Advances in Targeted Genome Editing (original) (raw)
. Author manuscript; available in PMC: 2013 Aug 1.
Published in final edited form as: Curr Opin Chem Biol. 2012 Jul 20;16(3-4):268–277. doi: 10.1016/j.cbpa.2012.06.007
Abstract
New technologies have recently emerged that enable targeted editing of genomes in diverse systems. This includes precise manipulation of gene sequences in their natural chromosomal context and addition of transgenes to specific genomic loci. This progress has been facilitated by advances in engineering targeted nucleases with programmable, site-specific DNA-binding domains, including zinc finger proteins and transcription activator-like effectors (TALEs). Recent improvements have enhanced nuclease performance, accelerated nuclease assembly, and lowered the cost of genome editing. These advances are driving new approaches to many areas of biotechnology, including biopharmaceutical production, agriculture, creation of transgenic organisms and cell lines, and studies of genome structure, regulation, and function. Genome editing is also being investigated in preclinical and clinical gene therapies for many diseases.
Introduction
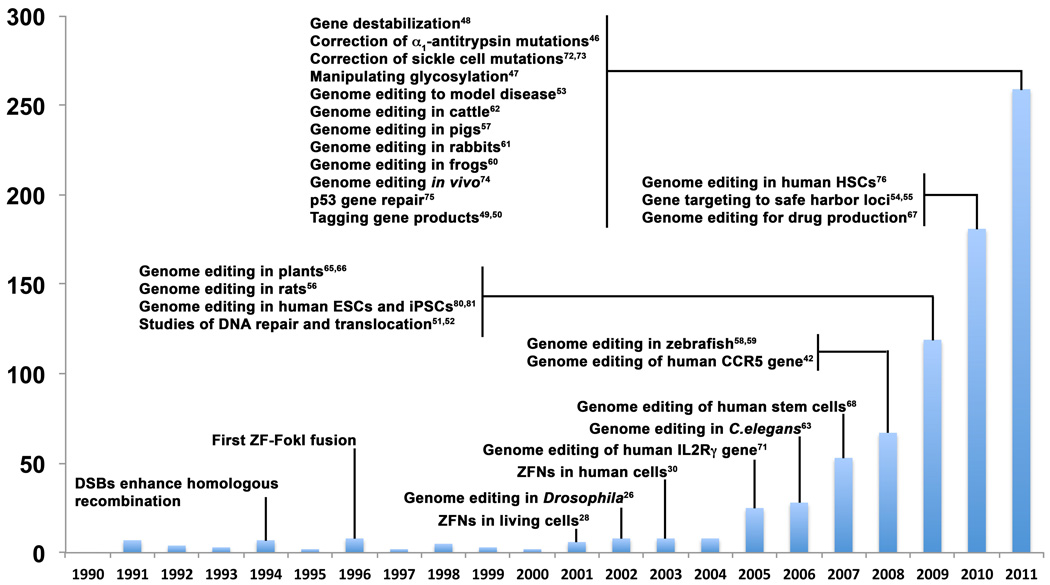
Genome editing is the introduction of a predetermined sequence change to the chromosomal DNA of a cellular genome. The instructions for almost all functions of living systems are encoded in the genome. Consequently, the ability to easily and precisely add, remove, or exchange DNA sequences within a cellular genome would theoretically enable routine reprogramming of biological systems for numerous applications relevant to all areas of biotechnology, including medicine, energy, and the environment. The editing of genome sequences in diverse cell types and species has recently become possible through the advent of synthetic nucleases that can be engineered to target almost any site in a complex genome. The enhancement of gene targeting through nuclease-mediated DNA cleavage has been known for over fifteen years, but genome editing has not been widely applied to diverse areas of biotechnology until recently (Figure 1). This rapid growth is the result of the increased availability of public and commercial sources for engineering targeted nucleases (Table 1), as well as significant progress in enhancing and monitoring genomic modifications. Despite the exponential growth of the use of this technology, current methods still do not fulfill the criteria of an ideal gene editing tool: 1) high frequency of desired sequence changes in the target cell population, 2) no off-target mutations, and 3) rapid and efficient assembly of nucleases that target any site in the genome at low cost. Progress in genome editing has been the subject of several comprehensive review articles [1–3]. Therefore this review emphasizes the most significant advances in genome editing in the last few years and the corresponding adoption of this technology for new applications. We also discuss the current challenges and future directions necessary to establish a genome editing technology that is sufficiently robust, efficient, specific, economical, and readily available for routine use in research and biotechnology.
Figure 1. Milestones in genome editing and accelerated progress in nuclease engineering.
An estimate of the number of articles referring to genome editing is shown for each year with specific reference to major advances. Data for this graph was obtained from the Web of ScienceSM by searching for articles referencing “zinc finger nuclease OR tale nuclease”.
Table 1.
Publicly Available Methods for Custom Nuclease Design and Assembly
| DNA-Binding Domain | Assembly Method | Description |
|---|---|---|
| Zinc Finger Proteins | Sigma-Aldrich CompoZr | Ready-made, pre-validated commercial ZFN constructs for targeted gene knockout or integration into a safe harbor locus54, 64 Pre-validated ZFN constructs against custom targets available within 10 weeks of purchase |
| Oligomerized Pool Engineering (OPEN)10 | Utilizes a publicly available molecular biology toolkit for end-user selection and validation of custom zinc finger arrays against a desired DNA sequence Requires molecular biology expertise | |
| Context-Dependent Assembly (CoDA)12 | A database of custom zinc finger arrays derived from previously identified OPEN zinc finger arrays that share a common zinc finger motif End- user can assemble the desired ZFN constructs using standard molecular biology techniques or commercial gene synthesis based on output from a webserver7 | |
| Modular Assembly (Barbas Kit)8 | Utilizes a toolkit of off-the-shelf synthetic zinc finger modules for most targetable DNA triplet sequences End-user assembles modules into custom arrays using standard molecular biology techniques or commercial gene synthesis based on output from a webserver6 | |
| Modular Assembly (Joung Kit) | Utilizes an extensive toolkit of off-the-shelf synthetic zinc finger modules from the Barbas8, Sangamo, and ToolGen5 set of zinc finger modules End-user assembles modules into custom arrays using standard molecular biology techniques or commercial gene synthesis based on output from a webserver7 | |
| Modular Assembly (Wolfe System)13 | Utilizes a toolkit of off-the-shelf synthetic zinc finger modules based on an engineered library of two-finger modules supplemented with additional one-finger modules End-user assembles modules into custom arrays using standard molecular biology techniques or commercial gene synthesis based on output from a webserver13 | |
| TAL Effectors | Cellectis Bioresearch | Pre-validated commercial TALEN constructs for targeted gene knockout, as well as for custom targets and available several weeks following order confirmation |
| Life Technologies | GeneArt® Precision TALs fused to various effector domains are supplied as Gateway® compatible entry clones for custom targets and available several weeks following order confirmation | |
| FLASH Assembly20 | High-throughput, automatable assembly of custom TALEs using a publicly available library of preassembled TAL arrays | |
| Modular Assembly (Voytas Kit)21 | Publicly available plasmid-based TALE monomer library that can generate arrays of 12–31 TALE repeats Custom arrays are assembled using rapid two-step Golden Gate molecular cloning; webserver is available to aid in target site selection21 | |
| Modular Assembly (Joung Kit) | Publicly available plasmid-based TALE monomer library that can generate arrays of any desired length Custom arrays are assembled in iterative ligation steps using Golden Gate molecular cloning; webserver is available to aid in target site selection7 | |
| Modular Assembly (Zhang Kit)18 | PCR-based TALE monomer library that can rapidly generate arrays of 18 TALE repeats using standard molecular biology techniques |
2. Targeted DNA-Binding Proteins
The engineering of enzymes that target specific sequences within complex genomes is a formidable challenge. The most successful approaches to date have been based on modular proteins in which a DNA-binding domain that recognizes the target DNA sequence is fused to an effector domain that catalyzes changes to the structure or function of the target gene. The DNA recognition domain is typically based on the structure of natural DNA-binding proteins, including zinc finger proteins and transcription activator-like effectors. These targeted DNA-binding proteins can be combined with effector domains to create functional enzymes, including synthetic transcription factors, methyltransferases, integrases, nucleases, and recombinases, that modify genes in many cell types and species.
2.1 Zinc Finger Proteins
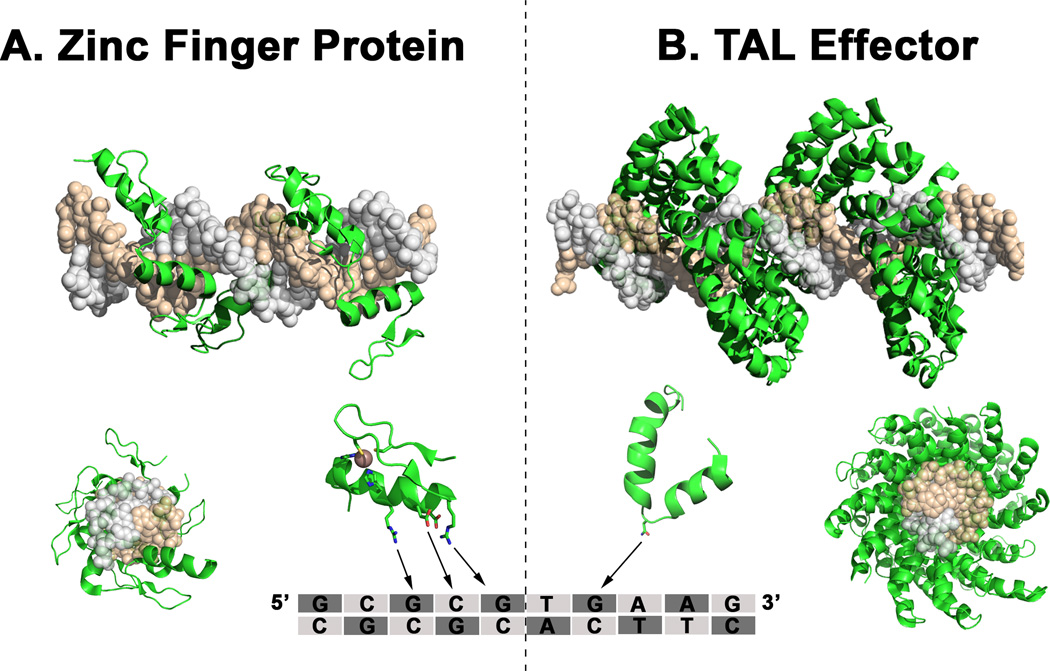
The Cys2-His2 zinc finger domain is the most common DNA-binding motif in the human proteome and consists of 30 amino acids in a ββα configuration, where the α-helix projects into the major groove of DNA and recognizes 3–4 contiguous nucleotide bases [4] (Figure 2A). The DNA-binding specificity of synthetic zinc finger domains has been extensively engineered through site-directed mutagenesis and rational design or the selection of large combinatorial libraries. Collectively, this work has yielded unique zinc finger domains with specificity for almost all of the 64 possible nucleotide triplets [4]. Significantly, the modular structure of the zinc finger motif permits the conjunction of several domains in series, allowing for the recognition and targeting of extended sequences in multiples of three nucleotides. As a result of this work, it is now theoretically possible to design synthetic zinc finger proteins to bind practically any target in the genome of any species.
Figure 2. Three dimensional structure of a Zinc Finger Protein and TAL effector.
(A) Front and lateral view of a six-finger zinc finger protein that consists of six tandem repeats of C2H2 zinc finger motifs, each consisting of approximately 30 amino acids. A single zinc finger, which recognizes 3 bp of DNA, consists of an α helix and two antiparallel β sheets that coordinate with a zinc ion through two histidine residues and two cysteine residues. Contacts with DNA are made through interactions with side chains on the α helix. (B) TAL effectors consist of repeats of 34 amino acids that recognize one single bp of DNA. Each of these units is formed by two nearly identical alpha helices flanking two variant amino acids, known as the repeat variable di-residue, that determine the binding specificity. The structures have been modeled using MacPyMOL with Protein Data Bank files 1P47 (crystal structure of tandem Zif268 molecules complexed to DNA) and 3UGM (structure of TAL effector PthXo1 bound to its DNA target).
Despite the numerous successful uses of engineered zinc finger proteins for regulating and editing genes in many species and cell types, the full potential of this technology has not yet been fulfilled. This has largely been attributed to the nuances of zinc finger protein engineering and failed attempts to adopt the methods in new laboratories. Although the source of the challenges in zinc finger engineering is still unclear, several new methods have become available in recent years that facilitate the rapid assembly and screening of numerous novel zinc finger proteins in parallel (Table 1). The modular assembly approach uses a single engineered zinc finger domain for each possible three base pair sequence [5,6]. The resulting zinc finger array is assembled from this library for any particular target sequence with the assistance of an online web server [6,7] and is then created with standard recombinant DNA methods or by commercial gene synthesis [8,9]. An alternative approach, known as “OPEN”, selects new zinc finger proteins from randomized libraries for each new target site [10]. Although this strategy entails considerably more effort and resources than modular assembly, it has been reported to generate functional zinc finger proteins with a higher frequency of success [10,11]. In 2010, a method labeled “context-dependent assembly” (CoDA), which recombines zinc finger domains that have previously been validated to work together, was suggested to be highly effective by accounting for interactions between zinc fingers while maintaining the simplicity of modular assembly [12]. An archive of optimized two-finger modules has also been described that generates highly successful proteins with a greater targeting range than CoDA [13]. Finally, engineered zinc finger proteins are available commercially for custom targets from Sigma-Aldrich Corporation’s CompoZr Zinc Finger Nuclease platform. This commercial service was created through partnership with Sangamo Biosciences, Inc. (Richmond, CA) and licensing of Sangamo’s proprietary methods for assembling zinc finger proteins.
2.2 TAL Effectors
The discovery of a simple modular DNA recognition code by transcription activator-like effectors (TALEs), reported in 2009, created another option for engineering programmable DNA-binding proteins [14,15]. TALEs are naturally occurring DNA-binding proteins produced by plant pathogenic bacteria to regulate host gene expression. In contrast to zinc finger domains of 30 amino acids that each recognize three base pairs, each TALE repeat consists of 34 amino acids and recognizes only a single base pair [14–16] (Figure 2B). DNA binding preference by each repeat is determined by only two hypervariable amino acids in positions 12 and 13, called repeat-variable diresidues [14, 15]. Like zinc finger domains, these modular TALE repeats can be linked together to recognize a specific DNA sequence and then fused with transcriptional activation domains or nuclease domains to direct enzyme activity to targeted chromosomal loci [17–20]. Several protocols have recently been described that enable rapid assembly of custom TALE repeat arrays in only a few days using publicly available reagents [18,20,21] (Table 1). Custom engineered TALEs have also become available commercially through Cellectis Bioresearch (Paris, France) and Life Technologies (Grand Island, NY). The rapid progress of TALE engineering relative to the development of synthetic zinc finger proteins has led many to suggest that this protein motif may be more amenable to reengineering, potentially due to a more modular structure [22]. However TALEs have been much less studied than zinc finger proteins, and continued work is necessary to fully understand the strengths and weaknesses of these different technologies.
3. Nuclease-Mediated Genome Editing
Although conventional homologous recombination can be used to introduce sequence changes into the genomic DNA of some species and cell types, this process is not efficient enough for most applications in which genome editing would be useful. However, the synthetic DNA-binding proteins described above can be used to engineer nucleases that can be targeted to almost any site in a cellular genome [1–3]. These nucleases create targeted double-strand breaks (DSBs) that stimulate natural DNA repair machinery to mend these breaks by non-homologous end joining (NHEJ). This repair pathway can be used to disrupt a gene or excise segments of genomes. Alternatively, the DSB also dramatically enhances the rate of homologous recombination at that locus and a homologous donor template can be delivered to the cells along with the nuclease to target gene addition to that site or make small substitutions to gene sequences. Recent advances in these techniques that have enabled more effective genome editing are described below.
3.1 FokI Endonuclease Domain
Engineered zinc finger proteins or TALEs can be fused to the catalytic domain of a restriction endonuclease to generate zinc finger nucleases (ZFNs) or TALE nucleases (TALENs) that create a DSB at the locus of interest. Because the nuclease acts as a dimer, two DNA-binding proteins must be engineered to target adjacent sequences, separated by a spacer region where the nuclease catalytic domain can dimerize and cleave the target DNA [1–3]. The catalytic domain most commonly used to induce the DSB is derived from the type IIS restriction endonuclease FokI. Several recent advances in the structure of the FokI domain have been made to increase its activity and specificity. First, a directed evolution strategy was used to identify a hyperactive FokI variant, named Sharkey, that increases cleavage activity in vitro and in vivo [23]. Second, several mutations have been described that prevent unwanted FokI homodimer formation and genotoxic cleavage of off-target sequences [24]. Although FokI heterodimer variants had previously been described to prevent homodimer formation, this was accompanied by lower catalytic activity. These new mutations appear to restore the lost nuclease activity while maintaining the strict requirement for heterodimer formation [24]. Finally, variants of FokI have been described that act as orthogonal obligate heterodimers, such that autonomous pairs of nucleases can be used together without cross-reactivity or homodimer formation [25].
3.2 Donor Vectors for Homologous Recombination
Although NHEJ-based repair of DSBs is sufficient for gene disruption [26] and the deletion of chromosomal segments [27], the introduction of new sequences to the nuclease target site requires a homologous donor repair template [28–30]. For certain applications, creating this homologous donor with homology arms of >700 base pairs may be complicated or laborious. Two new approaches have provided methods for simplification of this strategy. First, linear donor sequences with as little as 50 base pairs of homology were efficiently integrated into sites of nuclease cleavage in human cells [31]. Second, single-stranded DNA oligonucleotides, in combination with engineered nucleases, were used instead of a donor targeting vector to induce targeted point mutations, deletions or insertions of short sequences [32,33]. Importantly, oligonucleotide-based templates contain the minimum genetic information needed to introduce DNA sequence changes, therefore reducing the risk of off-target effects.
3.3 Enhancing Genome Editing
Current methods for nuclease-mediated genome editing do not allow for directing gene repair exclusively to either the NHEJ or homologous recombination pathway. Consequently, the inclusion of a donor vector with the nuclease treatment results in a cell population containing a mixture of cells modified by both pathways [34]. In order to better monitor this process, a “traffic light” reporter system was created in which NHEJ and homologous recombination events are differentially monitored by flow cytometric analysis of green and red fluorescence [35]. This study also identified factors that bias the balance of the two repair pathways and confirmed findings that creating single-strand breaks with a nickase, in contrast to DSBs with a nuclease, favors homology-directed repair and minimizes NHEJ [36–38]. The use of episomal fluorescent reporters of gene repair was later extended to enrich for cells modified at their endogenous locus by cytometric cell sorting [39]. Other methods that have been used to improve the efficiency of genome editing include the regulation of nuclease activity with small molecules to minimize toxicity [40] and transient hypothermia to increase nuclease expression levels [41].
4. Monitoring Specificity of Genome Editing
The usefulness of genome editing technologies is largely dependent on achieving single site specificity in the context of large and complex genomes. However, it is challenging to prove that no other sequences across the whole genome are unintentionally modified. This is particularly important given the observed cytotoxicity of many nucleases, presumably due to off-target DNA cleavage. Analysis had previously been limited to predicting potential off-target sites based on in vitro binding profiles [42]. To address these concerns, new methods have been developed for comprehensive mapping of nuclease activity in vitro [43] and in vivo [44,45]. Although the ZFNs analyzed in these studies acted primarily at their intended target site, many previously unknown off-target sites were also revealed. These off-target sites had high sequence homology to the intended ZFN binding site and therefore these methods will be valuable to designing improved nucleases. Additionally, advances in high-throughput DNA sequencing have enabled direct analysis of genomes with single base pair resolution. For example, a recent study sequenced the complete exome of a ZFN-treated clonal cell population and showed that only a single point mutation was created in this process [46].
5. Genome Editing in Basic Science and Biotechnology
The advent of genome editing has created a variety of new approaches that are progressively becoming routine methods to interrogate biological systems. The accessibility of commercially and publicly available custom nucleases has facilitated novel studies of protein glycosylation [47], gene destabilization [48], protein localization and dynamics [49,50], chromosomal translocation [51] and DNA repair [52]. Genome editing tools can be used to model human disease [53] or generate human [54] or mouse [55] isogenic cell lines that allow for robust and uniform transgene expression. Traditionally, gene targeting in animal models has been largely limited to mice, but engineered nucleases have enabled targeted gene modifications in rats [56], pigs [57], zebrafish [58,59], frogs [60], rabbits [61], cattle [62], flies [26], and worms [63]. Furthermore, gene targeting in zygotes is possible, independent of embryonic stem cells [64]. Genome editing in plants is providing new opportunities in agricultural biotechnology for the production of food and biofuels [65,66]. Finally, genome editing has been applied to the generation of apoptosis-resistant mammalian cells lines for improved biopharmaceutical production [67].
6. Genome Editing in Gene and Cell Therapy
The field of gene therapy has typically focused on the addition of new genes to cells, leading to a variety of challenges and obstacles. Genome editing has provided several distinct means for addressing the limitations of previous gene therapy approaches. First, transgenes can be added to specific “safe harbor” loci in the genome [68,69], in contrast to conventional gene delivery vectors that integrate randomly into chromosomal DNA. This approach was recently explored as a gene therapy for chronic granulomatous disease [70]. Alternatively, the disease-causing mutations can be directly corrected by genome editing, as has been done in studies of X-linked SCID [71], α1-antitrypsin deficiency [46], sickle cell anemia [72,73], hemophilia B [74], and p53-related cancer [75]. Genes may also be disrupted by genome editing to produce therapeutic phenotypes. For example, the HIV co-receptor CCR5 has been disrupted in both T cells [42] and hematopoietic stem cells [76] thus blocking HIV entry. Clinical trials are underway with this approach (NCT00842634, NCT01044654, and NCT01252641) and at the time of this review, data from the first phase 1 clinical trial has demonstrated improvement in several clinical parameters while being well tolerated. The HIV co-receptor CXCR4 has also been targeted in similar preclinical studies [77]. Furthermore, gene editing has been used to enhance cellular immunotherapy by disrupting endogenous T cell receptors [78,79] or the glucocorticoid receptor in T cells in a clinical trial for malignant glioma (NCT01082926). Finally, successful genome editing in human embryonic stem cells and induced pluripotent stem cells has provided new avenues for genetic correction or augmentation in regenerative medicine [68,80–82].
7. Conclusions
Genome editing is rapidly progressing towards a golden era of easily accessible, highly specific enzymes that can directly manipulate genomic targets of interest. In the last two years, there has been an explosion in the number and diversity of applications of this technology (Figure 1). Collectively these advances represent a paradigm shift in the way we manipulate and study complex genomes and cellular processes.
Several challenges and opportunities still remain as these technologies move towards widespread adoption. A large-scale study of the in vitro and in vivo DNA-binding properties of TALEs relative to zinc finger proteins would provide insightful information on the differential activity and capacity for reengineering of these scaffolds. There is still much work to be done to further improve both the specificity of engineered nucleases as well as the methods used to monitor off-target events, and advances in high-throughput sequencing are facilitating these efforts [43,45,46]. The structure and epigenetic state of the genomic target site are likely equally important as the engineered DNA-binding proteins [69], and this is a subject that has largely been understudied. The continued development of methods for controlling the mechanisms of DNA repair will enhance the robustness of genome editing and the uniformity of modified alleles [34–38]. Improved methods for efficient nuclease delivery, particularly in vivo [74], will be essential to translating genome editing into gene therapies. Recent evidence that AAV-based homologous donor vectors lead to enhanced homology-directed repair provides a promising path forward in this area [83–86]. Finally, the development of methods for genome editing that do not depend on DNA repair pathways, such as zinc finger recombinases [87–91], may ultimately improve the safety and specificity of genome editing.
Table 2.
Representative Applications of Genome Editing
| Type of modification | Species | Gene | Nuclease Source | Reference(s) |
|---|---|---|---|---|
| Gene knockout | Human | CCR5 | Sangamo | 19,42,76 |
| Rat | eGFP, IgM, Rab38 | Sangamo | 56 | |
| Pig | GGTA1 | Sangamo | 57 | |
| Zebrafish | GOL, NTL | Sangamo | 58 | |
| Frog | eGFP, NOG | Sangamo | 60 | |
| Rabbit | IgM | Sangamo | 61 | |
| Hamster | BAK, BAX | Sangamo | 67 | |
| Human | CXCR4 | Sangamo | 77 | |
| Human | TCR | Sangamo | 78, 79 | |
| Cattle | β-lactoglobulin | Sigma CompoZr | 62 | |
| Human | COSMC | Sigma CompoZr | 47 | |
| Zebrafish | KDRA | Bacterial 1-hybrid | 59 | |
| Human | VEGF-A, HoxB13, CFTR | OPEN | 10 | |
| Plants, Zebrafish | 20 unique genes | CoDA | 12 | |
| Drosophila | yellow | Modular assembly | 26 | |
| C. elegans | Nw | Modular assembly | 63 | |
| Gene addition | Human | H3f3b | Sangamo | 49 |
| Human, Monkey | CLTA, DNM2 | Sangamo | 50 | |
| Maize | IPK1, Zp15 | Sangamo | 65 | |
| Human | Factor IX | Sangamo | 74 | |
| Human | Oct4, AAVS1, PITX3 | Sangamo | 80,82 | |
| Human | MALAT1 | Sigma CompoZr | 48 | |
| Human | AAVS1 | Sigma CompoZr | 54, 68, 70 | |
| Mouse | ROSA26 | Sigma CompoZr | 64 | |
| Mouse | ROSA26 | Modular assembly | 55 | |
| Human | VEGF-A | OPEN | 10 | |
| Human | PIG-A | OPEN | 81 | |
| Genetic substitution | Human | α1-antitrypsin | Sangamo | 46 |
| Human | α-synuclein | Sangamo | 53 | |
| Human | IL2Rγ | Sangamo | 71 | |
| Human | β-globin | Sigma CompoZr | 72 | |
| Human | β-globin | OPEN | 73 | |
| Human | p53 | Yeast 1-hybrid | 75 | |
| Tobacco | SuR | Modular assembly, OPEN | 66 | |
| Drosophila | yellow | Modular assembly | 29 | |
| Chromosomal modification | Mouse | ROSA26, H3f3b | Sigma CompoZr, Sangamo | 52 |
| Human | IL2Rγ, AAVS1 | Sangamo | 51 |
Highlights.
- Genome editing is the process of modifying endogenous gene sequences and can be facilitated by engineered, targetable nucleases.
- Recent advances in methods for creating targeted DNA-binding proteins and enhancing nuclease activity have accelerated progress in genome editing.
- Genome editing has permitted novel studies of chromosomal translocation, DNA repair, gene splicing and destabilization, protein dynamics and localization, and post-translational modification through manipulation of endogenous gene sequences.
- New animal models have been developed for several species using targeted genome editing.
- Genome editing is being explored as a gene therapy for several diseases in preclinical studies, and cell-based therapies for HIV and cancer have progressed to clinical trials.
Acknowledgements
This work was supported by The Hartwell Foundation, a Basil O’Connor Starter Scholar Award from the March of Dimes, an NSF Faculty Early Career Development (CAREER) Award (1151035) and an NIH Director’s New Innovator Award (1DP2OD008586).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
*special interest
**outstanding interest
- 1.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nature reviews. Genetics. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 2.Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188:773–782. doi: 10.1534/genetics.111.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman SH, Maeder ML, Joung JK, Cathomen T. Zinc-finger nucleases for somatic gene therapy: the next frontier. Hum Gene Ther. 2011;22:925–933. doi: 10.1089/hum.2011.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pabo CO, Peisach E, Grant RA. Design and selection of novel Cys2His2 zinc finger proteins. Annu Rev Biochem. 2001;70:313–340. doi: 10.1146/annurev.biochem.70.1.313. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Lee HJ, Kim H, Cho SW, Kim JS. Targeted genome editing in human cells with zinc finger nucleases constructed via modular assembly. Genome research. 2009;19:1279–1288. doi: 10.1101/gr.089417.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandell JG, Barbas CF., 3rd Zinc Finger Tools: custom DNA-binding domains for transcription factors and nucleases. Nucleic Acids Res. 2006;34:W516–W523. doi: 10.1093/nar/gkl209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sander JD, Maeder ML, Reyon D, Voytas DF, Joung JK, Dobbs D. ZiFiT (Zinc Finger Targeter):an updated zinc finger engineering tool. Nucleic acids research. 2010;38:W462–W468. doi: 10.1093/nar/gkq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez B, Schwimmer LJ, Fuller RP, Ye Y, Asawapornmongkol L, Barbas CF., 3rd Modular system for the construction of zinc-finger libraries and proteins. Nat Protoc. 2010;5:791–810. doi: 10.1038/nprot.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Lee MJ, Kim H, Kang M, Kim JS. Preassembled zinc-finger arrays for rapid construction of ZFNs. Nature methods. 2011;8:7. doi: 10.1038/nmeth0111-7a. [DOI] [PubMed] [Google Scholar]
- 10.Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, et al. Rapid "open-source" engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez CL, Foley JE, Wright DA, Muller-Lerch F, Rahman SH, Cornu TI, Winfrey RJ, Sander JD, Fu F, Townsend JA, et al. Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Methods. 2008;5:374–375. doi: 10.1038/nmeth0508-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, Curtin SJ, Blackburn JS, Thibodeau-Beganny S, Qi Y, et al. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA) Nat Methods. 2011;8:67–69. doi: 10.1038/nmeth.1542. Describes a method for designing novel ZFNs by recombining pairs of zinc finger domains that were previously verified to be active. Provides a means for rapid synthesis of ZFNs while accounting for potential interactions of neighboring zinc fingers.
- 13.Gupta A, Christensen RG, Rayla AL, Lakshmanan A, Stormo GD, Wolfe SA. An optimized two-finger archive for ZFN-mediated gene targeting. Nat Methods. 2012 doi: 10.1038/nmeth.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 15.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 16.Mak AN, Bradley P, Cernadas RA, Bogdanove AJ, Stoddard BL. The Crystal Structure of TAL Effector PthXo1 Bound to Its DNA Target. Science. 2012;335:716–719. doi: 10.1126/science.1216211. Crystal structure of a TAL effector that elucidates the mechanism of DNA-binding and target site recognition.
- 17.Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186:757–761. doi: 10.1534/genetics.110.120717. This is one of the first examples of engineering TAL effector nucleases, which were verified to be active in a yeast-based reporter assay.
- 18.Zhang F, Cong L, Lodato S, Kosuri S, Church GM, Arlotta P. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol. 2011;29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. Describes the first example of genome editing endogenous human genes with TALENs and includes characterization of TALEN design and DNA recognition.
- 20.Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012 doi: 10.1038/nbt.2170. This paper describes a protocol for automatable, fast, high-throughput assembly of TALENs, presents the largest collection of TALENs to date, and reports an exceptionally high frequency of active TALENs at diverse target sites.
- 21.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic acids research. 2011;39:e82. doi: 10.1093/nar/gkr218. Describes a system for the rapid assembly of novel TALENs that can be completed in just a few days using reagents that are publicly available from Addgene. It also describes online software that can be used to guide TALEN design and target site selection.
- 22.Defrancesco L. Move over ZFNs. Nat Biotechnol. 2011;29:681–684. doi: 10.1038/nbt.1935. [DOI] [PubMed] [Google Scholar]
- 23.Guo J, Gaj T, Barbas CF., 3rd Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. Journal of molecular biology. 2010;400:96–107. doi: 10.1016/j.jmb.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyon Y, Vo TD, Mendel MC, Greenberg SG, Wang J, Xia DF, Miller JC, Urnov FD, Gregory PD, Holmes MC. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nature methods. 2011;8:74–79. doi: 10.1038/nmeth.1539. Provides an improved set of mutations to the FokI domain that reduce nuclease toxicity and off-target activity by preventing homodimer formation without compromising catalytic activity as was seen in earlier sets of mutations.
- 25.Sollu C, Pars K, Cornu TI, Thibodeau-Beganny S, Maeder ML, Joung JK, Heilbronn R, Cathomen T. Autonomous zinc-finger nuclease pairs for targeted chromosomal deletion. Nucleic Acids Res. 2010;38:8269–8276. doi: 10.1093/nar/gkq720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HJ, Kim E, Kim JS. Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res. 2010;20:81–89. doi: 10.1101/gr.099747.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibikova M, Carroll D, Segal DJ, Trautman JK, Smith J, Kim YG, Chandrasegaran S. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol. 2001;21:289–297. doi: 10.1128/MCB.21.1.289-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bibikova M, Beumer K, Trautman JK, Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- 30.Porteus MH, Baltimore D. Chimeric nucleases stimulate gene targeting in human cells. Science. 2003;300:763. doi: 10.1126/science.1078395. [DOI] [PubMed] [Google Scholar]
- 31.Orlando SJ, Santiago Y, DeKelver RC, Freyvert Y, Boydston EA, Moehle EA, Choi VM, Gopalan SM, Lou JF, Li J, et al. Zinc-finger nuclease-driven targeted integration into mammalian genomes using donors with limited chromosomal homology. Nucleic acids research. 2010;38:e152. doi: 10.1093/nar/gkq512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen F, Pruett-Miller SM, Huang Y, Gjoka M, Duda K, Taunton J, Collingwood TN, Frodin M, Davis GD. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nature methods. 2011;8:753–755. doi: 10.1038/nmeth.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radecke S, Radecke F, Cathomen T, Schwarz K. Zinc-finger nuclease-induced gene repair with oligodeoxynucleotides: wanted and unwanted target locus modifications. Mol Ther. 2010;18:743–753. doi: 10.1038/mt.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bozas A, Beumer KJ, Trautman JK, Carroll D. Genetic analysis of zinc-finger nuclease-induced gene targeting in Drosophila. Genetics. 2009;182:641–651. doi: 10.1534/genetics.109.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Certo MT, Ryu BY, Annis JE, Garibov M, Jarjour J, Rawlings DJ, Scharenberg AM. Tracking genome engineering outcome at individual DNA breakpoints. Nature methods. 2011;8:671–676. doi: 10.1038/nmeth.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnell Smith A, Takeuchi R, Pellenz S, Davis L, Maizels N, Monnat RJ, Jr, Stoddard BL. Generation of a nicking enzyme that stimulates site-specific gene conversion from the I-AniI LAGLIDADG homing endonuclease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5099–5104. doi: 10.1073/pnas.0810588106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Friedman G, Doyon Y, Wang NS, Li CJ, Miller JC, Hua KL, Yan JJ, Babiarz JE, Gregory PD, et al. Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme. Genome Res. 2012 doi: 10.1101/gr.122879.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez CL, Certo MT, Mussolino C, Goodwin MJ, Cradick TJ, McCaffrey AP, Cathomen T, Scharenberg AM, Joung JK. Engineered zinc finger nickases induce homology-directed repair with reduced mutagenic effects. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H, Um E, Cho SR, Jung C, Kim JS. Surrogate reporters for enrichment of cells with nuclease-induced mutations. Nature methods. 2011;8:941–943. doi: 10.1038/nmeth.1733. [DOI] [PubMed] [Google Scholar]
- 40.Pruett-Miller SM, Reading DW, Porter SN, Porteus MH. Attenuation of zinc finger nuclease toxicity by small-molecule regulation of protein levels. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000376. e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doyon Y, Choi VM, Xia DF, Vo TD, Gregory PD, Holmes MC. Transient cold shock enhances zinc-finger nuclease-mediated gene disruption. Nat Methods. 2010;7:459–460. doi: 10.1038/nmeth.1456. [DOI] [PubMed] [Google Scholar]
- 42.Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattanayak V, Ramirez CL, Joung JK, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nature methods. 2011;8:765–770. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petek LM, Russell DW, Miller DG. Frequent endonuclease cleavage at off-target locations in vivo. Mol Ther. 2010;18:983–986. doi: 10.1038/mt.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabriel R, Lombardo A, Arens A, Miller JC, Genovese P, Kaeppel C, Nowrouzi A, Bartholomae CC, Wang J, Friedman G, et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat Biotechnol. 2011;29:816–823. doi: 10.1038/nbt.1948. Analyzes the specificity of ZFN-mediated DNA cleavage by genome-wide deep sequencing of sequence tags integrated into DSBs. This is the first example of mapping specificity in vivo without predicting target sites a priori. Although many off-target events were detected, they typically had high sequence homology to the intended target site.
- 46.Yusa K, Rashid ST, Strick-Marchand H, Varela I, Liu PQ, Paschon DE, Miranda E, Ordonez A, Hannan NR, Rouhani FJ, et al. Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cells. Nature. 2011;478:391–394. doi: 10.1038/nature10424. This article uses ZFNs to correct a point mutation that causes α1-antitrypsin deficiency in human induced pluripotent stem cells that were subsequently differentiated into functioning liver cells. The study includes a comprehensive analysis of the effects of genome editing and genetic reprogramming on the genome, including exome sequencing and comparative genomic hybridization.
- 47.Steentoft C, Vakhrushev SY, Vester-Christensen MB, Schjoldager KT, Kong Y, Bennett EP, Mandel U, Wandall H, Levery SB, Clausen H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nature methods. 2011;8:977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 48.Gutschner T, Baas M, Diederichs S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome research. 2011;21:1944–1954. doi: 10.1101/gr.122358.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doyon JB, Zeitler B, Cheng J, Cheng AT, Cherone JM, Santiago Y, Lee AH, Vo TD, Doyon Y, Miller JC, et al. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nature cell biology. 2011;13:331–337. doi: 10.1038/ncb2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunet E, Simsek D, Tomishima M, DeKelver R, Choi VM, Gregory P, Urnov F, Weinstock DM, Jasin M. Chromosomal translocations induced at specified loci in human stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10620–10625. doi: 10.1073/pnas.0902076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simsek D, Brunet E, Wong SY, Katyal S, Gao Y, McKinnon PJ, Lou J, Zhang L, Li J, Rebar EJ, et al. DNA ligase III promotes alternative nonhomologous end-joining during chromosomal translocation formation. PLoS genetics. 2011;7 doi: 10.1371/journal.pgen.1002080. e1002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soldner F, Laganiere J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeKelver RC, Choi VM, Moehle EA, Paschon DE, Hockemeyer D, Meijsing SH, Sancak Y, Cui X, Steine EJ, Miller JC, et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome research. 2010;20:1133–1142. doi: 10.1101/gr.106773.110. Provides a comprehensive characterization of ZFN-mediated gene targeting to the AAVS1 locus, which can be used for robust and reproducible development of isogenic human cell lines.
- 55.Perez-Pinera P, Ousterout DG, Brown MT, Gersbach CA. Gene targeting to the ROSA26 locus directed by engineered zinc finger nucleases. Nucleic Acids Res. 2012;40:3741–3752. doi: 10.1093/nar/gkr1214. Provides a comprehensive characterization of ZFN-mediated gene targeting to the ROSA26 locus, which can be used for robust and reproducible development of isogenic mouse cell lines.
- 56.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. First example of genome editing in rats, providing a method for creating animal models with much greater physiological similarity to humans than the more commonly used mouse model.
- 57.Hauschild J, Petersen B, Santiago Y, Queisser AL, Carnwath JW, Lucas-Hahn A, Zhang L, Meng X, Gregory PD, Schwinzer R, et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12013–12017. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nature biotechnology. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young JJ, Cherone JM, Doyon Y, Ankoudinova I, Faraji FM, Lee AH, Ngo C, Guschin DY, Paschon DE, Miller JC, et al. Efficient targeted gene disruption in the soma and germ line of the frog Xenopus tropicalis using engineered zinc-finger nucleases. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7052–7057. doi: 10.1073/pnas.1102030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flisikowska T, Thorey IS, Offner S, Ros F, Lifke V, Zeitler B, Rottmann O, Vincent A, Zhang L, Jenkins S, et al. Efficient immunoglobulin gene disruption and targeted replacement in rabbit using zinc finger nucleases. PloS one. 2011;6:e21045. doi: 10.1371/journal.pone.0021045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu S, Luo J, Song Z, Ding F, Dai Y, Li N. Highly efficient modification of beta-lactoglobulin (BLG) gene via zinc-finger nucleases in cattle. Cell research. 2011;21:1638–1640. doi: 10.1038/cr.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morton J, Davis MW, Jorgensen EM, Carroll D. Induction and repair of zinc-finger nuclease-targeted double-strand breaks in Caenorhabditis elegans somatic cells. Proc Natl Acad Sci U S A. 2006;103:16370–16375. doi: 10.1073/pnas.0605633103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyer M, de Angelis MH, Wurst W, Kuhn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, et al. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature. 2009;459:437–441. doi: 10.1038/nature07992. [DOI] [PubMed] [Google Scholar]
- 66.Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK, Voytas DF. High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature. 2009;459:442–445. doi: 10.1038/nature07845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cost GJ, Freyvert Y, Vafiadis A, Santiago Y, Miller JC, Rebar E, Collingwood TN, Snowden A, Gregory PD. BAK and BAX deletion using zinc-finger nucleases yields apoptosis-resistant CHO cells. Biotechnol Bioeng. 2010;105:330–340. doi: 10.1002/bit.22541. [DOI] [PubMed] [Google Scholar]
- 68.Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, Ando D, Urnov FD, Galli C, Gregory PD, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 69.Lombardo A, Cesana D, Genovese P, Di Stefano B, Provasi E, Colombo DF, Neri M, Magnani Z, Cantore A, Lo Riso P, et al. Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat Methods. 2011;8:861–869. doi: 10.1038/nmeth.1674. [DOI] [PubMed] [Google Scholar]
- 70.Zou J, Sweeney CL, Chou BK, Choi U, Pan J, Wang H, Dowey SN, Cheng L, Malech HL. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood. 2011;117:5561–5572. doi: 10.1182/blood-2010-12-328161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 72.Zou J, Mali P, Huang X, Dowey SN, Cheng L. Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood. 2011;118:4599–4608. doi: 10.1182/blood-2011-02-335554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sebastiano V, Maeder ML, Angstman JF, Haddad B, Khayter C, Yeo DT, Goodwin MJ, Hawkins JS, Ramirez CL, Batista LF, et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells. 2011;29:1717–1726. doi: 10.1002/stem.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, Malani N, Anguela XM, Sharma R, Ivanciu L, et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475:217–221. doi: 10.1038/nature10177. This is the first example of genome editing in vivo and provides a therapeutic approach to hemophilia via gene delivery to the liver that could also be extended to many other diseases.
- 75.Herrmann F, Garriga-Canut M, Baumstark R, Fajardo-Sanchez E, Cotterell J, Minoche A, Himmelbauer H, Isalan M. p53 Gene repair with zinc finger nucleases optimised by yeast 1-hybrid and validated by Solexa sequencing. PloS one. 2011;6:e20913. doi: 10.1371/journal.pone.0020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. This is the first example of genome editing of hematopoietic stem cells and subsequent engraftment into an animal model. This approach to disrupting CCR5 is now in clinical trials as a cell-based therapy for HIV.
- 77.Yuan J, Wang J, Crain K, Fearns C, Kim KA, Hua KL, Gregory PD, Holmes MC, Torbett BE. Zinc-finger Nuclease Editing of Human cxcr4 Promotes HIV-1 CD4(+) T Cell Resistance and Enrichment. Mol Ther. 2012;20:849–859. doi: 10.1038/mt.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovitch B, et al. A foundation for"universal" T-cell based immunotherapy: T-cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119:5697–5705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, Reik A, Chu V, Paschon DE, Zhang L, Kuball J, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012 doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, Zeitler B, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zou J, Maeder ML, Mali P, Pruett-Miller SM, Thibodeau-Beganny S, Chou BK, Chen G, Ye Z, Park IH, Daley GQ, et al. Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell. 2009;5:97–110. doi: 10.1016/j.stem.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hendrie PC, Russell DW. Gene targeting with viral vectors. Mol Ther. 2005;12:9–17. doi: 10.1016/j.ymthe.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Handel EM, Gellhaus K, Khan K, Bednarski C, Cornu TI, Muller-Lerch F, Kotin RM, Heilbronn R, Cathomen T. Versatile and Efficient Genome Editing in Human Cells by Combining Zinc-Finger Nucleases With Adeno-Associated Viral Vectors. Hum Gene Ther. 2012;23:321–329. doi: 10.1089/hum.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellis BL, Hirsch ML, Porter SN, Samulski RJ, Porteus MH. Zinc-finger nuclease-mediated gene correction using single AAV vector transduction and enhancement by Food and Drug Administration-approved drugs. Gene Ther. 2012 doi: 10.1038/gt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asuri P, Bartel MA, Vazin T, Jang JH, Wong TB, Schaffer DV. Directed Evolution of Adeno-associated Virus for Enhanced Gene Delivery and Gene Targeting in Human Pluripotent Stem Cells. Mol Ther. 2012;20:329–338. doi: 10.1038/mt.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gordley RM, Gersbach CA, Barbas CF., 3rd Synthesis of programmable integrases. Proc Natl Acad Sci U S A. 2009;106:5053–5058. doi: 10.1073/pnas.0812502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gersbach CA, Gaj T, Gordley RM, Mercer AC, Barbas CF., 3rd Targeted plasmid integration into the human genome by an engineered zinc-finger recombinase. Nucleic Acids Res. 2011;39:7868–7878. doi: 10.1093/nar/gkr421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gordley RM, Smith JD, Graslund T, Barbas CF., 3rd Evolution of programmable zinc finger-recombinases with activity in human cells. J Mol Biol. 2007;367:802–813. doi: 10.1016/j.jmb.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 90.Gersbach CA, Gaj T, Gordley RM, Barbas CF., 3rd Directed evolution of recombinase specificity by split gene reassembly. Nucleic Acids Res. 2010;38:4198–4206. doi: 10.1093/nar/gkq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaj T, Mercer AC, Gersbach CA, Gordley RM, Barbas CF., III Structure-guided reprogramming of serine recombinase DNA sequence specificity. Proc Natl Acad Sci U S A. 2011;108:498–503. doi: 10.1073/pnas.1014214108. Describes a method for directed evolution of zinc finger recombinases to new target sequences. This approach has the potential for genome editing without inducing DNA damage repair.