation. These pathways merge at the β1-integrin, the level of which was increased by HGF in the cultured mutant livers. HGF treatment reversed the defects in cell proliferation and hepatic architecture in the Smad2+/−_; Smad3_+/− livers.
Smads serve as intracellular mediators of transforming growth factor β (TGF-β) signaling (reviewed in reference 21). There are three classes of Smad proteins, including receptor-activated or R-Smads, inhibitory Smads, and the common Smad, Smad4/Dpc4. Smad2 and Smad3 are R-Smads that relay signals from TGF-β and activin receptors. After phosphorylation by activated type I TGF-β receptors, Smad2 and/or Smad3 proteins translocate to the nucleus in concert with Smad4, where they serve as transcription factors that increase or decrease expression of TGF-β target genes (reviewed in references 21 and 33). Disruption of murine Smad2 led to gastrulation stage lethality (24, 31, 32), while mice lacking Smad3 were viable but suffered from impaired mucosal immune responses (7, 34) or colon cancer (38).
Although TGF-β is a well-known contributor to liver fibrosis and hepatocellular carcinoma (1, 14), little is known about its functions during normal liver development. Liver development commences with the formation of the hepatic bud, an outgrowth of the foregut endoderm. Endodermal cells migrate out into the surrounding mesenchyme to form the liver parenchyma, which later becomes the primary site of embryonic blood formation (reviewed in reference 34). The results presented here indicate that signals of the TGF-β superfamily are involved in liver outgrowth, as mice that lack one copy each of Smad2 and Smad3 exhibit abnormal liver development. This defect can be overridden by hepatocyte growth factor (HGF) in vitro, suggesting a parallel pathway operating during hepatogenesis.
MATERIALS AND METHODS
Generation of Smad2 +/− ; Smad3 +/− embryos.
Smad2 and Smad3 mutations were maintained on a mixed 129Svev/NIH Black Swiss background. The presence of the mutations was monitored by PCR as described previously (32, 34).
RT-PCR.
Total RNA was prepared from liver samples using RNA-Stat 60 (Tel-Test, Inc., Friendswood, Tex.) and subjected to a reverse transcription-PCR (RT-PCR) analysis using standard procedures. Integrins were amplified using the following primers: α2-integrin, 5′-GCAATGTGACCGTGATTCAG-3′ and 5′-TTGGACCCAAGGATTTTCTG-3′; αM-integrin, 5′-TGTGACAGGCACTTGAGAGG-3′ and 5′-CCATCCCATCTTTCCTGCTA-3′; αV-integrin, 5′-TTCAACCTGGACGTCGAAAG-3′ and 5′-TATCCTGCTTTGACCTCACA-3′; β3-integrin, 5′-GATGCAATCATGCAGGTTGC-3′ and 5′-TGTAGGCATCGATGATTAGC-3′; GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-ACAGCCGCATCTTCTTGTGC-3′ and 5′-TTTGATGTTAGTGGGGTCTCGC-3′. HGF was amplified using primers 5′-TGCCAGAAAGATATCCCGAC-3′ and 5′-AACTCGGATGTTTGGGTCAG-3′.
Histology, in situ hybridization, and immunohistochemistry.
Paraffin sectioning, hematoxylin and eosin (H&E) staining, in situ hybridization, and immunohistochemistry were performed by standard methods. The HGF probe used for in situ hybridization is a 1.7-kb _Xho_I/_Bam_HI fragment of the mouse HGF cDNA cloned into pBluescript (a gift from Bill LaRochelle). An anti-PCNA antibody was purchased from Signet (Dedham, Mass.), and a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay detection system was purchased from Intergen (Purchase, N.Y.). These were used according to the manufacturers' directions. Percentages of proliferative and apoptotic cells were the averages of counts from over 700 cells from each of three individuals with normal and Smad2+/−; Smad3+/− livers. An α-fetoprotein antibody was obtained from ICN and used as described previously (22). Smad2 and Smad3 antibodies, a kind gift from Akiko Hata, recognized epitopes in the MH1 domain of Smad2 and linker domain of Smad3, respectively. Confocal micrographs were taken on a Zeiss confocal microscope.
In vitro culture of embryonic liver.
Livers and heart mesenchyme were excised from day 10.5 (E10.5) embryos as described previously (22). These were cultured in BGJb medium (Gibco) on sterilized 0.8-μm filters (Millipore) supported by metal grids in organ culture dishes (Fisher). TGF-β and HGF were purchased from Research Genetics and Sigma, respectively.
Hepatocyte adhesion assays.
Livers were dissected from E13.5 wild-type and mutant embryos and dissociated with 330 μg of collagenase/ml. They were plated on chamber slides coated with collagen or fibronectin (Sigma) and cultured for 2 days. Slides were subsequently washed, fixed, and stained with either hematoxylin or rhodamine-conjugated phalloidin (Molecular Probes). For integrin inhibition analysis, RGD peptides were added at 15 μg/μl, while a 26-μg/μl RGD peptide concentration was sufficient to abolish hepatocytic adhesion.
Western blotting.
Twenty micrograms of each sample was run on 4 to 12% NuPAGE gels (Novex, San Diego, Calif.) and transferred to nitrocellulose according to the manufacturer's directions. β1-Integrin, Smad2, and horseradish peroxidase-coupled antimouse antibodies were purchased from Transduction Labs (Lexington, Ky.). Cyclin E and actin antibodies were purchased from Santa Cruz Biotechnology. The Smad3 antibody was purchased from Zymed, while antibodies to activated mitogen-activated protein (MAP) kinases were purchased from New England Biolabs. All of these were used as directed.
RESULTS
To examine potential genetic interactions between the two highly related genes Smad2 and Smad3, mice heterozygous for either Smad2 (Smad2+/−) or Smad3 (Smad3+/−) disruptions were interbred. However, only 1 doubly heterozygous (Smad2+/−; Smad3+/−) offspring was recovered out of 200 offspring analyzed, which was only 2% of the expected number. Ten out of 21 doubly heterozygous embryos examined between E8.5 and E10.5 suffered lethality due to patterning defects that will be described elsewhere. The other 11 double heterozygotes were indistinguishable from their siblings at these developmental stages.
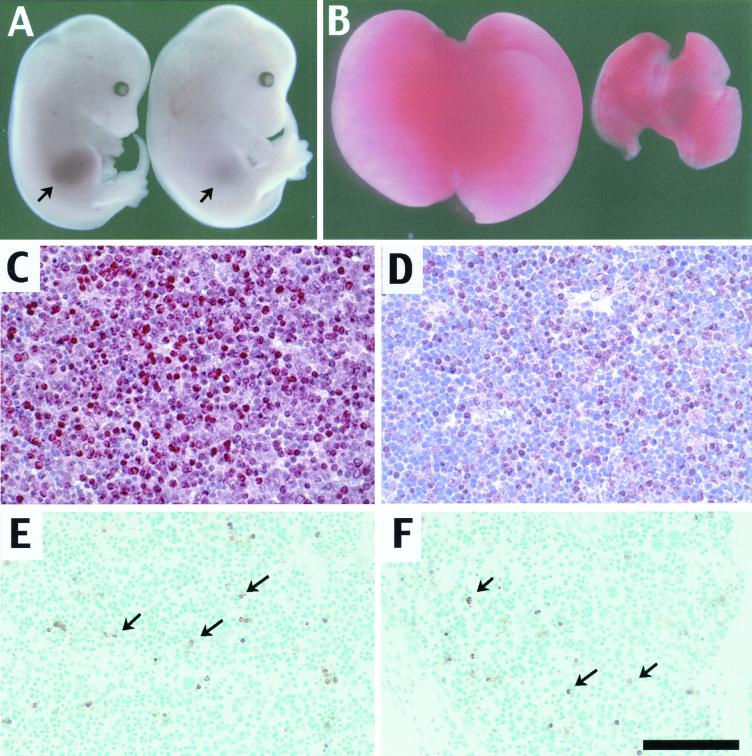
Eighty percent of the doubly heterozygous embryos examined at E14.5 appeared normal except that they exhibited a severely hypoplastic liver (Fig. 1A). Histological analysis of these animals suggested that there was no major defect elsewhere at this stage (data not shown). The other 20% of the embryos examined at this stage had additional craniofacial defects that will be described elsewhere, in addition to the liver hypoplasia. Livers from Smad2+/−; Smad3+/− animals were markedly smaller than normal but had the correct number of lobes and appeared red (Fig. 1B), suggesting that they could carry out the initial steps of liver development and hematopoiesis. This was confirmed by RT-PCR analysis of a number of hepatocytic lineage markers, including Hnf3β, cJun, Praja, Elf, Itih-4, and Cded, all of which were expressed normally in the mutant livers (not shown). E14.5 livers were examined with an antibody for proliferating cell nuclear antigen (PCNA). Fifty percent of the cells in normal livers were in a proliferative state, as judged by labeling with the PCNA antibody (Fig. 1C). This was reduced to 34% of liver cells of double heterozygotes, suggesting that the cells were in a less proliferative state (Fig. 1D). E14.5 livers were also examined for apoptotic cell death by TUNEL assay, but no detectable difference between doubly heterozygous and sibling livers was seen (Fig. 1E and F). In normal livers 7.5% of the cells were apoptotic versus 6.5% in the Smad2+/−; Smad3+/− mutants.
FIG. 1.
Animals doubly heterozygous for Smad2 and Smad3 exhibit liver defects. (A) Wild-type (left) and Smad2 +/− ; Smad3 +/− (right) E14.5 embryos. The doubly heterozygous embryo has a reduced liver (arrow) despite its somewhat larger size. (B) Livers dissected from E14.5 wild-type (left) and Smad2 +/− ; Smad3 +/− (right) embryos. (C and D) PCNA staining of E14.5 wild-type (C) and Smad2 +/− ; Smad3 +/− (D) liver sections. Red staining indicates the presence of PCNA-positive cells, which are reduced in number in the mutant livers. (E and F) TUNEL assay of E14.5 wild-type (E) and Smad2 +/− ; Smad3 +/− (F) livers. Stained cells (arrows) are seen in the same relative abundance in both samples. Bar (F), 2 mM for panel A, 800 μM for panel B, and 100 μM for panels C to F.
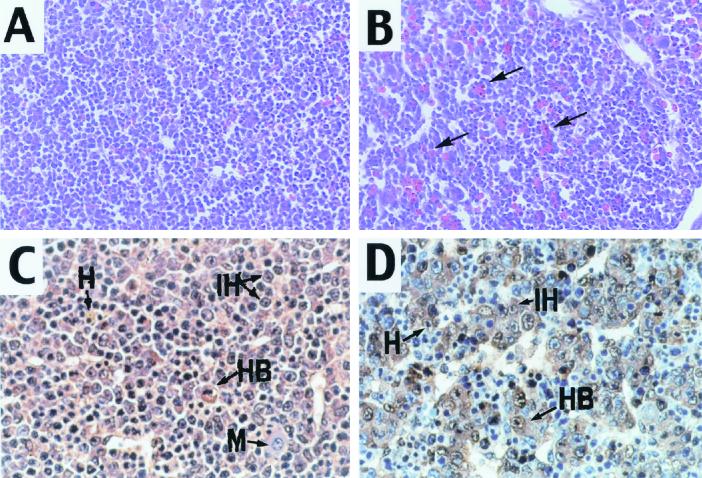
Histologically, E14.5 livers from Smad2+/−; Smad3+/− animals showed increases in the number of erythrocytes (Fig. 2A and B). In addition, the liver architecture was distorted, with an increase in the size of the sinusoidal spaces (Fig. 2A and B). The Smad2+/−; Smad3+/− livers, as well as those of sibling animals, were examined with the hepatocytic marker α-fetoprotein, which is normally expressed at the onset of the hepatocytic differentiation program (10). This staining revealed significant differences in the arrangement of hepatocytes between the mutant and control livers (Fig. 2C and D). In normal E14.5 livers, cords of differentiated hepatocytes were distributed throughout in the parenchyma (Fig. 2C), whereas the mutant hepatocytes were found in small clusters and cell plates were absent (Fig. 2D).
FIG. 2.
Smad2_+/−_ ; Smad3 +/− livers exhibit architectural defects. (A and B) H&E staining of wild-type (A) and Smad2 +/− ; Smad3 +/− (B) E14.5 livers. Note that the mutant liver exhibited abundant red blood cells (arrows) and dilated sinusoidal spaces. (C and D) α-Fetoprotein staining of wild-type (C) and Smad2 +/− ; Smad3 +/− (D) E14.5 livers. α-Fetoprotein (brown) labels hepatocytes, which form chords in the normal livers and small clusters in the mutants (D, arrows). H, hepatocytes; HB, hepatoblasts; IH, immature hepatocytes; M, monocytes. Magnifications, ×100 (A and B) and ×200 (C and D).
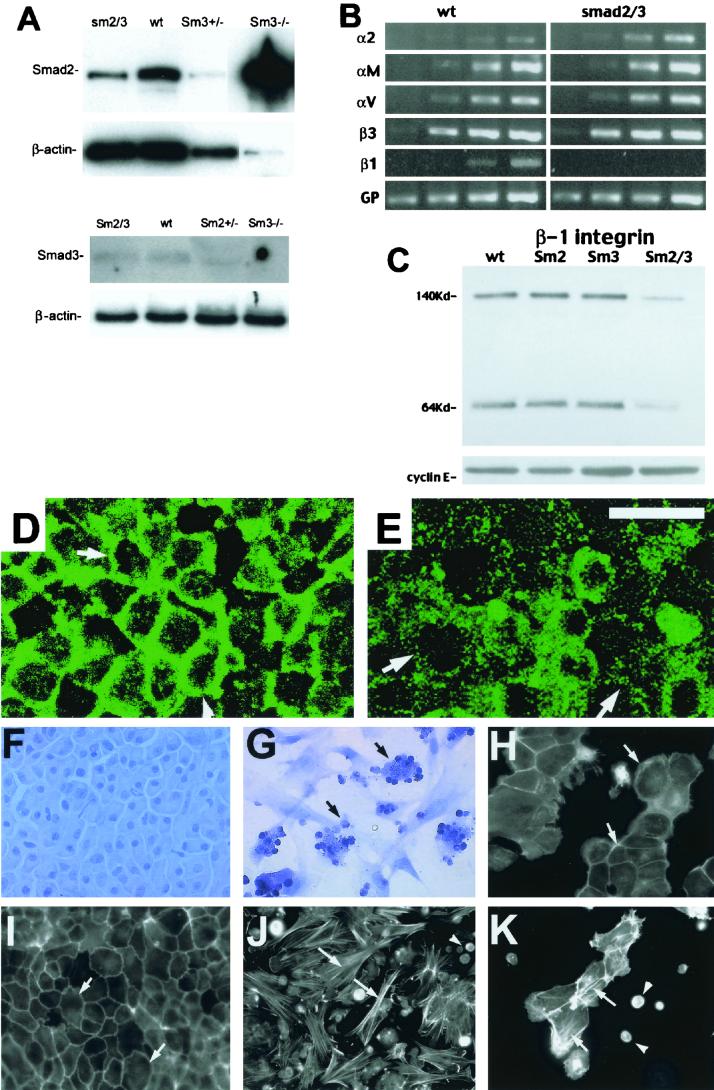
Western analysis was carried out to determine the expression of Smad2 and Smad3 in the normal and mutant livers. Interestingly, the Smad2 level increased dramatically in Smad3-homozygous livers but not in the Smad3-heterozygous livers (Fig. 3A). It may be this increased Smad2 expression that allows normal liver development in the Smad3−/− mice. Smad2 appears to be involved in this regulation, as the level of Smad2 is decreased in the Smad2+/−; Smad3+/− livers relative to the levels in wild-type and Smad3−/− livers (Fig. 3A). The level of Smad3 protein was slightly decreased in the doubly heterozygous livers, and Smad3 was absent in the Smad3-homozygous livers (Fig. 3A).
FIG. 3.
Perturbations of cellular adhesion in Smad2 +/− ; Smad3 +/− livers. (A) Western blots of Smad2 and Smad3, with β-actin shown as a loading control. Genotypes are as indicated. sm2/3, Smad2 +/− ; Smad3 +/−; wt, wild type. (B) RT-PCR analysis of integrin expression. Results shown are for increasing numbers of PCR cycles (from left to right, 20, 25, 30, and 35 cycles). Integrins are as indicated, with GAPDH (GP) as a control. (C) β-Integrin Western blot. Cyclin E is shown as a loading control. (D and E) E-cadherin staining of wild-type (D) and Smad2 +/− ; Smad3 +/− (E) livers. The membrane staining seen in the wild-type livers is partially lost in mutant livers (arrows). (F and G) Hematoxylin staining of wild-type (F) and Smad2 +/− ; Smad3 +/− (G) hepatocytes. Note the clustering of nonadherent Smad2 +/− ; Smad3 +/− hepatocytes (arrows in panel G). (H and I) Hepatocytes grown on collagen and stained with rhodamine-phalloidin. The wild-type hepatocytes (H) exhibit cortical actin (arrows), while the Smad2 +/− ; Smad3 +/− hepatocytes (I) show stress fibers (arrows). (J) Wild-type hepatocytes grown on fibronectin. The cells are more spread out, although they still exhibit cortical actin bundles (arrows). (K) Hepatocytes treated with RGD peptides, which cause the formation of stress fibers (arrows) and nonadherent cells (arrowheads). Bar (E), 10 μM for panels D and E and 26 μM for panels F to K.
The clustering of mutant hepatocytes suggested the cells might have altered adhesive properties. We therefore examined the expression and distribution of a number of adhesive proteins. Several integrins, including the α2-, αM-, αV-, and β3-integrins, appeared to be expressed normally in an RT-PCR analysis. Of note, expression of the β1-integrin in the doubly heterozygous livers appeared to be lost in this analysis (Fig. 3B). Western blot analysis shows that the β1-integrin protein concentration in the Smad2+/−; Smad3+/− livers was about 10% of the normal level (Fig. 3C). Interestingly, this was specific to the double heterozygotes and was not seen in the singly heterozygous livers (Fig. 3C), suggesting that the hepatocytes of Smad2+/−; Smad3+/− livers were unable to bind normally to the extracellular matrix.
Focal adhesions mediated by integrins are known to affect adherens junctions, which depend on cadherins (16, 23). We therefore examined E-cadherin localization using immunolabeling with an E-cadherin antibody to determine the intracellular localization of this molecule in the Smad2+/−; Smad3+/− livers. Confocal micrographs showed that E-cadherin is primarily membranous in sibling livers (Fig. 3D), but this membrane staining was lost from a substantial portion of the cells in Smad2+/−; Smad3+/− livers (Fig. 3E). Thus, E-cadherin is mislocalized in the mutant livers, suggesting a defect in cell-cell adhesion.
These data pointed to an adhesive defect in the Smad2+/−; Smad3+/− livers. We therefore cultured hepatocytes from normal and mutant livers to directly test cell adhesion. Wild-type hepatocytes were able to adhere and form epithelial sheets on collagen (Fig. 3F) and fibronectin (Fig. 3H), although cells grown on the latter were considerably more spread out than those grown on the collagen. The Smad2+/−; Smad3+/− cells failed to adhere well to these substrates. Most cells were small, rounded, and clustered, while some spread out and appeared fibroblastic in nature (Fig. 3G). Cell viability was monitored using trypan blue staining (not shown); results suggested that the smaller round cells were not dead but nonadherent. Staining with rhodamine-phalloidin showed that the normal hepatocytes contained primarily cortical actin bundles (Fig. 3I), further confirming their epithelial behavior. However, the Smad2+/−; Smad3+/− cells contained multiple stress fibers when cultured on collagen or fibronectin (Fig. 3J).
The discovery of decreased β1-integrin expression in the Smad2+/−; Smad3+/− livers was intriguing, because its expression is vital for liver development. β1-Integrin is a known TGF-β target (3, 13) that controls the response of hepatocytes to the extracellular matrix (19). ES cells that lacked it failed to colonize the liver in a chimeric analysis (11). Indeed, hepatocytes that lack β1-integrin exhibit a clustering phenotype (19) similar to that which we have documented in the Smad2+/−; Smad3+/− livers. In order to determine whether the reduction in the β1-integrin is the cause of the adhesion defect exhibited by the Smad2+/−; Smad3+/− hepatocytes, we attempted to decrease integrin activity in the wild-type hepatocytes by culturing them in the presence of RGD peptides, which are known to bind to and inhibit integrins.
We found that high concentrations of RGD peptides were sufficient to inhibit hepatocyte binding to fibronectin completely (not shown). In addition, intermediate concentrations of RGD peptides were sufficient to elicit stress fiber formation in the wild-type hepatocytes and interfere with their attachment to the substrate (Fig. 3K), suggesting that the loss of β1-integrin may play a role in the adhesion defect exhibited by the Smad2+/−; Smad3+/− hepatocytes. Despite this, the RGD peptide-treated wild-type cells (Fig. 3K) do not look like the Smad2+/−; Smad3+/− hepatocytes in terms of cluster formation (Fig. 3G). The difference between Smad2+/−; Smad3+/− cells and RGD peptide-treated wild-type cells suggests the presence of other adhesive defects in these cells, as indicated by the mislocalization of E-cadherin.
Our genetic analysis has revealed a role for Smad2 and Smad3 in liver development. To further dissect the role of Smad2 and Smad3 in hepatogenesis, we turned to liver organ culture. This technique has been used successfully to characterize the functions of other developmentally important molecules such as fibroblast growth factors, their receptors (12), and cytoskeletal proteins (22). This approach provided the added benefit that we could attempt a rescue of the hepatocytic defect in the Smad2+/−; Smad3+/− livers with added signaling factors.
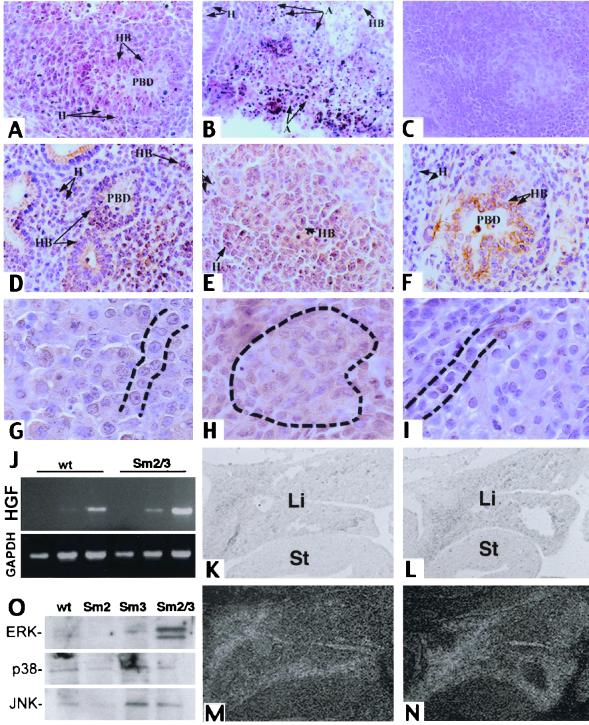
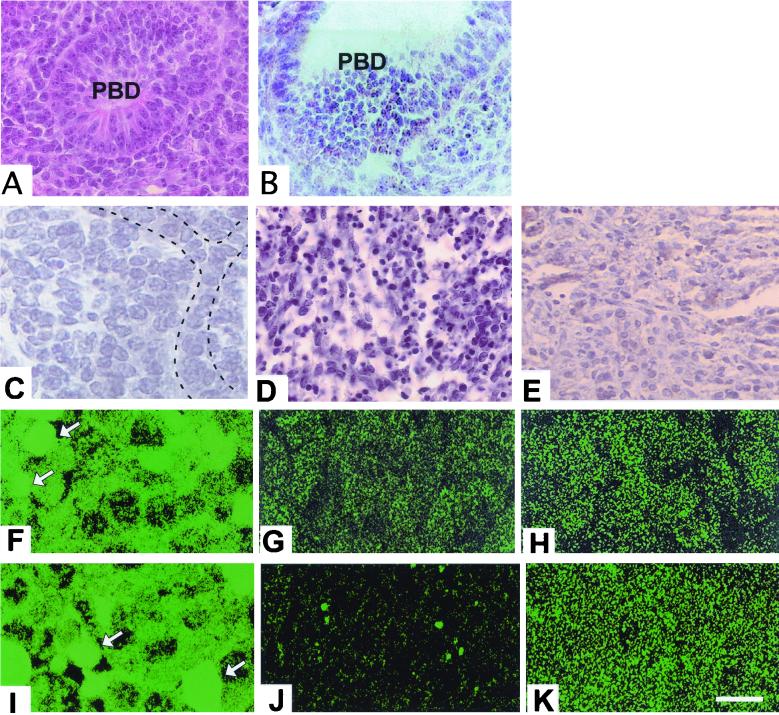
We therefore cultured livers from E10.5 Smad2+/−; Smad3+/− embryos and their siblings in an in vitro explant culture system (22). Embryonic tissues were cultured for 72 h, fixed, and processed for histology. Tissues from wild-type, Smad2+/−, and Smad3+/− embryos exhibited outgrowth of normal liver lobules with primitive bile ducts (Fig. 4A) marked by cytokeratin expression (Fig. 4D) and chords of hepatocytes (Fig. 4G). Conversely, explants isolated from Smad2+/−; Smad3+/− animals were incapable of developing normal liver tissue. They instead either suffered extensive cell death (Fig. 4B) or failed to develop normal liver architecture (Fig. 4C), exhibiting widespread cytokeratin expression (Fig. 4E) and replacement of hepatocytic chords by clusters (Fig. 4H).
FIG. 4.
Rescue of the Smad2 +/− ; Smad3 +/− phenotype by HGF in explant culture. (A to E, G, and H) Explants cultured in the absence of HGF. (F and I) Explants cultured with 5 ng of HGF/ml. (A to C) H&E-stained paraffin sections of wild-type (A) and Smad2 +/− ; Smad3 +/− (B and C) explants. (D to F) Cytokeratin-stained sections of explants. (D) Wild-type explant with cytokeratin-positive primitive bile ducts. (E) Smad2 +/− ; Smad3 +/− explant exhibiting widespread cytokeratin labeling. (F) Smad2 +/− ; Smad3 +/− explant cultured with HGF exhibiting cytokeratin-positive bile ducts. (G) Wild-type explant exhibiting normal hepatocyte arrangement in chords (dashed lines). (H) Smad2 +/− ; Smad3 +/− explant with clustered hepatocytes (dashed outline). (I) Chords of hepatocytes can be seen (dashed lines) in a mutant explant that has been treated with HGF. (J) RT-PCR analysis of HGF expression in E13.5 embryos. sm2/3, Smad2 +/− ; Smad3 +/−. GAPDH is shown as a control. (K to N) In situ analysis of HGF expression in E11.5 livers. (K and L) Bright-field images of wild-type (K) and Smad2 +/− ; Smad3 +/− (L) livers; (M and N) corresponding dark-field images. (O) MAP kinase activity in the mutant livers. Liver proteins from E14.5 animals were examined with antibodies to phosphorylated MAP kinases as shown. wt, wild type; PBD, primary bile duct; A, apoptotic cell death; H, hepatocytes; HB, hepatoblasts; Li, liver; St, stomach. Magnifications, ×100 (A to F), ×250 (G to I), and ×45 (J to M).
Given that the Smad2+/−; Smad3+/− livers exhibited a phenotype in culture, we wished to find out if this defect could be corrected by addition of exogenous signaling molecules. We first added HGF, which has been shown through genetic analysis to be a potent hepatotrophic growth factor that is essential for liver development. It has been used to promote bile duct development in rats (30), and mice that lack either HGF or its receptor, cMet, exhibit defects in liver development due to reduced hepatocyte differentiation, resulting in embryonic lethality (reviewed in reference 4). HGF is also known to activate Smad2 in cultured cells (8). Addition of HGF to the culture medium was sufficient to fully revert the doubly heterozygous explants to the wild-type phenotype. HGF treatment allowed Smad2+/−; Smad3+/− explants to form morphologically normal liver lobules organized around bile ducts (Fig. 4F) with normal hepatocytic chords (Fig. 4I). To determine whether HGF was expressed in the Smad2+/−; Smad3+/− livers, we performed an RT-PCR analysis of HGF expression (Fig. 4J) which showed slightly higher expression of HGF in the mutant livers. We have confirmed this result through in situ hybridization on E11.5 wild-type and Smad2+/−; Smad3+/− embryos. These results suggest that HGF expression in the mutant livers (Fig. 4L and N) is comparable to or greater than that in the wild-type livers (Fig. 4K and M) and that the observed phenotype is not a result of deficient HGF signaling. We have also seen normal expression of the HGF receptor, c-Met (not shown). To detect downstream changes in HGF signaling, we examined the activity of MAP kinases in the normal and mutant livers. Liver proteins from Smad2+/−; Smad3+/− embryos and their siblings were probed with antibodies to phosphorylated MAP kinases. An increase in the concentration of active p42 and p44, but not in that of p38 or JNK, was seen (Fig. 4O).
TGF-β1 was capable of inducing bile duct development when added to Smad2+/−; Smad3+/− livers (Fig. 5B) but did not fully rescue the mutant phenotype, as the bile ducts were larger than normal (Fig. 5B). The hepatocytes still had little cytoplasm, did not form the normal plates seen the wild-type livers, and tended to undergo apoptosis (Fig. 5D). We also examined the effects of added insulin on the mutant livers. Insulin induces hepatocytic growth in wild-type explants, as did HGF (not shown). Insulin also induces bile duct development in Smad2+/−; Smad3+/− mutants (not shown) but was unable to rescue the hepatocytic defects. Insulin instead caused the formation of highly abnormal tissues in the mutant explants (Fig. 5E). Therefore, only HGF could correct the defect caused by the haploid loss of Smad2 and Smad3.
FIG. 5.
Rescue of the Smad2 +/− ; Smad3 +/− phenotype is specific to HGF and does not involve the activation of Smad2 or Smad3. (A and B) H&E-stained sections of Smad2 +/− ; Smad3 +/− explants cultured with HGF (A) and TGF-β1 (B). PBD, primitive bile duct. (C) Smad2 +/− ; Smad3 +/− explant cultured with HGF exhibiting normal hepatocytic chords (dashed lines). (D) TGF-β1-treated mutant explant exhibiting cell death. (E) Insulin-treated Smad2 +/− ; Smad3 +/− explant showing abnormal development. (F to K) Confocal images of explants cultured in the absence of HGF (F and I) or with HGF addition (G, H, J, and K). (F to H) Smad2 staining of wild-type (F and G) and Smad2 +/− ; Smad3 +/− (H) explants. The amount of Smad2 was reduced in the HGF-treated wild-type explant (G). (I to K) Smad3 staining of wild-type (I and J) and Smad2 +/− ; Smad3 +/− (K) explants. HGF treatment reduced the level of Smad3 in the wild type (J). Bar (K), 50 μM for panels A, B, D, and E, 25 μM for panel C, and 10 μM for panels F to K.
One mechanism by which HGF might rescue the defect observed in our culture system might be through the activation of the Smad2 and Smad3 remaining in the Smad2+/−; Smad3+/− livers. We therefore examined the intracellular localization of Smad2 in the liver explants with and without HGF treatment. Wild-type livers cultured in the absence of HGF exhibited abundant staining with an antibody directed against Smad2 (Fig. 5F), which appeared nuclear in some but not all cells (Fig. 5F). However, the apparent abundance of Smad2 dropped precipitously in the wild-type explants that were treated with HGF (Fig. 5G). HGF failed to cause nuclear accumulation of Smad2 in the compound heterozygous livers (Fig. 5H). Similar results were seen for Smad3, which was also found in wild-type livers in relative abundance (Fig. 5I) and which exhibited a reduced level in wild-type explants treated with HGF (Fig. 5J). HGF did not seem to promote nuclear accumulation of Smad3 in the mutant explants (Fig. 5K).
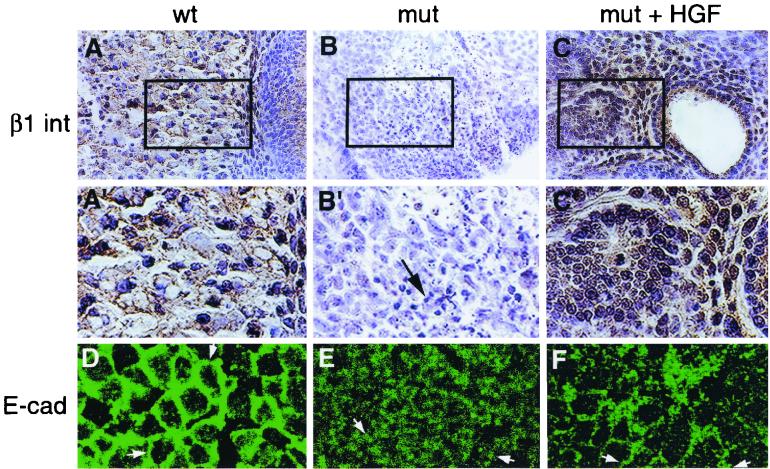
HGF was clearly able to direct normal liver development in the Smad2+/−; Smad3+/− mutants, but whether it did so by activating Smad-responsive genes was unclear. HGF can increase the expression of the β1-integrin in cultured cells (15). In view of the vital roles for HGF and β1-integrin in liver development (9, 11, 19, 28), it was possible that HGF treatment caused a similar increase in β1-integrin expression, resulting in the rescue of the hepatic phenotype of the Smad2+/−; Smad3+/− mutants. Wild-type explants exhibited abundant labeling with an antibody to the β1-integrin (Fig. 6A and A′), which was largely lost in the mutants (Fig. 6B and B′). Treatment with HGF restored the expression of this integrin both in the primitive bile ducts and the liver parenchyma (Fig. 6C and C′).
FIG. 6.
HGF rescues the level of β1-integrin and E-cadherin in explant culture. (A to C) Sections were immunostained for the β1-integrin (brown staining). (A) Wild type (wt). (B) Smad2 +/− ; Smad3 +/− explant cultured in the absence of added growth factors. (C) Smad2 +/− ; Smad3 +/− explant cultured in the presence of HGF. The widespread staining for the integrin seen in the wild type (A) was largely lost in the mutant (mut) (B) but was restored by HGF treatment (C). (A′ to C′) Enlarged rectangular areas of panels A to C, respectively. Arrow (B′), dying cells. (D to F) Confocal images of explants labeled with an E-cadherin antibody. (D) Wild type. E-cadherin was localized to the membrane (arrows). (E) Smad2 +/− ; Smad3 +/− explant. E-cadherin was predominantly cytoplasmic (arrows). (F) Smad2 +/− ; Smad3 +/− explant. HGF treatment returned the E-cadherin to the membrane (arrows). Magnifications, ×125 (A to C) and ×280 (A′ to C′ and D to F).
The restoration of β1-integrin expression therefore suggested a return to normal behavior for the mutant hepatocytes. To further confirm this, the localization of E-cadherin was examined in the presence and absence of HGF treatment. Explants from sibling control animals exhibited membranous staining of E-cadherin (Fig. 6D), but those derived from doubly heterozygous animals were considerably different. Instead of staining at the membrane, the mutants exhibited punctate staining within the cell interior (Fig. 6E). However, HGF treatment was sufficient to restore the E-cadherin staining to the wild-type membranous domain (Fig. 6F). It is likely that a return to normal adhesiveness allowed for the more-normal architectural and proliferative properties of the mutant explants.
DISCUSSION
We have reported a novel phenotype of a midgestational defect in the liver development of Smad2+/−; Smad3+/− mice. Through a three-pronged approach using genetics, organ culture, and primary hepatocyte culture, we have delineated the relationship between factors vital for liver development, i.e., HGF, β1-integrin, and Smad2 and -3. We showed that wild-type levels of both Smad2 and Smad3 were required in concert for normal hepatic development. Dual haploinsufficiency for both genes resulted in profound liver defects that eventually killed the embryos.
The results seen here suggested that Smad2 and Smad3 function in a highly cooperative fashion, as loss of one allele of each elicits defects of greater severity than disruption of both alleles of Smad3. The appearance of this phenotype is somewhat curious, as neither allele alone will cause the defects seen. This is likely due to the high degree of homology between these two Smads (36) and the nonamplifiable nature of the Smad signal. Other signal transduction systems depend on enzymatic cascades, such as those that lead to activation of MAP kinases (6). However, Smad proteins translocate from the plasma membrane to the cell nucleus directly. The intracellular concentrations of these mediators are therefore critical, and this explains why Smad2 and Smad3 mutants exhibit haploinsufficiency phenotypes (2, 24) and why Smad1 and Smad2 are subjected to ubiquitination and degradation to control their intracellular concentrations (17, 37). In addition, we detected a dramatic increase in the level of the Smad2 protein in the Smad3-homozygous liver (Fig. 2A). It is possible that this increase in Smad2 expression is what allows normal liver development in the Smad3−/− animals. Interestingly, this increase is not seen in the Smad3 heterozygotes. Thus, when there are disruptions in both these genes, their combined protein levels may drop too low to maintain normal development.
We did recover one viable doubly heterozygous animal during the course of this study. This animal, a male, was bred to Smad2+/− and Smad3+/− females. We thought that it might carry modifiers that reduced the severity of the defect caused by the loss of Smad2 and Smad3 and might therefore give rise to additional viable doubly heterozygous mice. Indeed, a modifier that affected the phenotype of mice lacking TGF-β1 was found (5). However, we failed to detect any other viable Smad2+/−; Smad3+/− animals in the offspring of this male, and an examination of the Smad2+/−; Smad3+/− embryos fathered by this animal failed to reveal any change in phenotype.
There exists a paucity of mechanistic insight concerning the role of TGF-β ligands in liver specification and outgrowth. None of the TGF-β knockout mutants exhibited liver defects (26, 27, 29), suggesting that multiple ligands of the TGF-β superfamily are required for liver development. TGF-β1 was unable to effect a full reversal of the Smad2+/−; Smad3+/− mutant phenotype, although it was sufficient to elicit the appearance of bile ducts. This suggests that it can indeed function in the developing liver but must do so in conjunction with other ligands to direct normal development.
An interesting result of this study is that HGF can rescue the compound Smad2/Smad3 haploinsufficiency in organ culture. It did not alter the adhesion of primary Smad2+/−; Smad3+/− hepatocytes, however (not shown). HGF is known to function in liver development. Embryos that lack it have livers with reduced dimensions and cellularity (28), and ES cells deficient in HGF are incapable of contributing to the hepatic compartment (28). Although HGF can activate Smad2 under some conditions, it did not appear to do so here. Addition of HGF to wild-type liver explants resulted in a disappearance of Smad2 and Smad3. It would be tempting to speculate that this was the consequence of Smad2 and Smad3 activation, as Smad2 is known to be ubiquitinated and destroyed upon activation (18). However, application of TGF-β did not result in the destruction of Smad2 and Smad3 (S. P. S. Monga and L. Mishra, unpublished observations). Instead, HGF appears to activate a parallel pathway that can supplant many functions of the TGF-β superfamily during liver outgrowth. Indeed, HGF expression appears to be increased somewhat in the mutant livers, as are the activities of p42 and p44 MAP kinases. This may be due to compensatory gene regulation in the Smad2+/−; Smad3+/− livers. Liver development may be abnormal in the mutant embryos because endogenous mechanisms are insufficient to increase HGF expression to the level needed to overcome the loss of TGF-β signals. These data make it clear that neither pathway activates the other but instead that they operate in parallel.
The β1-integrin is reduced in the Smad2+/−; Smad3+/− mutants and is restored by HGF treatment. β1-Integrin expression is essential for an ES cell contribution to the liver in chimeric embryos (9). Indeed, hepatocytes in which the α3β1-integrin has been eliminated by antisense treatment exhibit a clustering phenotype similar to what we have reported here (19). Moreover, both TGF-β and HGF are known to induce the β1-integrin gene (2, 13, 15). β1-Integrin is likely to play an important role in hepatocytic adhesion to the extracellular matrix, as RGD peptides were sufficient to disrupt hepatocytic adhesion to fibronectin. However, there are clearly other molecules involved in the adhesive defect encountered in the Smad2+/−; Smad3+/− hepatocytes, as RGD peptide treatment of wild-type hepatocytes only partially phenocopied the Smad2+/−; Smad3+/− phenotype.
The Smad2+/−; Smad3+/− hepatocytes may also have a defect in cell-cell adhesion, a result of improper E-cadherin localization, not seen in the wild-type cells treated with RGD peptides. Such adhesive defects may be a secondary result of defects in the extracellular matrix. However, we have conducted an RT-PCR analysis of extracellular matrix components that has shown several molecules, including collagen VI, connective tissue growth factor, fibronectin, vitronectin, gelatinase B, and others, to be expressed normally in the mutant livers (not shown). These results suggest that the adhesive defects seen in the Smad2+/−; Smad3+/− hepatocytes are a primary result of Smad2 and Smad3 haploinsufficiency and are not secondary to other defects.
These results raise a number of interesting questions about the nature of the TGF-β signal operating in liver development and the mechanism by which is can be supplanted by HGF. We are using genetic and biochemical experiments to answer these and other questions about liver development and TGF-β signaling.
ACKNOWLEDGMENTS
We thank John C. Thompson for his technical assistance for this work.
M. Weinstein and S. P. S. Monga contributed equally to this work.
REFERENCES
- 1.Abou-Shady M, Baer H U, Friess H, Berberat P, Zimmermann A, Graber H, Gold L I, Korc M, Buchler M W. Transforming growth factor betas and their signaling receptors in human hepatocellular carcinoma. Am J Surg. 1999;177:209–215. doi: 10.1016/s0002-9610(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft G S, Yang X, Glick A, Weinstein M, Letterio J J, Mizel D E, Anzano M, Greenwell-Wild T, Wahl S M, Deng C, Roberts A B. Mice lacking SMAD 3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 3.Azuma M, et al. Expression of integrin subunits in normal and malignant human salivary gland cell clones and its regulation by transforming growth factor-beta 1. Cancer Lett. 1996;109:91–99. doi: 10.1016/s0304-3835(96)04430-8. [DOI] [PubMed] [Google Scholar]
- 4.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 5.Bonyadi M, Rusholme S A, Cousins F M, Su H C, Biron C A, Farrall M, Akhurst R J. Mapping of a major genetic modifier of embryonic lethality in TGF-beta 1 knockout mice. Nat Genet. 1997;15:207–211. doi: 10.1038/ng0297-207. [DOI] [PubMed] [Google Scholar]
- 6.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 7.Datto M B, Frederick J P, Pan L, Borton A J, Zhuang Y, Wang X F. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Caestecker M P, Parks W T, Frank C J, Castagnino P, Bottaro D P, Roberts A B, Lechleider R J. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–1592. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 10.Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman J R, Zaret K S. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch E, Iglesias A, Potocnik A J, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 12.Jung J, Zheng M, Goldfarb M, Zaret K S. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 13.Kagami S, Kuhara T, Yasutomo K, Okada K, Loster K, Reutter W, Kuroda Y. Transforming growth factor-beta (TGF–beta) stimulates the expression of beta1 integrins and adhesion by rat mesangial cells. Exp Cell Res. 1996;229:1–6. doi: 10.1006/excr.1996.0336. [DOI] [PubMed] [Google Scholar]
- 14.Kanzler S, Lohse A W, Keil A, Henninger J, Dienes H P, Schirmacher P, Rose-John S, zum Buschenfelde K H, Blessing M. TGF–β1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol. 1999;276:G1059–G1068. doi: 10.1152/ajpgi.1999.276.4.G1059. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami-Kimura N, Narita T, Ohmori K, Yoneda T, Matsumoto K, Nakamura T, Kannagi R. Involvement of hepatocyte growth factor in increased integrin expression on HepG2 cells triggered by adhesion to endothelial cells. Br J Cancer. 1997;75:47–53. doi: 10.1038/bjc.1997.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levenberg S, Katz B Z, Yamada K M, Geiger B. Long-range and selective autoregulation of cell-cell or cell-matrix adhesions by cadherin or integrin ligands. J Cell Sci. 1998;111:347–357. doi: 10.1242/jcs.111.3.347. [DOI] [PubMed] [Google Scholar]
- 17.Lin X, Liang M, Feng X H. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in TGF–beta signaling. J Biol Chem. 2000;275:36818–36822. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 18.Lo R S, Massague J. Ubiquitin-dependent degradation of TGF–beta-activated Smad2. Nat Cell Biol. 1999;1:472–478. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- 19.Lora J M, Rowader K E, Soares L, Giancotti F, Zaret K S. Alpha3beta1-integrin as a critical mediator of the hepatic differentiation response to the extracellular matrix. Hepatology. 1998;28:1095–1104. doi: 10.1002/hep.510280426. [DOI] [PubMed] [Google Scholar]
- 20.Massague J. TGF–beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 21.Massague J, Wotton D. Transcriptional control by the TGF–beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra L, Cai T, Yu P, Monga S P, Mishra B. Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene. 1999;18:353–364. doi: 10.1038/sj.onc.1202313. [DOI] [PubMed] [Google Scholar]
- 23.Monier-Gavelle F, Duband J L. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by beta1 and beta3 integrins in migrating neural crest cells. J Cell Biol. 1997;137:1663–1681. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 25.Ozawa M, Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol. 1990;111:1645–1650. doi: 10.1083/jcb.111.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proetzel G, Pawlowski S A, Wiles M V, Yin M, Boivin G P, Howles P N, Ding J, Ferguson M W, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanford L P, Ormsby I, Gittenberger-de Groot A C, Sariola H, Friedman R, Boivin G P, Cardell E L, Doetschman T. TGF-beta2 knockout mice have multiple developmental defects that are nonoverlapping with other TGF-beta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 29.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 31.Waldrip W R, Bikoff E K, Hoodless P A, Wrana J L, Robertson E J. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng C X. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Natl Acad Sci USA. 1998;95:9378–9383. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrana J L, Attisano L. The Smad pathway. Cytokine Growth Factor Rev. 2000;11:5–13. doi: 10.1016/s1359-6101(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Letterio J J, Lechleider R J, Chen L, Hayman R, Gu H, Roberts A B, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF–beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaret K. Early liver differentiation: genetic potentiation and multilevel growth control. Curr Opin Genet Dev. 1998;8:526–531. doi: 10.1016/s0959-437x(98)80006-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF–beta response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Kavsak P, Abdollah S, Wrana J L, Thomsen G H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Richardson J A, Parada L F, Graff J M. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]