Biology and Biophysics of the Nuclear Pore Complex And Its Components (original) (raw)
. Author manuscript; available in PMC: 2015 Mar 19.
Abstract
Nucleocytoplasmic exchange of proteins and ribonucleoprotein particles occurs via nuclear pore complexes (NPCs) that reside in the double membrane of the nuclear envelope (NE). Significant progress has been made the past few years in obtaining better structural resolution of the 3-D architecture of the NPC with the help of cryo-electron tomography and atomic structures of domains from nuclear pore proteins (nucleoporins). Biophysical and imaging approaches have helped elucidate how nucleoporins act as a selective barrier in nucleocytoplasmic transport. Nucleoporins act not only in trafficking of macromolecules but also in proper microtubule attachment to kinetochores, in the regulation of gene expression and signaling events associated with, for example, innate and adaptive immunity, development and neurodegenerative disorders. Recent research has also been focused on the dynamic processes of NPC assembly and disassembly that occur with each cell cycle. Here we review emerging results aimed at understanding the molecular arrangement of the NPC and how it is achieved, defining the roles of individual nucleoporins both at the NPC and at other sites within the cell, and finally deciphering how the NPC serves as both a barrier and a conduit of active transport.
I. Introduction
In interphase eukaryotic cells, trans-cription takes place in the cell nucleus while proteins are synthesized in the cytoplasm. Exchange of material between these two cellular compartments occurs via nuclear pore complexes (NPCs) located in the double membrane of the nuclear envelope (NE). NPCs support passive diffusion of small molecules and ions and facilitate receptor-mediated translocation of proteins and ribonucleoprotein complexes. Overall, the vertebrate NPC is a ~120 MDa protein complex made up ~30 different proteins called nucleoporins (or Nups) that are repetitively arranged as distinct subcomplexes to form the NPC (Cronshaw, J. M. et al., 2002; Lim, R. Y., and Fahrenkrog, B., 2006; Rout, M. P. et al., 2000; Schwartz, T. U., 2005; Tran, E. J., and Wente, S. R., 2006). In the plane of the NE, the eightfold symmetric central framework of the NPC embraces a central pore that is ~50 nm long and is narrowest (~40 nm) at the NE midplane (Beck, M. et al., 2004; Beck, M. et al., 2007; Stoffler, D. et al., 2003). Attached to the central framework are cytoplasmic filaments and a nuclear basket (Fig. 1). We begin, here, by reviewing recent exciting advances towards the elucidation of NPC architecture at the ultrastructural and atomic level by electron tomography and X-ray crystallography. These inroads into NPC structure lay important groundwork for understanding the function of the nuclear pore and we will overview progress that has been made in our understanding how the NPC acts as a selective barrier for macromolecular cargo. We will also discuss recent insights into the function of individual nucleoporins in nuclear organization that go beyond their well-characterized role in nucleocytoplasmic transport. Last but not least, we will review recent progress in addressing how the NPC disassembles and assembles at the beginning and end of mitosis, respectively.
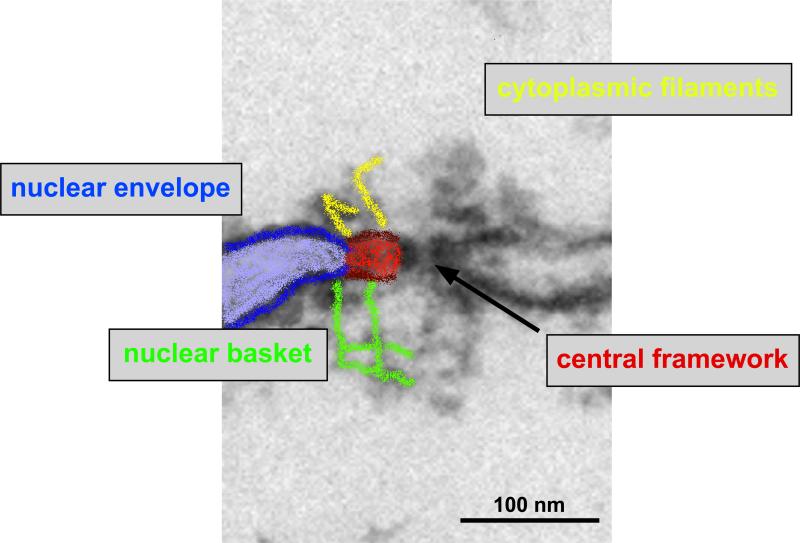
Figure 1.
Electron micrograph with partially overlayed schematic representation of a cross-sectioned nuclear pore complex. The major structural components include the central framework, the cytoplasmic filaments and a nuclear basket.
II. Nuclear pore complex structure
A. Overall nuclear pore complex architecture
The NPC is a highly complex structure and electron microscopy (EM) and, more recently, cryo-electron tomography (CET) have proven to be the methods of choice to study intact NPCs at high resolution. The NPC consists of an approximately cylindrical central framework, eight cytoplasmic filaments and a nuclear basket composed of eight filaments that join into a distal ring (Fig. 1). Early EM studies provided 3D reconstructions of the central framework using negatively stained and frozen-hydrated NPCs from Xenopus laevis oocyte NEs (Akey, C. W., and Radermacher, M., 1993; Hinshaw, J. E. et al., 1992) or frozen-hydrated yeast cells (Yang, Q.et al., 1998). The central framework of the NPC (also called the spoke complex) resides between the inner and outer nuclear membranes, anchored where these parallel membrane bilayers curve to meet each other. Early structural studies showed that cytoplasmic and nuclear ring moieties are integral to the central framework. Two recent CET studies in Xenopus oocyte isolated nuclei (Stoffler, D. et al., 2003) and in intact, transport-competent nuclei isolated from Dictyostelium discoideum (Beck, M. et al., 2004; Beck, M. et al., 2007) have improved the resolution of the central framework to 8 - 9 nm and revealed the first reconstructions of peripheral, flexible components of the NPC, i.e. the cytoplasmic filaments and the nuclear basket. In Dictyostelium, the cytoplasmic filaments have a length of ~35 nm and the nuclear basket is about 60 nm long. Together with the ~50 nm central framework, the NPC therefore has a total length of about 150 nm with the outer diameter of the structure being 125 nm (Beck, M. et al., 2004; Beck, M. et al., 2007). The overall linear dimensions of the NPC varies between species, whereas the overall 3D architecture appears to be evolutionarily conserved (Fahrenkrog, B. et al., 1998; Kiseleva, E. et al., 2004; Yang, Q. et al., 1998).
Enclosed by the central framework is the hourglass-shaped central pore of the NPC with a diameter of 60 - 70 nm at its cytoplasmic and nuclear periphery and ~45 nm in the midplane of the NPC/NE (Beck, M. et al., 2004; Beck, M. et al., 2007; Panté, N., and Kann, M., 2002; Stoffler, D. et al., 2003). This central pore mediates all exchange between the cytoplasm and the nucleus and enables transport of macromolecules with diameters of up to 39 nm (Panté, N., and Kann, M., 2002). Increasing concentrations of signal-carrying cargoes selectively interferes with the passage of other molecules that utilize a facilitated pathway, but not with the diffusion of inert molecules and vice versa, suggesting that passive and facilitated transport across the NPC proceed via routes that are sterically non-overlapping (Naim, B. et al., 2007). Whether these two routes exist in the central pore [i.e., facilitated transport along the walls of the central pore and passive diffusion through a narrow diffusion tube located at the pore center (Peters, R., 2005)], or whether passive diffusion might additionally utilize peripheral channels (Akey, C. W., and Radermacher, M., 1993; Beck, M. et al., 2004; Hinshaw, J. E. et al., 1992; Stoffler, D. et al., 2003) remains to be seen.
Peripheral channels of the NPC have a diameter of about 8 nm and have been implicated in the diffusion of small molecules and ions (Feldherr, C. M., and Akin, D., 1997; Hinshaw, J. E. et al., 1992) and/or in trafficking of integral membrane proteins to the inner nuclear membrane (Soullam, B., and Worman, H. J., 1995). However, the more recent observation that the cytoplasmic openings of the peripheral channels are not topologically continuous with the nuclear openings (Stoffler, D. et al., 2003) challenges the view that they act as transport channels. Other potential roles have been proposed, such as in maintenance the NE electrical conductance (Danker, T. et al., 1999; Enss, K.et al., 2003; Mazzanti, M. et al., 2001; Shahin, V. et al., 2001) or as buffer zones that accommodate deformations of the central framework upon translocation of large cargoes (Fahrenkrog, B., and Aebi, U., 2003).
B. The nuclear pore complex at atomic level
Based on secondary structure prediction, nucleoporins have been grouped into three classes (Devos, D. et al., 2006). The transmembrane group, which contains transmembrane α-helices and a cadherin-fold, comprises the outermost features of the NPC central framework and at least some members of this group are thought to help anchor the NPC in the NE. The second group of nucleoporins contain β-propeller and α-solenoid folds and these nucleoporins localize towards the inside of the NPC, whereas the third class harbors the conserved sequence motif of phenylalanine-glycine (FG)-repeats (see Section III and IV) in combination with a coiled-coil fold and may contribute to the formation of the NPC's inner central framework and the peripheral structures (Devos, D. et al., 2006; Schwartz, T. U., 2005; Tran, E. J., and Wente, S. R., 2006). Other less frequent structural motifs found in nucleoporins are zinc-finger domains as in Nup153 and RanBP2/Nup358 (Higa, M. M. et al., 2007) or RNA-recognition motifs as in Nup35 (Handa, N. et al., 2006).
β-propellers are predicted in a third of the nucleoporins, and in fact seven-bladed β-propellers have been resolved from the N-terminal domains (NTD) of the human nucleoporins Nup133 and Nup214 and its yeast homologue Nup159p by X-ray crystallography (Berke, I. C. et al., 2004; Napetschnig, J. et al., 2007; Weirich, C. S. et al., 2004). Proteins with β-propeller folds participate in diverse cellular functions and serve as platforms for multiple dynamic protein-protein interactions. Along this line, yeast Nup133p and Nup159p both play roles in mRNA export from the nucleus, and deletion or mutations in their NTDs impair their functions in mRNA export, probably by preventing the association of multiple mRNA export factors with the NPC (Berke, I. C. et al., 2004; Weirich, C. S. et al., 2004).
The NTD of human Nup133 furthermore contains an amphipathic α-helical motif capable of sensing membrane curvature (Drin, G. et al., 2007). This motif corresponds to an exposed loop, which connects two blades of the β-propeller and folds into an α-helix upon interacting with small liposomes. Whether the membrane curvature sensor in Nup133 serves to recognize the topology of the nuclear pore membrane to anchor the NPC during interphase or to recognize small vesicles containing NE fragments critical for NE reassembly after mitosis, or both, remains to be seen (Drin, G. et al., 2007).
The NTD of human Nup214, in comparison to its yeast homologue Nup159p, consists of two distinct structural elements: the β-propeller and a 30-residue C-terminal extended peptide segment (Napetschnig, J. et al., 2007). This extension binds to the bottom of the β-propeller with low affinity and has been suggested to play an “auto-inhibitory” role in NPC assembly.
The first crystal structure obtained for a nucleoporin was the NPC targeting domain of human Nup98 (Hodel, A. E. et al., 2002). This domain, similar to the nuclear pore targeting domain of its yeast homologue Nup116p, consists of a six-stranded β-sheet sandwiched against a two-stranded β-sheet and flanked by two α-helical regions (Hodel, A. E. et al., 2002; Robinson, M. A. et al., 2005). This domain exhibits multiple conformations and is stabilized only when bound to a ligand, i.e. Nup96 and Nup145p-C in the case of Nup98 and Nup116p, respectively (Robinson, M. A. et al., 2005). Conformational diversity might allow Nup98 and Nup116p to bind to multiple targets within the NPC or to associate and dissociate fast from the NPC to increase the mobility of the nucleoporins, as described for Nup98, which shuttles in an transcription-dependent manner (Griffis, E. R. et al., 2002; Griffis, E. R. et al., 2004).
The attempt to crystallize the first subcomplex of the NPC, the Nup62 complex, yielded the structure of the α-helical coiled-coil domain of only one of its components, rat Nup58/45 (Melcak, I. et al., 2007). Nup58/45 forms tetramers in the crystal structure consisting of two antiparallel dimers. Each dimer consists of two α-helices that are connected by a short loop. The intradimer interactions are of hydrophobic nature, whereas two dimers associate through hydrophilic residues. The tetramer can adopt various conformations leading to a lateral displacement between tetramers suggesting an intermolecular sliding mechanism (Melcak, I. et al., 2007). The Nup62 complex has recently been mapped to the cytoplasmic periphery of the NPC's central pore (Schwarz-Herion, K. et al., 2007), so that sliding of Nup58/45, and most likely of Nup62 and Nup54 as well, could contribute to modulating the diameter of the central pore in response to transport activity (Melcak, I. et al., 2007). While definitely intriguing, further experimental data will be required to uphold this model.
C. Nuclear pore complex density and distribution
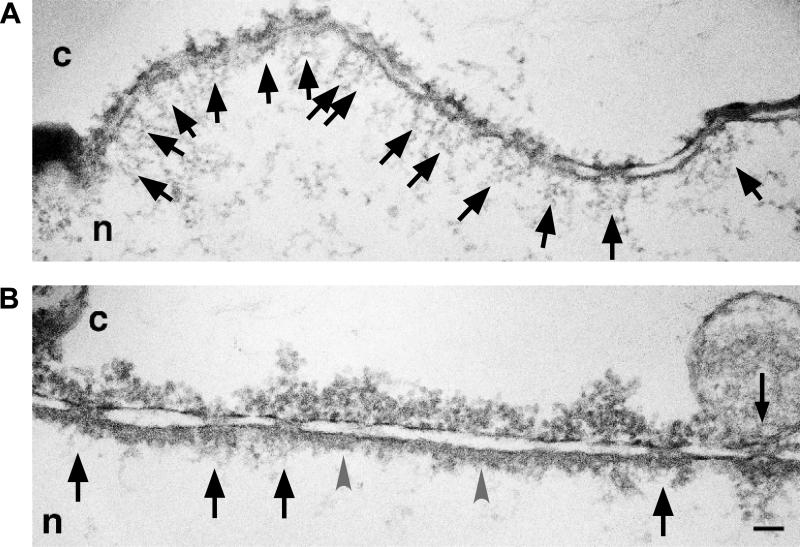
The number of NPCs per cell varies greatly with cell size and activity. Yeast cells have ~200 NPCs, proliferating human cells ~3000-5000 and a mature Xenopus oocyte ~5 × 107 (Gorlich, D., and Kutay, U., 1999). A comprehensive ultrastructural study using freeze-fracture EM of yeast cells in combination with 3D reconstruction has shown that the distribution of yeast NPCs in the NE is not equidistant, but rather clustered into regions of higher density (Winey, M. et al., 1997). The number of NPCs was found to increase steadily, beginning in G1-phase and peaking in S-phase of the cell cycle, suggesting that NPC assembly occurs continuously throughout the cell cycle (see Section V) (Winey, M. et al., 1997). Similarly, the density of NPCs increases through-out the cell cycle in HeLa S3 cells (Maeshima, K. et al., 2006). Interestingly, these HeLa S3 cells exhibit large subdomains in the NE devoid of NPCs. These “pore-free islands” are present in telophase and G1 nuclei and are enriched in the inner nuclear membrane proteins emerin and lamin A/C, but not lamin B (Maeshima, K. et al., 2006). Knock-down of lamin A/C by RNAi resulted in the disappearance of the pore-free islands, whereas upregulation of lamin A/C facilitated the formation of pore-free islands. Although the physiological relevance of pore-free islands remains to be elucidated, in HeLa cells the presence of such regions correlates with lower proliferative activity. Consistent with this, embryonic cells lack lamin A/C and have a high density of NPC along with high proliferative activity (Maeshima, K. et al., 2006; Maul, G. G. et al., 1980). Further indication of a relationship between lamin expression and pore density was obtained using Xenopus oocytes, whose giant nuclei lack lamin A/C and exhibit a high density of NPCs. Overexpression of human lamin A in these oocyte nuclei leads to the appearance of stretches in the NE that are devoid of NPCs (B.Fahrenkrog and B. Maco, unpublished results; Fig. 2).
Figure 2.
Electron micrographs of cross-sections along a nuclear envelope of isolated Xenopus oocyte nuclei. (A) The nuclear envelope of a stage 6 nucleus is characterized by a high density of nuclear pore complexes (black arrows). (B) Overexpression of human lamin A in these Xenopus oocyte nuclei causes a decrease in nuclear pore complex (black arrows) density and a thickened nuclear lamina (grey arrowheads). c, cytoplasm; n, nucleus. Scale bar, 100 nm.
Recent studies using mouse embryonic stem (ES) cells addressed the adaptation of NPC structure and density during cardiac differentiation (Perez-Terzic, C. et al., 2003; Perez-Terzic, C. et al., 2007). Accordingly, NPC density increases somewhat when ES cells differentiate into proliferative cardiomyocytes. With a few significant exceptions, genes encoding components of the nucleocytoplasmic transport machinery, i.e. nuclear transport receptors, nucleoporins and Ran-related factors, were found to be broadly down regulated in cardiomyocytes derived from ES cells compared to the undifferentiated state of ES cells, supporting the notion that changes in transport occur concomitantly (and maybe help to drive) differentiation. Further differences have been observed between stem-cell derived cardiomyocytes and adult heart-isolated cardiomyocytes, which have a density of ~15 NPCs/μm2 and ~28 NPCs/μm2 respectively. While the overall diameter and height of the NPC are similar in both cell types, there is greater central density in the NPCs of stem-cell derived cardiomyocytes, indicative of greater transport activity (Perez-Terzic, C. et al., 2003).
Drosophila Nup154, the homologue of rat Nup155, is essential for gametogenesis (Gigliotti, S. et al., 1998) and regulated expression of a testis-specific isoform of RanBP2/Nup358, BS-63, and Nup50/Npap60 may also influence gamete/testis maturation (Hogarth, C. et al., 2005).
Another case in which the nuclear transport machinery appears to be involved in cellular fate is found in malignant cells resistant to chemotherapy (Lewin, J. M. et al., 2007). Multidrug resistance commonly limits efficiency in treating malignant cells with chemotherapy and is classically described as a plasma membrane phenomenon. However, multidrug resistant cells specifically exclude chemotherapy from the nucleus and have now been shown to exhibit an increased number of NPCs compared to drug-sensitive cells (Lewin, J. M. et al., 2007). The increase in NPC number somehow correlates with the exclusion of chemotherapeutic drugs from the nucleus, suggesting that nuclear export is selectively enhanced in the resistant cells. The mechanism by which NPCs export chemotherapy remains elusive, but inhibition of nucleocytoplasmic transport with injection of wheat germ agglutinin can reverse multidrug resistance in these cells (Lewin et al., 2007). All together, these data indicate that regulation of NPC number, composition and nucleo-cytoplasmic transport may drive and influence more cellular processes than previously assumed.
III. Nucleoporin function(s)
A. FG-nucleoporins and nucleo-cytoplasmic transport
FG-repeat domains are found in about one third of the nucleoporins and mediate the interaction between soluble transport receptors loaded with signal-bearing cargo and the NPC. These FG-repeat domains also likely contribute to the selective barrier that limits diffusion through the NPC (see section IV). Atomic structures of FG-repeat peptides in complex with, for example importin β, NTF2 or the putative mRNA export factor TAP/NXF1, have consistently shown that the interaction between FG-repeats and the different transport receptors involves primarily the phenylalanine ring of the FG-repeat core and hydrophobic residues on the surface of the receptor. Hydrophilic linker between individual FG-motifs, which constitute the majority of amino acid mass in the overall FG-domain, appear to influence the strength of the binding and allow simultaneous binding of several FG-cores to the receptor (Liu, S. M., and Stewart, M., 2005).
Based on biophysical measurements, the FG-repeat domains of yeast nucleoporins were found to be natively unfolded, i.e. having no or only little secondary structure. Similarly, FG-repeat domains of human, fly, worm and other yeast species are most likely disordered based on their amino acid composition (Denning, D. P., and Rexach, M. F., 2007). This notion is further supported by immuno-EM studies on two vertebrate FG-repeat nucleoporins, Nup153 and Nup214, which suggested that FG-repeat domains are flexible and mobile within the NPC (Paulillo, S. M. et al., 2005; Paulillo, S. M. et al., 2006). Atomic force microscopy (AFM) studies on recombinantly expressed FG-repeat domain of human Nup153 further revealed that this ~700 residue domain in fact is an extended molecule with a length of ~180 nm, resembling an unfolded polypeptide chain (Lim, R. Y., and Aebi, U., 2005).
Nup153 and Nup214 are both known to play roles in distinct nucleocytoplasmic transport pathways and to interact with a number of nuclear transport receptors via their FG-repeats (Ball, J. R., and Ullman, K. S., 2005; Bernad, R. et al., 2006; Hutten, S., and Kehlenbach, R. H., 2006; Sabri, N. et al., 2007; van Deursen, J. et al., 1996). The location of the FG-repeat domains of Nup153 and Nup214 in the NPC shifts in a transport-dependent manner, further supporting their role in nucleocytoplasmic transport (Paulillo et al., 2005). Systematic deletion of FG-repeat regions in yeast nucleoporins revealed, however, that yeast NPCs are able to compensate the loss of about 50% of their FG-repeats with only little effect on distinct nuclear transport pathways, indicating that FG-repeats are highly redundant within the NPC, that individual FG-nucleoporins appear critical for specific nuclear transport pathways but not for bulk nucleocytoplasmic transport, and/or that other interaction sites for transport receptors exist within the NPC.
Besides playing important roles in nucleocytoplasmic transport, FG-repeat domains may have other functions as well. The crystal structure of the RRM domain of mouse Nup35 revealed that all three FG-sequences of this nucleoporin are in ordered secondary structure elements and that these FG-sequences do not interact with transport receptors, such as importin β, but rather with, for example, the integral membrane protein Ndc1. Thus the FG-sequences of Nup35 may contribute to the formation of the NPC's central framework (Handa, N. et al., 2006).
B. Nucleoporins and kinetochores
A well-studied and conserved subcomplex of the NPC is the vertebrate Nup107-160 complex and its yeast homologue the Nup84p complex. The Nup107-160 complex is composed of nine nucleoporins and resides on both sides of the central framework of the NPC (Belgareh, N. et al., 2001; Krull, S. et al., 2004; Loiodice, I. et al., 2004; Orjalo, A. V. et al., 2006). The Nup107-160 complex seems to represent the core element of the central framework, since depletion of any member of this NPC subcomplex in nuclear reconstitution assays or by RNAi led to the assembly of NPC-free nuclei or nuclei with severe deficiencies in NPC formation (Boehmer, T. et al., 2003; Harel, A. et al., 2003b; Loiodice, I. et al., 2004; Walther, T. C. et al., 2003a) (see Section V).
A fraction of the Nup107-160 complex is targeted to kinetochores from prophase to late anaphase (Belgareh, N. et al., 2001; Loiodice, I. et al., 2004), to spindle poles and proximal spindle fibers in prometaphase mammalian cells and throughout reconstituted spindles in Xenopus egg extracts (Orjalo, A. V. et al., 2006). Anchoring of the human Nup107-160 complex to kinetochores is mediated by the Ndc80 complex, which is part of the outer kinetochore and involved in formation and maintenance of stable kinetochore-microtubule (MT) interaction, and CENP-F, which is also involved in MT attachment (Zuccolo, M. et al., 2007). Kinetochores depleted of the Nup107-160 complex fail to establish proper MT attachment, which leads to a checkpoint-dependent mitotic delay (Zuccolo, M. et al., 2007).
Another nucleoporin recruited to kinetochores and the spindle in mitosis is RanBP2/Nup358 in complex with RanGAP1 (Joseph, J. et al., 2004; Joseph, J. et al., 2002; Matunis, M. J. et al., 1998). RNAi approaches revealed that the RanBP2/RanGAP1 complex is involved in chromosome congression and segregation, stable kinetochore-MT association, and kinetochore assembly (Askjaer, P. et al., 2002; Joseph, J. et al., 2004; Salina, D. et al., 2003). The nuclear export receptor CRM1 provides the anchoring site for RanBP2 and RanGAP1 at the kinetochores (Arnaoutov, A. et al., 2005), and the Nup107-160 complex in turn is required for the recruitment of CRM1, RanBP2 and RanGAP1 to the kinetochores (Zuccolo, M. et al., 2007). Therefore, the Nup107-160 complex helps to recruit distinct kinetochore subcomplexes required for stable kinetochore-MT interaction.
C. Nucleoporins and transcription
In the past few years it became evident that the NPC plays a role in chromatin organization in the nucleus. In this context, the nuclear periphery and the NPCs have been considered a zone of gene repression caused by the presence of heterochromatin and silencing factors (Brown, C. R., and Silver, P. A., 2007). Consistently, in S. cerevisiae, two nucleoporins, Nup60p and Nup145p-C, are required for repression of the silent mating type loci HML and HMR and for the proper silencing of telomeres (Brown, C. R., and Silver, P. A., 2007; Feuerbach, F. et al., 2002; Galy, V. et al., 2000). However, yeast NPCs can also positively regulate gene expression by preventing the spread of heterochromatin regions and by recruiting actively transcribed genes to the nuclear periphery (Cabal, G. G. et al., 2006; Casolari, J. M. et al., 2005; Casolari, J. M. et al., 2004; Ishii, K. et al., 2002; Luthra, R. et al., 2007; Menon, B. B. et al., 2005; Schmid, M. et al., 2006), indicating a function for nucleoporins in transcription activation as well as gene silencing. Most importantly, the same set of nucleoporins, namely Nup2p and the Nup84p complex, can have repressive and activating functions (Dilworth, D. J. et al., 2005; Ishii, K. et al., 2002; Menon, B. B. et al., 2005; Schmid, M. et al., 2006; Therizols, P. et al., 2006), and it will be interesting to see how their dual functions in gene expression are regulated at the molecular level.
In higher eukaryotes, the first clues to function of nucleoporins in transcription came from studies with the chimeric NUP98-HOXA9 protein, a chromosomal translocation product that occurs in myelodysplastic syndromes and acute myeloid leukemia. These studies consistently showed that NUP98-HOXA9 acts as an aberrant transcription factor, with the N-terminal FG-repeat domain of Nup98 enhancing the transcriptional activity of the DNA binding domain derived from the transcription factor HoxA9 (Ghannam, G. et al., 2004; Kasper, L. H. et al., 1999). The NUP98 gene has been found to fuse with 19 different fusion partners causing different forms of acute and myeloid leukemia. Recently it became evident that NUP98 fusions can also act as trans-repressors of transcription (Bai, X. T. et al., 2006). The intranuclear localization of these fusion proteins (Kasper, L. H. et al., 1999) suggests that transcription regulation by the NUP98 fusion may not occur at the NPC or the NE. Additionally, it is not yet clear whether transcriptional regulation is the sole role of Nup98 sequences in oncogenic fusions or how this ability to modulate transcription relates to the role of endogenous Nup98. It is notable, however, that in yeast, human Nup98 was found capable of stimulating the transcription of a reporter gene at the nuclear periphery (Menon, B. B. et al., 2005).
Nup153 and Nup98 both dynamically interact with the NPC and their mobility within the cell appears transcription-dependent (Griffis, E. R. et al., 2002; Griffis, E. R. et al., 2004). The transcription factor PU.1, which is expressed in several hematopoietic cell lineages and plays a pivotal role in the differentiation of myeloid cells and lymphocytes, is proposed to be imported into the nucleus via direct interaction with Nup153 (Zhong, H. et al., 2005). Binding of PU.1 to Nup153 is stimulated by RanGTP, but is independent of any nuclear transport receptor of the karyopherin family. In the presence of a source of energy (and presumably elevated RanGTP levels), PU.1 associates with the nuclear side of the NPC (Zhong, H. et al., 2005), suggesting that PU.1-dependent active genes might be targeted to the NPC, at least in part. More direct evidence that Nup153 in fact targets genes directly to the NPC came from a recent study on dosage compensation in Drosophila, a phenomenon that is distinguished by the hypertranscription of the male X chromosome (Mendjan, S. et al., 2006). Proteins of the dosage compensation complex (DCC) were found to associate with NPC components, in particular Nup153 and Mtor, the Drosophila homologue of the mammalian nucleoporin Tpr. Nup153 and Mtor/Tpr are part of the NPC's nuclear basket, and knock-down of Nup153 or Mtor resulted in a shift of DCC localization away from the nuclear rim, coinciding with a loss of dosage compensation in male cells (Mendjan, S. et al., 2006).
The putative transcription factor ELYS was identified as binding partner of the Nup107-160 complex and found to localize to NPCs during interphase and to kinetochores in mitosis (Rasala, B. A. et al., 2006). ELYS, and its homologue in C. elegans named Mel-28, is required for NPC assembly (see Section V; (Fernandez, A. G., and Piano, F., 2006; Franz, C. et al., 2007; Galy, V. et al., 2006; Rasala, B. A. et al., 2006)), and as a DNA binding protein it potentially targets active genes to the NPC. Taken together, recent results lend support to the notion that NPCs act in gene gating (Blobel, G., 1985) and that locating genes directly to the NPC is indeed a ubiquitous, evolutionary conserved mechanism for regulating gene expression.
D. Nucleoporins, the immune system and Parkinson's disease
The nucleoporin Nup96 is autocatalytically cleaved from a Nup98/Nup96 precursor protein, which results in the two nucleoporins Nup96 and Nup98 (Enninga, J. et al., 2003). Nup96, like Nup98, localizes to both sides of the NPC, and is a component of Nup107-160 complex (Enninga, J. et al., 2002). Both Nup96 and Nup98 are induced by interferons (Enninga, J. et al., 2002). Transgenic heterozygous Nup96+/− mice show downregulation of interferon-regulated genes and defects in the mRNA export of major players of immune response, MHCI and MHCII gene products, coinciding with alterations in MHC-related T cell function. Additionally, B cell function is impaired in Nup96+/− mice, resulting in Nup96+/− cells and mice highly susceptible to viral infection. Therefore Nup96 appears to function in antiviral response and in innate and adaptive immunity (Faria, A. M. et al., 2006).
A homologue of Nup96 has recently been identified in Arabidopsis thaliana, but in contrast to vertebrates the AtNup98 and AtNup96 genes locate to different chromosomal regions (Mans, B. J. et al., 2004; Zhang, Y., and Li, X., 2005). AtNup96 is required for basal defense and constitutive resistance response to pathogens (Li, X. et al., 2001; Zhang, Y. et al., 2003), indicating a conserved function of Nup96 in immune response. Similarly conserved is the function of Nup96 in mRNA export: plants depleted for Nup96 accumulate polyadenylated RNA within their nuclei (Parry, G. et al., 2006). Moreover, plants depleted for Nup96 and Nup160, another component of the Nup107-160 complex, exhibit pleiotropic growth defects implicating these nucleoporins in hormone signaling (Parry, G. et al., 2006). In different contexts, other roles for nucleoporins in signaling and development have been documented: a genome-wide RNA interference screen in Drosophila identified Nup153 and Nup98 as positive regulators of the Hedgehog signaling pathway (Nybakken, K. et al., 2005). Additionally, Drosophila Nup154 was found to play a critical role in oogenesis due to its interaction with the germline specific protein Cup, which is implicated in multiple aspects of female gametogenesis (Grimaldi, M. R. et al., 2007).
A pleiotropic role in cell function has also been suggested for the nucleoporin RanBP2/Nup358. RanBP2 is a large modular protein and several molecular partners with distinct functions interacting with specific domains of RanBP2 have been identified. Several roles of RanBP2 have emerged that implicate RanBP2 in nucleocytoplasmic transport (Bernad, R. et al., 2004; Yokoyama, N. et al., 1995), protein biogenesis (Ferreira, P. A. et al., 1996; Ferreira, P. A. et al., 1997), the formation of the mitotic spindle and NE assembly (Askjaer, P. et al., 2002), and the integration of NE breakdown with kinetochore formation and maturation during early mitotic progression (Salina, D. et al., 2003). Some of the protein partners interact with RanBP2 in a tissue-specific manner, such as a subset of G protein-coupled receptors, the red/green opsin, in photosensory neurons (Ferreira, P. A. et al., 1996; Ferreira, P. A. et al., 1997) or the kinesins KIF5B and KIF5C selectively in the central nervous system (CNS) (Cai, Y. et al., 2001).
CNS-selective effects of RanBP2 may underlie the pathogenesis of certain neuropathies, in particular Parkinson's disease (PD). The Parkin protein, which has E3 ubiquitin ligase activity, has been implicated in autosomal recessive juvenile Parkinsonism, and RanBP2 has been identified as target for Parkin leading to the ubiquitination of RanBP2 and its subsequent proteosomal degradation (Um, J. W. et al., 2006). Abnormal processing of RanBP2 by Parkin might therefore play a role in PD pathogenesis. RanBP2 itself posses SUMO-E3 ligase activity (Pichler, A. et al., 2002), and it will therefore be interesting to see if RanBP2-mediated sumoylation or the loss of it contributes to PD progression, in particular since NPC-regulated sumoylation appears to also play a role in other cellular processes, such as DNA repair and cytokinesis (Makhnevych, T. et al., 2007; Palancade, B. et al., 2007).
Haploinsufficient RanBP2+/− mice show a selective reduction of hexokinase type I (HKI) in the CNS, whereas skeletal muscle, spleen and liver levels of HKI remained largely unaffected. HKI is a key player in glucose metabolism and ATP production, and RanBP2 appears to prevent the inhibition of HKI and its degradation by binding the HKI antagonist COX 11 (Aslanukov, A. et al., 2006). Haploinsufficiency in RanBP2 consequently promotes the destabilization and degradation of HKI, decreases ATP production and, hence, reduces the responsiveness of neurons. These observations may help to explain how targeting of RanBP2 for degradation by Parkin (Um, J. W. et al., 2006) contributes to the pathophsyiological mechanisms underlying Parkinsonism and other neurodegenerative disorders.
Together, mouse models as for Nup96 and RanBP2 present a powerful tool to link cellular pathways, as well as pathophysiological states, to the NPC –and, in doing so, expose roles for nucleoporins that had not been anticipated.
IV. Selective cargo translocation across the nuclear pore complex
Whereas small molecules (e.g. H2O and ions) can diffuse freely through the NPC, large cargoes (>40kDa) require the assistance of soluble transport receptor molecules, known collectively as karyopherins (Kaps; also called importins, exportins and transportins) to be effectively chaperoned through the NPC (Stewart, M., 2007). Appropriate macromolecules (i.e. cargo) are identified through a short sequence of residues known as nuclear localization/export signals (i.e. NLS/NES), which exhibit binding interactions with the Kaps. Import of NLS-cargo into the nucleus usually entails the use of importin α, which acts as an adaptor to importin β through an importin β-binding (IBB) domain (Gorlich, D. et al., 1996). However, there are some proteins that can bind directly with importin β. The directionality of nucleocytoplasmic transport is driven by an asymmetric distribution of the two nucleotide states of Ran (GTP/GDP) (reviewed in (Gorlich, D., and Kutay, U., 1999; Macara, I. G., 2001; Weis, K., 2002)). RanGTP is found predominantly in the nucleus, and functions to release NLS-cargo from its import receptor by binding to the import receptor itself (Gorlich, D. et al., 1996). The importin-RanGTP complex is then recycled back into the cytoplasm. Similarly, trimeric complexes formed by an export receptor, its cargo and RanGTP, are ferried to the cytoplasm. Once in the cytoplasm, RanGAP (together with RanBP1 and RanBP2/Nup358) catalyzes GTP hydrolysis, which drives the disassembly of the complexes (reviewed in (Gorlich, D., and Kutay, U., 1999; Macara, I. G., 2001; Weis, K., 2002)). In this manner, the receptors are recycled while a large pool of RanGDP in the cytoplasm is constantly replenished (Stewart, M., 2007; Weis, K., 2002).
A current controversy remains as to how passage through the NPC is obstructed for non-NLS/NES harboring molecules that do not bind to the karyopherins or have a non-canonical means of transport (Paine, P. L. et al., 1975). Hence, the selection criterion for transport through the NPC is not simply based on size exclusion per se, and alludes to the presence of a selective gating mechanism within the NPC that simultaneously prevents the passive passage of molecules while promoting the translocation of receptor-mediated cargo. In this section, we will review the various concepts and supporting evidence that have led to the current understanding of selective gating, as well as highlight outstanding aspects of the NPC which need to be addressed in order to provide for a more refined description of NPC function.
A. The NPC as a selective gate
Initial EM-based structural studies linked the biophysical origin of the selective gate to the presence of a “central plug” or “transporter” module located within the NPC (Akey, C. W., 1990; Feldherr, C. M., and Akin, D., 1997). With its nanometer-resolution imaging capability and its ability to be used in physiologically relevant environments, AFM was subsequently used to resolve the basis of the central plug, but these studies resulted in controversy (Jaggi, R. D. et al., 2003a; Jaggi, R. D. et al., 2003b; Mooren, O. L. et al., 2004; Stoffler, D. et al., 1999; Wang, H., and Clapham, D. E., 1999). Today, by using state-of-the-art CET, it has been shown that the central plug most likely represents cargo caught in transit (Beck, M. et al., 2004; Beck, M. et al., 2007; Stoffler, D. et al., 2003).
Several lines of evidence now indicate that the key constituents of the NPC selective gate consist of FG-repeat nucleoporins and reside at both the cytoplasmic and nuclear peripheries surrounding the central pore (Rout, M. P. et al., 2000). Cargo selection relies on binding interactions that occur between karyopherins and the FG-motifs (Bayliss, R. et al., 2000; Bayliss, R. et al., 2002; Bednenko, J.et al., 2003; Liu, S. M., and Stewart, M., 2005). Instead of possessing any well-defined structure, the FG-repeat domains exhibit large Stokes radii and are natively unfolded. Accordingly, AFM-based stretching experiments (i.e. single molecule force spectroscopy (SMFS)) show that the FG-repeat domains exhibit a highly flexible entropic elasticity (Lim, R. Y. et al., 2006; Lim, R. Y. et al., 2007). Interestingly, studies reveal a high level of functional redundancy between the various FG-repeat domains in the NPC: 1) the asymmetric FG-nucleoporins have been shown to be dispensable for nucleocytoplasmic transport (Strawn, L. A. et al., 2004; Zeitler, B., and Weis, K., 2004); 2) the direction of transport through the NPC can be inverted by reversing the gradient of RanGTP (Nachury, M. V., and Weis, K., 1999); 3) active transport is able to proceed in NPCs lacking cytoplasmic filaments (i.e. FG-rich RanBP2/Nup358) (Walther, T. C. et al., 2002); and perhaps most tellingly, 4) the selective gating mechanism has been found to remain functional even after 50% FG-repeats have been depleted (Strawn, L. A. et al., 2004).
B. Current models of selective gating
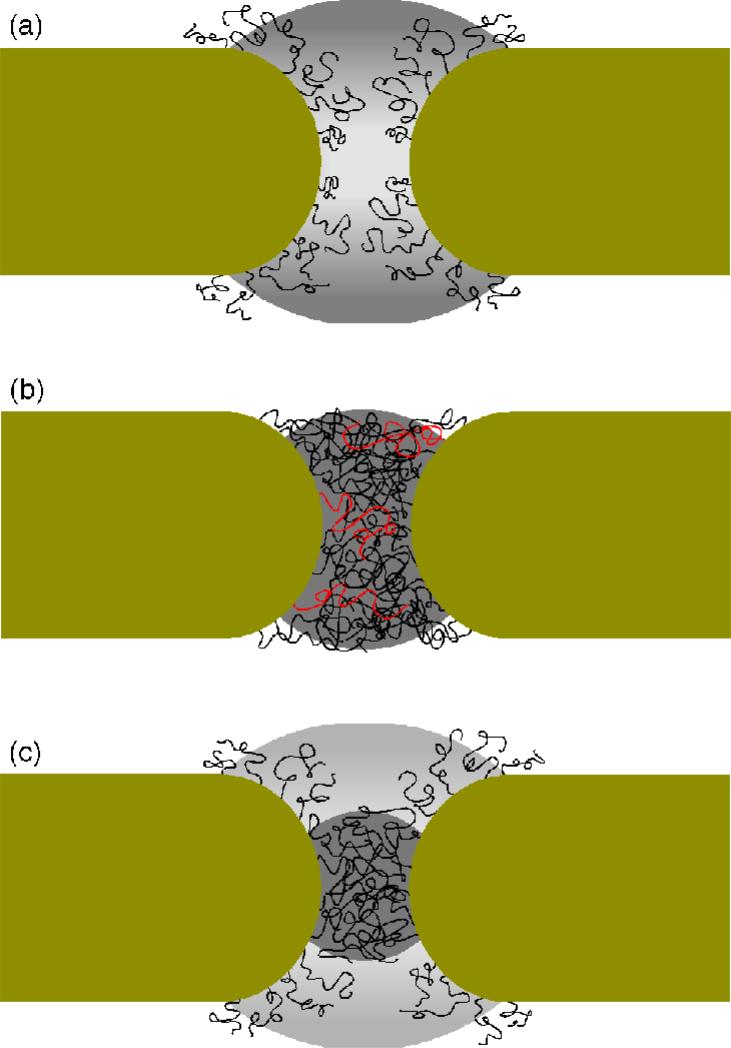
The manner in which the FG-repeat domains contribute to the selective gating of the NPC is widely speculated and has been the subject of several reviews (Fahrenkrog, B., and Aebi, U., 2003; Lim, R. Y. H. et al., 2006a; Stewart, M., 2007; Suntharalingam, M., and Wente, S. R., 2003; Weis, K., 2003). As illustrated in Fig. 3, it is generally agreed that the FG-repeat domains form the physical constituents of the underlying barrier (Ben-Efraim, I., and Gerace, L., 2001; Macara, I. G., 2001; Ribbeck, K., and Gorlich, D., 2002; Rout, M. P. et al., 2000). The Brownian affinity gating model (Rout, M. P. et al., 2000) or virtual gating (Rout, M. P. et al., 2003)) proposes that the entropic behavior of peripheral FG-repeat domains acts as a substantial barrier to inert cargo. Translocation is anticipated for receptor-mediated cargoes due to interactions between the FG-repeats and the transport receptors (Bayliss, R. et al., 2000; Bayliss, R. et al., 2002) which increases the residence time and probability of entry into the NPC. In a similar manner, the “oily-spaghetti” model (Macara, I. G., 2001) postulates that noninteracting FG-repeat domains are pushed aside by cargo complexes but otherwise obstruct the passage of passive cargo. The selective phase model (Ribbeck, K., and Gorlich, D., 2002) predicts that FG-repeat domains attract each other via hydrophobic inter-FG-repeat interactions to form a hydrophobic gel or meshwork. This interpretation is based on experiments which show that the addition of hydrophobic solvents disrupts the meshwork and triggers a non-selective opening of the central pore (Ribbeck, K., and Gorlich, D., 2002; Shulga, N., and Goldfarb, D. S., 2003). Hence, it is predicted that passive, more hydrophilic material is obstructed while hydrophobic cargo complexes are able to “dissolve” through the sieve-like meshwork. Most recently, Patel et al have proposed a two-gate model that combines elements of both Brownian gating and the selective phase (Patel, S. S. et al., 2007). Based on the observation that the centrally located yeast FG-repeat domains (i.e. GLFG) exhibited cohesion as opposed to the peripheral yeast FG-repeat domains (i.e. FxFG) that did not, the authors’ deduced that the more centralized FG-repeat domains formed a cohesive meshwork while the peripheral FG-repeat domains functioned as an entropic barrier.
Figure 3.
Main models of selective gating in the NPC. (A) The Brownian/virtual gating model (Rout, M. P. et al., 2003; Rout, M. P. et al., 2000) predicts that the entropic fluctuations of the unfolded FG-domains form an effective barrier to passive cargo. Although the central pore appears unobstructed, the highly stochastic motion of the elongated FG-domains (shaded area) generates a high-density FG-domain entropic barrier or “cloud” that surrounds and extends beyond the immediate peripheries of the NPC (dark). (B) The selective phase model predicts that hydrophobic interactions between the FG-repeats drive the FG-domains to form an randomly interconnected gel-like meshwork within the central pore that acts as a sieve to passive, hydrophilic cargo (Ribbeck, K., and Gorlich, D., 2002). Receptor-cargo complexes can dissolve through and negotiate the meshwork by breaking the “links” between the FG-domains via receptor-FG interactions. The gray area denotes the “range” of the meshwork while three FG-domains are drawn in red to emphasize that the FG-domains have to be elongated in order to cross-link with each other. (C) By combining aspects of Brownian gating and the selective phase, the two-gate model suggests that the more central GLFG-domains form a cohesive meshwork in the central pore while the peripheral FxFG-domains give rise to an entropic barrier (Patel, S. S. et al., 2007). The shaded areas represent the locations of the two gates.
C. In Vitro studies of FG-domain function
In spite of progress in characterization of the NPC and its individual components, an accurate picture of how selective gating is achieved by the FG-repeat domains remains unclear due to a general lack of information about FG-domain behavior in the context of the NPC. The source of this ambiguity stems in part from the difficulty in trying to visualize the FG-repeat domains in vivo, which is evident given the lack of resolution, even when using state-of-the-art structural techniques such as CET, to detect the FG-repeat domains (Beck, M. et al., 2004; Beck, M. et al., 2007; Stoffler, D. et al., 2003). Direct imaging of the NPC with AFM is also limited in resolution and chemical sensitivity due to the complexity of the NPC and its cellular environment (Jaggi, R. D. et al., 2003a; Jaggi, R. D. et al., 2003b; Mooren, O. L. et al., 2004; Stoffler, D. et al., 1999; Wang, H., and Clapham, D. E., 1999). Presently, only immunogold-EM has been able to provide positional information of the FG-repeat domains and has been used to show that the FG-repeat domains of Nup153 and Nup214 (Paulillo, S. M. et al., 2005) appear diffuse and mobile within the nuclear and cytoplasmic peripheries of the NPC, respectively. The FG-repeat domains themselves have so far been directly visualized only in isolation as individual biopolymers by AFM (Lim, R. Y. H. et al., 2006a).
To reproduce the contextual dimensions of the NPC (i.e the FG-repeat domains are anchored to the NPC surface and not free-floating in solution), Lim et al developed an experimental platform that allowed for the collective, biophysical behavior of surface tethered FG-repeat domains to be probed at the nanoscopic level (Lim, R. Y. et al., 2006). In support of the Brownian affinity model (Rout, M. P. et al., 2003; Rout, M. P. et al., 2000), they found that FG-domain clusters of Nup153 (i.e. cNup153) are entropically dominated and resemble a polymer brush (Halperin, A. et al., 1992; Milner, S. T., 1991; Zhao, B., and Brittain, W. J., 2000). Being surface-anchored, the molecular chains exhibit a predisposed net directionality normal to the surface because of lateral packing constraints, which causes them to stretch away from the surface i.e. forming a brush. This provided an explanation as to how FG-repeat domains could give rise to an effective repulsive entropic barrier in and around the NPC. The observation that the extended brush-like conformation of the FG-repeat domains collapses in hexanediol provides an explanation as to why NPCs appear to reversibly open and close when the same reagent is added/removed (Patel, S. S. et al., 2007; Ribbeck, K., and Gorlich, D., 2002; Shulga, N., and Goldfarb, D. S., 2003). This was substantiated with SMFS-AFM analysis, which showed that individual Nup153 FG-domain molecules could be reversibly stretched and relaxed without any change to its intrinsic entropic elasticity i.e. resembling a worm-like chain (Bustamante, C. et al., 1994; Marko, J. F., and Siggia, E. D., 1995). These measurements indicate a lack of intra-FG interactions within each individual FG-repeat domain and provide a nanomechanical verification of the natively unfolded conformation of this domain. In comparison, SMFS analysis detected an interaction between importin β and cNup153 when importin β-modified AFM tips were used (Lim, R. Y. et al., 2007) and, further, provided evidence for multiple points of contact of importin β with the cNup153 region. This is in agreement with the fact that importin β consists of five hydrophobic FG-binding sites (Bayliss, R. et al., 2000; Bayliss, R. et al., 2002; Bednenko, J. et al., 2003; Liu, S. M., and Stewart, M., 2005) (with an additional five binding sites predicted by molecular dynamics (MD) simulations (Isgro, T. A., and Schulten, K., 2005)) that can be simultaneously occupied (Isgro, T. A., and Schulten, K., 2005). This led to the suggestion that 1) cooperativity between FG-repeat domains arises from FG-receptor interactions instead of FG-FG interactions, and 2) binding promiscuity allows for a “capture” mechanism that involves the coiling or wrapping of the FG-domain(s) around receptor molecules (Lim, R. Y. et al., 2007).
At the macroscopic level Frey et al showed that the yeast FG-nucleoporin, Nsp1p, can be cast in the form of a macroscopic hydrogel (Frey, S. et al., 2006) to lend support to the “selective phase” model (Ribbeck, K., and Gorlich, D., 2002). Remarkably, the authors showed that a saturated hydrogel made of Nsp1p FG-repeats can reproduce the permeability properties of NPC (Frey, S., and Gorlich, D., 2007). The in vivo relevance of such as gel, however, remains in question since Nsp1p does not gelify under more physiological conditions (Patel, S. S. et al., 2007). In order to investigate the cohesiveness of different FG-nucleoporins, Patel et al devised a low affinity assay, which could detect the binding of CFP-nucleoporins to GST-nucleoporins immobilized on Sepharose beads. Interestingly, they found that only GLFG-domains showed weak cohesive interactions whereas FxFG-domains (such as in Nsp1p) did not. By overexpressing the FG-repeat domains in yeast cells, the authors observated a similar pattern of interactions in vivo, albeit not visualized in the context of the NPC. A systematic depletion of FG-repeat domains in yeast showed, however, that the NPCs displayed similar qualitative “leakiness” in all the cases studied, which indicated that the FG-repeat domains in both peripherally and centrally anchored nucleoporins play an important role in maintaining the selective gating mechanism. These findings led to the conclusion that FxFG domains on both faces of the NPC act as an entropic repulsive barrier while the GLFG-domains form a cohesive meshwork in the NPC's central pore (Patel, S. S. et al., 2007).
D. In silico studies of the FG-domains and barrier function
Computational studies have also been useful in providing additional insight into the possible aspects of FG-repeat domain behavior. By modelling a cross-linked network, Bickel and Bruinsma showed that a receptor molecule would have a lower, and not higher, mobility than a passive molecule due to its attachments to the FG-repeat domains (Bickel, T., and Bruinsma, R., 2002). In agreement with the cohesive properties observed between GLFG-domains (Patel, S. S. et al., 2007), Kustanovich and Rabin predicted that FG- repeat domains would exhibit low equilibrium affinities for each other (Kustanovich, T., and Rabin, Y., 2004). In support of brush-like behavior for FG-domains (Lim, R. Y. et al., 2006), Nielsen et al showed that the conformational entropy of non-interacting FG-repeat domains was enough to provide for a robust barrier around the NPC by modelling the FG-repeat domains as surface grafted, polymeric random coils (Nielsen, B. et al., 2006). By solving a rigorous mathematical model of transport through the NPC, Zilman et al. showed that selectivity, efficiency, directionality, and robustness of nucleocytoplasmic transport could be explained by combining the interaction strengths of binding to the flexible FG-repeat domains with the physics of diffusion inside a channel (Zilman, A. et al., 2007). Besides finding that NPC selectivity arises from a balance between the probability and the speed of receptor-mediated cargo complexes, they propose that the competition between specific receptor-mediated versus non-specific interaction for FG-binding also contributes to the selectivity of the NPC mechanism. In contrast to predictions of the selective phase model, the authors find the inherent flexibility of the FG-repeat domains to play a key role in maintaining the high throughput and relative robustness (i.e.insensitive to FG-repeat deletions) of the NPC.
Using molecular dynamics simulations, Isgro and Schulten predicted that additional hydrophobic binding spots could exist on the transport receptors importin β (Isgro, T. A., and Schulten, K., 2005), NTF2 (Isgro, T. A., and Schulten, K., 2007a) and the Cse1p:Kap60p:RanGTP complex (Isgro, T. A., and Schulten, K., 2007b) that could enhance receptor interactions with the FG-repeats. Besides requiring several binding spots, the authors predict that close physical proximity between binding spots on each receptor molecule is likely to be an important criterion for transport selection. Furthermore, by using identical FG-repeat peptides derived from both FxFG domains (i.e. Nsp1p) and GLFG-domains (i.e. Nup116p) in all three studies, these simulations show that both classes of FG-domains interact with overlapping binding spots on importin β, NTF2 and Cse1p, respectively. Indeed, such overlap has been experimentally observed previously for importin β (Bayliss, R. et al., 2002).
E. Kinetic aspects of nucleocytoplasmic transport
Selective gating appears to be a rapid process given the relatively short residence times of receptor-cargo complexes at the NPC as shown by single molecule fluorescence microscopy (Kubitscheck, U. et al., 2005; Yang, W. et al., 2004). By directly monitoring the transport of a model protein substrate (i.e. NLS-2xGFP) through individual NPCs in permeabilized HeLa cells, Yang et al showed that movement through the NPC is bidirectional, resembling a random walk whereby the import substrate spends the majority of its ~10 ms interaction time within the central pore (Yang, W. et al., 2004). Kubitscheck et al obtained kinetic data regarding the dwell times of the nuclear transport receptors NTF2 (5.8 ms) and transportin (7.2 ms) at their respective NPC binding sites (Kubitscheck, U. et al., 2005). They observed that the dwell times decreased from 5.8 ms to 5.2 ms for NTF2, and 7.2 ms to 5.6 ms for transportin when each respective transport receptor was bound to specific transport substrates, indicating that translocation is accelerated for receptor-cargo complexes. By comparing their data with known bulk transport rates, they suggested that nucleocytoplasmic transport proceeds via multiple parallel pathways within each NPC.
More recently, Yang et al showed that the transport efficiency and import time of cargo was modulated by importin β concentration and suggested that in vivo mechanisms that altered the expression levels of the receptor could dramatically affect transport rates (Yang, W., and Musser, S. M., 2006). In addition, the recent findings of Paradise et al (Paradise, A. et al., 2007) and Timney et al (Timney, B. L. et al., 2006) revealed that karyopherins compete non-specifically with other cytosolic structures and proteins. By colliding with a large number of non-specific partners in the crowded cytosolic environment, karyopherins could be “shielded” from the FG-repeat domains. Indeed, Timney et al reported that non-specific competition resulted in each karyopherin having to take about 10 s to search for and successfully import the appropriate cargo - a hundred times longer than previous estimates which ignored such effects (Timney, B. L. et al., 2006). Based on the 5-10 ms residence/dwell time at the NPC (Kubitscheck, U. et al., 2005; Yang, W. et al., 2004), this led the authors to suggest that the limiting factor in cargo transport arises from the receptor having to seek out specific partners (i.e. cargo) in the milieu of non-specific interactions instead of the actual process of translocation through the NPC. To obtain a direct thermodynamic perspective of nuclear transport, Kopito and Elbaum conducted quantitative transport measurements in reconstituted nuclei and showed that nuclear accumulation follows Michaelis-Menten first-order kinetics as a function of the cytoplasmic cargo concentration (Kopito, R. B., and Elbaum, M., 2007). Importantly, this suggests that 1) the fate of a protein population led by receptor-mediated transport is dictated by the NLS, and 2) individual molecules are free to shuttle back and forth through the NPC.
F. Towards an understanding of FG-domain behavior in the NPC
While the biophysical behavior of the FG-repeat domains in vivo remains unsubstantiated, the effect of non-physiological reagents (e.g. hexanediol) to abolish the NPC barrier as observed in transport assays (Patel, S. S. et al., 2007; Ribbeck, K., and Gorlich, D., 2002; Shulga, N., and Goldfarb, D. S., 2003) provides an important clue to their physiologically-relevant conformations. By observing that the FG-repeat domains “collapse” in hexanediol (Lim, R. Y. H. et al., 2006b), it can be inferred that the FG-repeat domains are predominantly extended to a degree in the NPC in the midst of ongoing receptor-FG interactions. How then does the movement of karyopherins (and cargo) through the NPC occur? To a achieve a rational mechanistic picture of how the NPC selectively gates nuclear transport, it will be essential to understand how the different FG-domain conformations (i.e. gel vs. brush) simultaneously prevent the passage of passive molecules while promoting the translocation of receptor-cargo complexes through the NPC (i.e. definition of selective gating) at the observed transport rates (~5 ms). Thus, newer structural/biophysical techniques will be required to elucidate even finer dynamic, molecular details of FG-repeat domain behavior within the NPC and how they respond to the biochemical interactions that govern nucleocytoplasmic transport.
V. Nuclear Pore Complex Assembly and Disassembly
Consideration of the massive, ornate structure of the NPC and its central role in creating distinct nuclear and cytoplasmic environments leads to the question of how this macromolecular machine is assembled with each cell division. In a proliferating human cell, thousands of NPCs are formed de novo during each cell cycle. NPCs are assembled both concomitantly with membrane recruitment to the newly forming nuclei, as well as after the chromatin is fully enclosed by the two lipid bilayers that comprise the NE. Whether these are truly distinct modes of assembly remains to be determined. If so, in post-mitotic cells or in organisms with a “closed mitosis” only the latter path is relevant. The process of NPC assembly is extremely rapid; in the Xenopus egg extract system, NPCs are estimated to form at the rate of ~140 per minute (D'Angelo, M. A. et al., 2006). Given the observation that during an “open mitosis” NPC components disperse into subunits and individual components, as well as evidence that formally rules out NPC splitting to create new NPCs (D'Angelo, M. A. et al., 2006), a pathway of self-assembly clearly exists. Many key players and steps in this process are now known, although significant gaps remain to be elucidated.
A. Building a nuclear pore: who's on first?
One strategy to set the stage for understanding NPC formation has been to delineate the order of nucleoporin recruitment during post-mitotic nuclear assembly (Bodoor, K. et al., 1999; Haraguchi, T. et al., 2000). Certain nucleoporins are thought to be present, albeit initially on a restricted region of the chromatin surface, from the very beginning since they reside at the kinetochore during mitosis (see Section IIIB). These include the Nup107-Nup160 complex (Belgareh, N. et al., 2001) and a newly-identified associated protein, ELYS/MEL28, as well as RanBP2/Nup358 (Joseph, J. et al., 2002). Broader recruitment of Nup107 and Nup133 has been reported to also occur very early –during anaphase, similar to Nup153 and before Nup62 (Belgareh, N. et al., 2001).
Membrane recruitment naturally brings with it integral membrane proteins of the NPC, although notably the recruitment of transmembrane proteins (or at least their stable association with the nuclear rim) does not occur simultaneously. Recruitment of POM121 and likely Ndc1, which appears to localize to the same vesicle population in Xenopus egg extracts (Mansfeld, J. et al., 2006), is an early event, whereas gp210 does not accumulate until later in the nuclear assembly process (Bodoor, K. et al., 1999).
mAb414 reactivity is detected relatively late during nuclear reconstitution in egg extracts (Antonin, W. et al., 2005); although this antibody recognizes at least four nucleoporins, the bulk of its reactivity usually reflects Nup62 levels. Nup214 was noted to arrive after Nup62 when these nucleoporins were tracked individually in NRK cells (Bodoor, K. et al., 1999). Nup155 arrives relatively late in the assembly process as well, although in its absence nucleoporins that get recruited earlier do not accumulate at the rim, suggesting that Nup155 plays a critical role in stabilizing interactions that lead to NPC formation (Franz, C. et al., 2005). Tpr is also a late-arriving nucleoporin (Bodoor, K. et al., 1999; Haraguchi, T. et al., 2000; Hase, M. E., and Cordes, V. C., 2003), but in this case its presence is dispensable for the core structure of the NPC (Frosst, P. et al., 2002; Hase, M. E., and Cordes, V. C., 2003; Shibata, S. et al., 2002).
Another approach to understanding the early steps of nuclear pore formation has been to observe this process at high resolution, using electron microscopy. Analysis of nuclear assembly using transmission EM led to the notion that an intermediate structure, termed a “pre-pore”, forms on the surface of chromosomes independent of membranes (Sheehan, M. A. et al., 1988). Further visual analysis in both Xenopus and Drosophila systems, using scanning EM, has provided a more detailed conceptual framework of the structural stages of pore assembly (Goldberg, M. W. et al., 1997; Kiseleva, E. et al., 2001). The molecular composition of NPC assembly intermediates is not yet known, although there is speculation that the Nup107-160 complex is a good candidate for forming a pre-pore-type structure (Walther, T. C. et al., 2003a). Revisiting these structures in combination with immuno-detection techniques will result in a more integrated picture of nucleoporin recruitment and the step-wise assembly of the NPC [(see (Drummond, S. P. et al., 2006)].
B. Nuclear Pore building blocks: the transmembrane proteins
Integral membrane proteins of the nuclear pore are predicted to play unique and essential roles in NPC formation, as such proteins seem likely to be involved in facilitating creation of the pore itself (or, described from a different perspective, in joining the inner and outer nuclear membranes) and in anchoring the soluble NPC building blocks to this site of the NE. In the Xenopus egg extract system, depletion of POM121-containing vesicles results in an early block to NE assembly: vesicles appear to bind the chromatin, but do not fuse (Antonin, W. et al., 2005). This phenotype precludes direct analysis of the role for POM121 in nuclear pore formation. Nonetheless, an interesting layer of regulatory cross-talk between POM121 and the Nup107-160 subcomplex was observed (see below). Knockdown approaches in mammalian cells have not led to a unified view on the role of POM121; the degree of impairment in NE/NPC assembly may depend on the extent of depletion (Antonin, W. et al., 2005; Imreh, G. et al., 2003; Stavru, F. et al., 2006b). In any case, the observation that POM121 appears to be restricted to vertebrates indicated a priori that another transmembrane protein would likely play a pivotal role. Indeed, the integral membrane protein Ndc1 (Chial, H. J. et al., 1998) has emerged in several studies as an important player in NPC assembly (Lau, C. K.et al., 2004; Madrid, A. S. et al., 2006; Mansfeld, J. et al., 2006; Stavru, F. et al., 2006a). Even depletion of Ndc1, however, does not lead to an absolute defect in NPC assembly; this was best illustrated by a C. elegans strain bearing a deletion that disrupts the ORF of NDC1. This mutant strain has high embryonic and larval mortality and dramatically reduced mAb414 reactivity at the nuclear rim, but rare survivors can be propagated (Stavru, F. et al., 2006a).
RNAi-directed depletion of the third metazoan transmembrane protein, gp210, in mammalian cells did not have a significant effect on NPCs in some studies (Eriksson, C.et al., 2004; Mansfeld, J. et al., 2006; Stavru, F. et al., 2006b), but did alter NPC and NE phenotype in another case (Cohen, M. et al., 2003). In one study, simultaneous knockdown of gp210 and Ndc1 was found to have a synergistic effect (Mansfeld, J. et al., 2006). Likewise, in yeast, Ndc1p was found to be partially redundant with POM152p (Madrid, A. S. et al., 2006). Results in Xenopus egg extracts have been complicated by initial mis-identification of the C-terminus of this orthologue (Antonin, W. et al., 2005; Drummond, S. P., and Wilson, K. L., 2002). In C. elegans, gp210 is required for viability, but the NPC phenotype associated with its depletion is primarily a problem in NPC positioning rather than formation (Cohen, M. et al., 2003). Tissue-specific expression patterns for gp210 (Olsson, M. et al., 2004) indicate that it may be more likely to modulate rather than to dictate NPC structure. One overriding theme of these studies is that redundancy is built into the NPC assembly pathway, ensuring that NPC formation is a robust biological process (Kitano, H., 2004; Stavru, F. et al., 2006a).
C. Collaboration between nucleoporins in NPC assembly
Understanding how the transmembrane components of the nuclear pore are linked to the soluble pore building blocks is key to understanding NPC assembly. One such connection exists between Ndc1 and Nup35 (sometimes referred to as Nup53 as it is the homologue of yeast Nup53p), which associate in a manner independent from the Nup35-Nup93 interaction (Mansfeld, J. et al., 2006). Nup35 itself has been implicated as an important player in NPC assembly (Hawryluk-Gara, L. A. et al., 2005). Although this nucleoporin is not a transmembrane protein, it appears to be in close apposition to the nuclear membrane and associates with lamin B. Indeed, overexpression of the yeast homologue, Nup53p, causes accumulation of extramembrane structures within the nucleus, in which Nup53p is found (Marelli, M. et al., 2001). Ndc1p is also targeted to these extra-membrane structures and the C-terminal region of Nup53p that is functionally implicated in membrane recruitment is also important for the interaction with Ndc1p.
A complex relationship appears to exist between the Nup107-160 complex and POM121 during NE formation. The observations are 1) when the Nup107-160 complex is depleted, POM121 is no longer recruited to the NE during assembly (Harel, A. et al., 2003b) and 2) the arrest in NE assembly seen when membrane vesicles are depleted of POM121 is dependent on the Nup107-160 complex (Antonin, W. et al., 2005). Although there is not evidence for a direct interaction between the Nup107-160 complex and POM121 itself, these interesting interdependent phenotypes have been proposed to suggest that chromatin-associated Nup107-160 complex exerts negative feedback on NE formation, which is relieved by the presence of POM121.
Whatever the exact nature of this regulatory relationship, the Nup107-160 complex is clearly playing an important collaborative role in creating the NPC. When this NPC subunit is depleted, the membranes that enclose chromatin in an in vitro nuclear assembly system completely lack NPCs (Antonin, W. et al., 2005; Harel, A. et al., 2003b; Walther, T. C. et al., 2003a) and knockdown of Nup107 in mammalian cells leads to severe NPC defects as well (Boehmer, T. et al., 2003; Walther, T. C. et al., 2003a). This NPC subunit is thought to eventually create an important core aspect of the nuclear pore. Interestingly, this complex, which shares two members with COPII and possesses additional general features similar to coatomer complexes, is hypothesized to form a coat-like structure at the pore membrane [(Devos, D. et al., 2004); see also (Antonin, W., and Mattaj, I. W., 2005)].
D. Peripheral pore structures
Tpr is proposed to be the critical building block of the nuclear pore basket in vertebrates, with its recruitment to the NPC dependent on Nup153 (Frosst, P. et al., 2002; Hase, M. E., and Cordes, V. C., 2003; Krull, S. et al., 2004). The role of Nup153, however, may be complicated by the presence of more than one population of this nucleoporin at the NPC. One possibility is that a stably associated population of Nup153 at the nuclear ring moiety is dedicated to tethering Tpr to form the core basket, while another population dynamically associates with the NPC (Ball, J. R., and Ullman, K. S., 2005; Fahrenkrog, B., and Aebi, U., 2003). The basket structure itself is conserved in yeast (Fahrenkrog, B. et al., 1998; Kiseleva, E. et al., 2004), although its composition is not well-defined in this organism. Tpr homologues in S. cerevisae, Mlp1p/Mlp2p, have been described as being on intranuclear filaments connected to the nuclear pore (Strambio-de-Castillia, C. et al., 1999), leaving open the question of how the basket structure itself is formed in this case. These proteins may have the potential for dual (multiple) localization, as Tpr has been reported to be on intranuclear filaments/channels in certain metazoan cells (Cordes, V. C. et al., 1997; Fontoura, B. M. et al., 2001; Zimowska, G. et al., 1997) and Mlp2p binds to the spindle pole body in yeast (Niepel, M. et al., 2005).
Based on knockdown analysis in cultured human cells, the partner proteins Nup214 and Nup88 are proposed to play a key role in recruiting RanBP2/Nup358 to create the cytoplasmic filaments of the NPC (Bernad, R. et al., 2004). This does not seem to be the case in Drosophila cells, where knocking down Nup214 leaves functions ascribed to RanBP2/Nup358 intact (Forler, D. et al., 2004). Likewise in nuclei assembled in Xenopus egg extract, depletion of Nup214 does not interfere with RanBP2/Nup358 targeting to the NPC (Walther, T. C. et al., 2002). Corresponding ultrastructural analysis suggested that Nup214 and RanBP2/Nup358 localize to distinct structures on the cytoplasmic face of the NPC (Walther, T. C. et al., 2002). The apparent discrepancy in the interdependence of Nup214 and RanBP2/Nup358 targeting may be due to the role of Nup88, which is proposed to anchor each of these nucleoporins. Nup88 levels are closely linked to those of Nup214, but in certain cases, Nup88 may be present in enough excess to recruit RanBP2/Nup358 to the NPC when Nup214 (and certain amounts of Nup88) are depleted. A central role for Nup88 in organizing the features of the cytoplasmic face of the NPC is underscored by its role in anchoring Nup98 to this site as well; in contrast, interactions with Nup96, a member of the Nup107-160 complex, target Nup98 to the nuclear face of the nuclear pore (Griffis, E. R. et al., 2003). Only a fraction of known interactions between nucleoporins have been highlighted here, but almost all of such contacts ultimately contribute to NPC assembly. Gaining an even more complete map of this interaction network is an important step in understanding the process of NPC assembly and its overall structure.
E. Regulation of NPC assembly
NPC assembly is regulated at several levels. One particularly striking observation is that the calcium chelator BAPTA completely prevents NPC assembly in the Xenopus nuclear reconstitution assays (Macaulay, C., and Forbes, D. J., 1996). The early observation that EGTA does not phenocopy BAPTA (Macaulay, C., and Forbes, D. J., 1996) suggests that it is not absolute calcium levels, but rather a burst of calcium, which may not be quickly enough quenched by EGTA, that is required during NPC assembly. Whether this reflects a calcium flux requirement for inner and outer membrane fusion or some earlier step has not been formally addressed. Among many possibilities, calcium could be involved in a SNARE-related event (Baur, T. et al., 2007) or in modulating the calcium-binding protein Cdc31p/centrin, which was identified as a component of yeast nuclear pores (Rout, M. P. et al., 2000) but not found, as yet, at the vertebrate NPC. A role for calcium, though intriguing, is ill-defined and there is evidence against a requirement for lumenal calcium stores during the process of NE/NPC assembly (Marshall, I. C. et al., 1997). Indeed, the dearth of information on the molecular events that underlie BAPTA inhibition leave open the possibility that this small molecule in fact exerts its effects on nuclear pore assembly via a mechanism distinct from calcium chelation. Whatever the mechanism, an important clue may lie in the observation that depletion of the Nup107-160 complex gives rise to a similar morphological phenotype as BAPTA inhibition.
In addition to post-translational modification, which will be discussed below, association with transport receptors has proven to provide another important layer of regulation for nucleoporins. Considered in this context, the transport receptors serve as chaperones and, in doing so, guide the spatial and temporal order of nuclear pore protein recruitment. This chaperone role of transport receptors has also been proposed to be important to formation of an FG-domain based hydrogel within the confines of the NPC (Frey, S., and Gorlich, D., 2007). Just as in the case of nucleocytoplasmic trafficking, the small GTPase Ran works as a switch to regulate nucleoporin association with importin β, the transport receptor best studied in the context of nuclear pore assembly. In fact, this role of Ran is intimately linked to the role of chromosomal DNA as the surface on which the NE and NPCs are assembled. This is because the guanine nucleotide exchange factor (GEF) for Ran, RCC1, is targeted to chromatin and its activity is stimulated by histones (Nemergut, M. E. et al., 2001). Thus, RanGEF activity is high in the vicinity of chromosomes, in turn creating a gradient of RanGTP even in the absence of a nuclear membrane [(Kalab, P. et al., 2006; Kalab, P. et al., 2002), but see (Gorlich, D. et al., 2003)]. RanGTP modulates the binding activity of importin β, promoting the release of associated proteins, such as Nup107, Nup153, and RanBP2/Nup358 (Walther, T. C. et al., 2003b), as well as (presumably) other factors required for fusion of the NE and assembly of nuclear pores. Yet, additional regulation of importin β appears to be at play: RanQ69L, which reverses the inhibitory effects of excess importin β on nuclear membrane fusion, does not reverse its ability to inhibit NPC insertion in a pre-assembled NE (Harel, A. et al., 2003a). A role for importin β, and for Ran, in NPC assembly is also found in yeast (Ryan, K. J. et al., 2003; Ryan, K. J. et al., 2007), suggesting similarity in fundamental regulatory mechanisms despite certain differences in NPC assembly due to the open vs. closed configuration of mitosis. A chaperone-like role is not restricted to importin β: in yeast, Kap121p helps to target Nup53p and is involved in NPC remodeling at mitosis (Lusk, C. P. et al., 2002; Makhnevych, T. et al., 2003); transportin may aid in escorting Nup153 to the NPC (Nakielny, S. et al., 1999).
Other components of the reforming nucleus impinge on nuclear pores as well. This was recently illustrated by the observation that patches of newly-formed NEs are initially pore-free and correspond to regions that are enriched in underlying lamin A/C and have lower levels of lamin B (Maeshima, K. et al., 2006) (see Section II.C). Whether this is a case of inhibiting NPC assembly in particular regions or of preventing NPC anchorage in certain domains of the lamina network remains to be addressed. And, in either case, the molecular mechanism and mediating proteins have yet to be explored. Nonetheless, this report underscores the many levels of nuclear assembly that are simultaneously orchestrated and cross-regulated.
F. Clues from a second site for NPC assembly
Although chromatin is typically the favored scaffold on which to build NPCs, an alternate site for NPC assembly exists in the annulate lamellae (AL). These cytoplasmic membrane cisternae house tightly arrayed NPCs. AL formation is more pronounced in rapidly proliferating cells and has been proposed to bea storage site for NPC components. These NPC-like complexes might seem poised to contribute to NPC formation at the NE itself during membrane expansion, but at least under certain circumstances, this does not appear to be the case. Specifically, in Drosophila embryos the excess nucleoporins, presumably the stores for new NPC formation, were found to be largely soluble rather than AL associated (Onischenko, E. A. et al., 2004). There was no decrease in AL-associated pore complexes concomitant with increases in NPC numbers. Although NPC formation at the AL may be a separable event, rather than a prelude to the appearance of NPCs at the NE, understanding what initiates this chromatin-independent assembly process and how it differs at this site is a way of gaining insight into the pathways that converge to create NPC structure.
Experimental manipulations that lead to increased AL formation include increasing levels of RanGTP (Harel, A. et al., 2003a; Walther, T. C. et al., 2003b). Similarly, Ran-coated beads are sufficient to direct formation of double membrane bilayer replete with nuclear pores (Zhang 2002), consistent with the notion that local levels of RanGTP direct NE/NPC formation to the chromatin surface and are capable of driving this process at other sites as well. Another interesting observation is that inhibition of microtubule formation and/or kinesin function prevents NPC assembly, but does not perturb formation of a nuclear envelope or NPC-containing AL (Ewald, A. et al., 2001). This suggests that delivery of certain components, perhaps a vesicle population, to the chromatin surface is facilitated by microtubules whereas this same component is either not needed at the AL or is incorporated independently of microtubules.
G. Nuclear Pore assembly is never-ending
Beyond the fact that new NPCs are assembled throughout much of the cell cycle, the dynamic nature of NPC components reveals that this structure is not assembled to a static end-point but rather is continuously remodeled. Dynamic association with the NPC was first observed for Nup153 (Daigle, N. et al., 2001) and for Nup98 (Griffis, E. R. et al., 2002). An extensive survey of this property with respect to nucleoporins later revealed that several nucleoporins move on and off the NPC structure (Rabut, G. et al., 2004; Tran, E. J., and Wente, S. R., 2006). Interestingly, this movement has been shown in certain cases to be dependent on ongoing transcription (Griffis, E. R. et al., 2002; Griffis, E. R. et al., 2004). NPC structure has additional layers of dynamics as well. For instance, large-scale conformational rearrangements have been observed by scanning EM (Goldberg, M. W. et al., 2000; Kiseleva, E. et al., 1998; Kiseleva, E. et al., 1996), by AFM (Shahin, V. et al., 2005; Stoffler, D. et al., 1999), and by CTE (Beck, M. et al., 2004). In addition, domains within individual pore proteins have been found to be (or have the potential to be) flexibly arranged within the NPC (Fahrenkrog, B. et al., 2002; Paulillo, S. M. et al., 2005; Paulillo, S. M. et al., 2006; Schwarz-Herion, K. et al., 2007), indeed, the dynamic arrangement of these domains is likely central to trafficking and the selectivity of the nuclear pore (see Section IV). Functional alterations of the NPC, such as the greater upper limit of cargo diameter in proliferating vs. quiescent cells, may also reflect specific reconfiguration of NPC structure (Feldherr, C. M., and Akin, D., 1990, 1991).
H. Deconstructing the nuclear pore complex
Several pore proteins are targets of phosphorylation at mitosis. These include both integral membrane proteins of the NPC, such as Ndc1 (Mansfeld, J. et al., 2006; Stavru, F. et al., 2006a) and gp210 (Favreau, C. et al., 1996), as well as non-membrane anchored, such as Nup35, Nup153, Nup98, Nup62, Nup214, and Nup107-160 complex members(Belgareh, N. et al., 2001; Glavy, J. S. et al., 2007; Lusk, C. P. et al., 2007; Macaulay, C. et al., 1995; Walther, T. C. et al., 2003b). A phosphorylation-dephosphorylation switch has long been thought to aide in driving NPC disassembly and re-assembly. Indeed, when isolated nuclei from Drosophila embryos are incubated with cdc2-cyclin, several pore proteins are released (Onischenko, E. A. et al., 2005). It is difficult to formally prove that this is due to nucleoporins being direct targets of cdc2-cyclin, but clearly they are responsive to a mitotic signaling cascade driven by phosphorylation events. Hallberg and colleagues also recently demonstrated that a phosphomimetic mutation in gp210, at a serine (1880) known to be phosphorylated at mitosis, interfered with its incorporation into the NPC (Onischenko, E. A. et al., 2007). Consistent with these observations, phosphatase activity is implicated in the process of post-mitotic nuclear pore assembly. In Drosophila embryos, okadaic acid inhibits NPC assembly, suggesting that PP1 and/or PP2A are involved (Onischenko, E. A. et al., 2005).
In certain organisms, nuclear pore remodeling at mitosis is limited to discrete, but still significant, rearrangements. In Aspergillus nidulans these changes lead to an increase in the diffusion cut-off of the NPC, whereas in S. cerevisae mitotic changes at the nuclear pore appear more subtle and selectively alter particular transport paths (De Souza, C. P. et al., 2004; Marelli, M. et al., 1998). In organisms that undergo open mitosis, changes in the diffusion cut-off of the NPC herald the prophase-to-prometaphase transition (Lenart, P., and Ellenberg, J., 2006; Lenart, P. et al., 2003) and correspond to an early wave of nucleoporin exit from the NPC (Lenart, P. et al., 2003), perhaps stimulated by phosphorylation. Extensive dispersal of the NPC components ensues and, concomitantly, the membranes that enclose the nucleus are also remodeled, allowing complete intermixing between cytoplasmic and the nuclear space. Although Ran is not implicated in the initial steps of NPC remodeling, it has recently been shown to have a regulatory role in mitotic nuclear membrane remodeling (Muhlhausser, P., and Kutay, U., 2007).
Mitotic membrane remodeling brings up the question of the fate of integral membrane proteins of the nuclear pore at mitosis. Direct observation of a GFP fusion with the inner nuclear membrane protein LBR in COS-7 cells indicated that this protein is ultimately intermixed with endoplasmic reticulum (ER) at mitosis (Ellenberg, J. et al., 1997), as was also the case for two other residents of the inner nuclear membrane (LAP1 and LAP2) as well as for the nucleoporin gp210 (Yang, L. et al., 1997). This intermixing could be explained by a passive process in which interactions that normally serve to keep the nuclear membranes distinct from the ER are lost at mitosis, allowing lateral diffusion between these membrane domains. In another study, however, LAP1 and LAP2 were found to have distinct localizations at mitosis (Maison, C. et al., 1997). Whether differing results are due to the timing of detection within mitosis or to the specific isoforms detected or to some technical issue is not clear. There is independent evidence from HeLa cells for distinct vesicle populations (Chaudhary, N., and Courvalin, J. C., 1993), however, an alternate interpretation is that such vesicles are derived artificially from microdomains within a contiguous ER-like network (Collas, I., and Courvalin, J. C., 2000).
Studies using nuclei reconstituted in Xenopus egg extract have implicated the coatomer complex, COPI, in nuclear disassembly (Cotter, L. et al., 2007; Liu, J. et al., 2003; Prunuske, A. J. et al., 2006). Given the role of COPI in forming vesicles at the Golgi (Bethune, J. et al., 2006), this brings up the possibility of a more active mechanism for dismantling the nuclear membrane and, at least in some cases, delivering it to the ER. It is possible that COPI-mediated vesicle formation occurs only local to the nuclear pores and represents one of two distinct routes of membrane disassembly (the second being passive lateral diffusion). Indeed, Allen and colleagues saw evidence of both vesicles and ER-like tubules when they examined the mitotic breakdown of reconstituted nuclei by field emission scanning EM (Cotter, L. et al., 2007). Vesicles enriched in certain proteins such as POM121 and Ndc1, but not necessarily the highly mobile pore membrane protein gp210, may have escaped observation in earlier studies, but could account for the presence of distinct vesicle populations present in Xenopus egg extract. Alternatively, COPI components may play a non-canonical role, perhaps participating in the establishment or maintainance of microdomains within the ER network.
Interestingly, impairing the function of many nucleoporins results in NE abnormalities, and likewise alterations in particular membrane proteins that reside in the NE/ER results in phenotypic differences at the NPC (Franz, C. et al., 2005; Lewis, A. et al., 2007; Liu, Q. et al., 2007; Miao, M. et al., 2006; Stavru, F. et al., 2006a). A recent example of the latter is the protein Apq12p; when this gene is deleted in yeast, NPCs appear to be imbedded in only the inner nuclear membrane (Scarcelli, J. J. et al., 2007). This advance in identification of important players in proper NPC assembly in fact exposes the general gap in our understanding of how fusion between the inner and outer nuclear membranes is coordinated as the core framework of the NPC coalesces. Future study of membrane dynamics at the nuclear envelope and how this is integrated with NPC assembly and disassembly will yield new insight into this important central question.
VI. Concluding remarks
Although it is not yet possible to build a complete molecular picture of the nuclear pore complex, the structures of individual nucleoporins and NPC subcomplexes are indispensable to our growing understanding of NPC assembly and nucleocytoplasmic transport. Being able to merge this information with CET on intact nuclei will allow for detection of distinct functional states of the NPC and may reveal changes in the configuration of individual NPC subcomplexes during nucleocytoplasmic transport. Single molecule approaches in combination with immuno-labelling will provide additional insight into the molecular composition of individual NPCs and variation among tissues or even from NPC to NPC. Newer structural and biophysical techniques in combination with molecular stimulation will be required to elucidate even finer molecular details of the NPC, such as the identity of the anchoring sites and the exact numbers of FG-repeat domains per NPC and FG-repeat domain behavior in a cellular context. Recent data also points to intriguing roles for nucleoporins that are distinct from, but perhaps coordinated with, their roles at the NPC. As nucleoporins are studied in further detail, it is likely that the scope of their roles will continue to hold surprises. Finally, as more detailed knowledge of how the NPC is put together emerges, this will go hand-in-hand with a deeper understanding of the molecular architecture of the NPC and in turn will aide in building a comprehensive model of trafficking through the NPC.
Acknowledgements
This worked was supported by a grant from the Swiss National Science Foundation (to B.F.), the M.E. Müller Foundation and the Kanton Basel Stadt, and from the N.I.H. (GM61275) and the Leukemia and Lymphoma Society (to K.U.).
References
- Akey CW. Visualization of transport-related configurations of the nuclear pore transporter. Biophys J. 1990;58:341–355. doi: 10.1016/S0006-3495(90)82381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey CW, Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol. 1993;122:1–19. doi: 10.1083/jcb.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell. 2005;17:83–92. doi: 10.1016/j.molcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Antonin W, Mattaj IW. Nuclear pore complexes: round the bend? Nat Cell Biol. 2005;7:10–12. doi: 10.1038/ncb0105-10. [DOI] [PubMed] [Google Scholar]
- Arnaoutov A, Azuma Y, Ribbeck K, Joseph J, Boyarchuk Y, Karpova T, McNally J, Dasso M. Crm1 is a mitotic effector of Ran-GTP in somatic cells. Nat Cell Biol. 2005;7:626–632. doi: 10.1038/ncb1263. [DOI] [PubMed] [Google Scholar]
- Askjaer P, Galy V, Hannak E, Mattaj IW. Ran GTPase cycle and importins alpha and beta are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol Biol Cell. 2002;13:4355–4370. doi: 10.1091/mbc.E02-06-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanukov A, Bhowmick R, Guruju M, Oswald J, Raz D, Bush RA, Sieving PA, Lu X, Bock CB, Ferreira PA. RanBP2 modulates Cox11 and hexokinase I activities and haploinsufficiency of RanBP2 causes deficits in glucose metabolism. PLoS Genet. 2006;2:e177. doi: 10.1371/journal.pgen.0020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai XT, Gu BW, Yin T, Niu C, Xi XD, Zhang J, Chen Z, Chen SJ. Trans-repressive effect of NUP98-PMX1 on PMX1-regulated c-FOS gene through recruitment of histone deacetylase 1 by FG repeats. Cancer Res. 2006;66:4584–4590. doi: 10.1158/0008-5472.CAN-05-3101. [DOI] [PubMed] [Google Scholar]
- Ball JR, Ullman KS. Versatility at the nuclear pore complex: lessons learned from the nucleoporin Nup153. Chromosoma. 2005;114:319–330. doi: 10.1007/s00412-005-0019-3. [DOI] [PubMed] [Google Scholar]
- Baur T, Ramadan K, Schlundt A, Kartenbeck J, Meyer HH. NSF- and SNARE-mediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J Cell Sci. 2007;120:2895–2903. doi: 10.1242/jcs.010181. [DOI] [PubMed] [Google Scholar]
- Bayliss R, Littlewood T, Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell. 2000;102:99–108. doi: 10.1016/s0092-8674(00)00014-3. [DOI] [PubMed] [Google Scholar]
- Bayliss R, Littlewood T, Strawn LA, Wente SR, Stewart M. GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J Biol Chem. 2002;277:50597–50606. doi: 10.1074/jbc.M209037200. [DOI] [PubMed] [Google Scholar]
- Beck M, Forster F, Ecke M, Plitzko JM, Melchior F, Gerisch G, Baumeister W, Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science. 2004;306:1387–1390. doi: 10.1126/science.1104808. [DOI] [PubMed] [Google Scholar]
- Beck M, Lucic V, Forster F, Baumeister W, Medalia O. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. 2007 doi: 10.1038/nature06170. [DOI] [PubMed] [Google Scholar]
- Bednenko J, Cingolani G, Gerace L. Importin beta contains a COOH-terminal nucleoporin binding region important for nuclear transport. J Cell Biol. 2003;162:391–401. doi: 10.1083/jcb.200303085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgareh N, Rabut G, Bai SW, van Overbeek M, Beaudouin J, Daigle N, Zatsepina OV, Pasteau F, Labas V, Fromont-Racine M, Ellenberg J, Doye V. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J Cell Biol. 2001;154:1147–1160. doi: 10.1083/jcb.200101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Efraim I, Gerace L. Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import. J Cell Biol. 2001;152:411–417. doi: 10.1083/jcb.152.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke IC, Boehmer T, Blobel G, Schwartz TU. Structural and functional analysis of Nup133 domains reveals modular building blocks of the nuclear pore complex. J Cell Biol. 2004;167:591–597. doi: 10.1083/jcb.200408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernad R, Engelsma D, Sanderson H, Pickersgill H, Fornerod M. Nup214-Nup88 nucleoporin subcomplex is required for CRM1-mediated 60 S preribosomal nuclear export. J Biol Chem. 2006;281:19378–19386. doi: 10.1074/jbc.M512585200. [DOI] [PubMed] [Google Scholar]
- Bernad R, van der Velde H, Fornerod M, Pickersgill H. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol Cell Biol. 2004;24:2373–2384. doi: 10.1128/MCB.24.6.2373-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethune J, Wieland F, Moelleken J. COPI-mediated transport. J Membr Biol. 2006;211:65–79. doi: 10.1007/s00232-006-0859-7. [DOI] [PubMed] [Google Scholar]
- Bickel T, Bruinsma R. The nuclear pore complex mystery and anomalous diffusion in reversible gels. Biophys J. 2002;83:3079–3087. doi: 10.1016/s0006-3495(02)75312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci U S A. 1985;82:8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoor K, Shaikh S, Salina D, Raharjo WH, Bastos R, Lohka M, Burke B. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J Cell Sci. 1999;112(Pt 13):2253–2264. doi: 10.1242/jcs.112.13.2253. [DOI] [PubMed] [Google Scholar]
- Boehmer T, Enninga J, Dales S, Blobel G, Zhong H. Depletion of a single nucleoporin, Nup107, prevents the assembly of a subset of nucleoporins into the nuclear pore complex. Proc Natl Acad Sci U S A. 2003;100:981–985. doi: 10.1073/pnas.252749899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Silver PA. Transcriptional regulation at the nuclear pore complex. Curr Opin Genet Dev. 2007;17:100–106. doi: 10.1016/j.gde.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bustamante C, Marko JF, Siggia ED, Smith S. Entropic Elasticity of Lambda-Phage DNA. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin JC, Hurt EC, Nehrbass U. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature. 2006;441:770–773. doi: 10.1038/nature04752. [DOI] [PubMed] [Google Scholar]
- Cai Y, Singh BB, Aslanukov A, Zhao H, Ferreira PA. The docking of kinesins, KIF5B and KIF5C, to Ran-binding protein 2 (RanBP2) is mediated via a novel RanBP2 domain. J Biol Chem. 2001;276:41594–41602. doi: 10.1074/jbc.M104514200. [DOI] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Drubin DA, Rando OJ, Silver PA. Developmentally induced changes in transcriptional program alter spatial organization across chromosomes. Genes Dev. 2005;19:1188–1198. doi: 10.1101/gad.1307205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004;117:427–439. doi: 10.1016/s0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- Chaudhary N, Courvalin JC. Stepwise reassembly of the nuclear envelope at the end of mitosis. J Cell Biol. 1993;122:295–306. doi: 10.1083/jcb.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chial HJ, Rout MP, Giddings TH, Winey M. Saccharomyces cerevisiae Ndc1p is a shared component of nuclear pore complexes and spindle pole bodies. J Cell Biol. 1998;143:1789–1800. doi: 10.1083/jcb.143.7.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Feinstein N, Wilson KL, Gruenbaum Y. Nuclear pore protein gp210 is essential for viability in HeLa cells and Caenorhabditis elegans. Mol Biol Cell. 2003;14:4230–4237. doi: 10.1091/mbc.E03-04-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collas I, Courvalin JC. Sorting nuclear membrane proteins at mitosis. Trends Cell Biol. 2000;10:5–8. doi: 10.1016/s0962-8924(99)01697-9. [DOI] [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Rackwitz HR, Franke WW. Identification of protein p270/Tpr as a constitutive component of the nuclear pore complex-attached intranuclear filaments. J Cell Biol. 1997;136:515–529. doi: 10.1083/jcb.136.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter L, Allen TD, Kiseleva E, Goldberg MW. Nuclear Membrane Disassembly and Rupture. J Mol Biol. 2007 doi: 10.1016/j.jmb.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo MA, Anderson DJ, Richard E, Hetzer MW. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312:440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- Daigle N, Beaudouin J, Hartnell L, Imreh G, Hallberg E, Lippincott-Schwartz J, Ellenberg J. Nuclear pore complexes form immobile networks and have a very low turnover in live mammalian cells. J Cell Biol. 2001;154:71–84. doi: 10.1083/jcb.200101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker T, Schillers H, Storck J, Shahin V, Kramer B, Wilhelmi M, Oberleithner H. Nuclear hourglass technique: an approach that detects electrically open nuclear pores in Xenopus laevis oocyte. Proc Natl Acad Sci U S A. 1999;96:13530–13535. doi: 10.1073/pnas.96.23.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CP, Osmani AH, Hashmi SB, Osmani SA. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr Biol. 2004;14:1973–1984. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Denning DP, Rexach MF. Rapid evolution exposes the boundaries of domain structure and function in natively unfolded FG nucleoporins. Mol Cell Proteomics. 2007;6:272–282. doi: 10.1074/mcp.M600309-MCP200. [DOI] [PubMed] [Google Scholar]
- Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D, Dokudovskaya S, Williams R, Alber F, Eswar N, Chait BT, Rout MP, Sali A. Simple fold composition and modular architecture of the nuclear pore complex. Proc Natl Acad Sci U S A. 2006;103:2172–2177. doi: 10.1073/pnas.0506345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth DJ, Tackett AJ, Rogers RS, Yi EC, Christmas RH, Smith JJ, Siegel AF, Chait BT, Wozniak RW, Aitchison JD. The mobile nucleoporin Nup2p and chromatin-bound Prp20p function in endogenous NPC-mediated transcriptional control. J Cell Biol. 2005;171:955–965. doi: 10.1083/jcb.200509061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Rutherford SA, Sanderson HS, Allen TD. High resolution analysis of mammalian nuclear structure throughout the cell cycle: implications for nuclear pore complex assembly during interphase and mitosis. Can J Physiol Pharmacol. 2006;84:423–430. doi: 10.1139/y05-148. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Wilson KL. Interference with the cytoplasmic tail of gp210 disrupts “close apposition” of nuclear membranes and blocks nuclear pore dilation. J Cell Biol. 2002;158:53–62. doi: 10.1083/jcb.200108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J, Levay A, Fontoura BM. Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol Cell Biol. 2003;23:7271–7284. doi: 10.1128/MCB.23.20.7271-7284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J, Levy DE, Blobel G, Fontoura BM. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science. 2002;295:1523–1525. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- Enss K, Danker T, Schlune A, Buchholz I, Oberleithner H. Passive transport of macromolecules through Xenopus laevis nuclear envelope. J Membr Biol. 2003;196:147–155. doi: 10.1007/s00232-003-0632-0. [DOI] [PubMed] [Google Scholar]
- Eriksson C, Rustum C, Hallberg E. Dynamic properties of nuclear pore complex proteins in gp210 deficient cells. FEBS Lett. 2004;572:261–265. doi: 10.1016/j.febslet.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Ewald A, Zunkler C, Lourim D, Dabauvalle MC. Microtubule-dependent assembly of the nuclear envelope in Xenopus laevis egg extract. Eur J Cell Biol. 2001;80:678–691. doi: 10.1078/0171-9335-00207. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B, Aebi U. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat Rev Mol Cell Biol. 2003;4:757–766. doi: 10.1038/nrm1230. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B, Hurt EC, Aebi U, Pante N. Molecular architecture of the yeast nuclear pore complex: localization of Nsp1p subcomplexes. J Cell Biol. 1998;143:577–588. doi: 10.1083/jcb.143.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrog B, Maco B, Fager AM, Koser J, Sauder U, Ullman KS, Aebi U. Domain-specific antibodies reveal multiple-site topology of Nup153 within the nuclear pore complex. J Struct Biol. 2002;140:254–267. doi: 10.1016/s1047-8477(02)00524-5. [DOI] [PubMed] [Google Scholar]
- Faria AM, Levay A, Wang Y, Kamphorst AO, Rosa ML, Nussenzveig DR, Balkan W, Chook YM, Levy DE, Fontoura BM. The nucleoporin nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity. 2006;24:295–304. doi: 10.1016/j.immuni.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Favreau C, Worman HJ, Wozniak RW, Frappier T, Courvalin JC. Cell cycle-dependent phosphorylation of nucleoporins and nuclear pore membrane protein Gp210. Biochemistry. 1996;35:8035–8044. doi: 10.1021/bi9600660. [DOI] [PubMed] [Google Scholar]
- Feldherr CM, Akin D. The permeability of the nuclear envelope in dividing and nondividing cell cultures. J Cell Biol. 1990;111:1–8. doi: 10.1083/jcb.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldherr CM, Akin D. Signal-mediated nuclear transport in proliferating and growth-arrested BALB/c 3T3 cells. J Cell Biol. 1991;115:933–939. doi: 10.1083/jcb.115.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldherr CM, Akin D. The location of the transport gate in the nuclear pore complex. J Cell Sci. 1997;110(Pt 24):3065–3070. doi: 10.1242/jcs.110.24.3065. [DOI] [PubMed] [Google Scholar]
- Fernandez AG, Piano F. MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr Biol. 2006;16:1757–1763. doi: 10.1016/j.cub.2006.07.071. [DOI] [PubMed] [Google Scholar]
- Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–640. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- Ferreira PA, Nakayama TA, Travis GH. Interconversion of red opsin isoforms by the cyclophilin-related chaperone protein Ran-binding protein 2. Proc Natl Acad Sci U S A. 1997;94:1556–1561. doi: 10.1073/pnas.94.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerbach F, Galy V, Trelles-Sticken E, Fromont-Racine M, Jacquier A, Gilson E, Olivo-Marin JC, Scherthan H, Nehrbass U. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat Cell Biol. 2002;4:214–221. doi: 10.1038/ncb756. [DOI] [PubMed] [Google Scholar]
- Fontoura BM, Dales S, Blobel G, Zhong H. The nucleoporin Nup98 associates with the intranuclear filamentous protein network of TPR. Proc Natl Acad Sci U S A. 2001;98:3208–3213. doi: 10.1073/pnas.061014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forler D, Rabut G, Ciccarelli FD, Herold A, Kocher T, Niggeweg R, Bork P, Ellenberg J, Izaurralde E. RanBP2/Nup358 provides a major binding site for NXF1-p15 dimers at the nuclear pore complex and functions in nuclear mRNA export. Mol Cell Biol. 2004;24:1155–1167. doi: 10.1128/MCB.24.3.1155-1167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C, Askjaer P, Antonin W, Iglesias CL, Haselmann U, Schelder M, de Marco A, Wilm M, Antony C, Mattaj IW. Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. Embo J. 2005;24:3519–3531. doi: 10.1038/sj.emboj.7600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, Galy V, Hetzer M, Mattaj IW, Antonin W. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 2007;8:165–172. doi: 10.1038/sj.embor.7400889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Gorlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. 2007;130:512–523. doi: 10.1016/j.cell.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Frey S, Richter RP, Gorlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- Frosst P, Guan T, Subauste C, Hahn K, Gerace L. Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export. J Cell Biol. 2002;156:617–630. doi: 10.1083/jcb.200106046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Askjaer P, Franz C, Lopez-Iglesias C, Mattaj IW. MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr Biol. 2006;16:1748–1756. doi: 10.1016/j.cub.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Galy V, Olivo-Marin JC, Scherthan H, Doye V, Rascalou N, Nehrbass U. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature. 2000;403:108–112. doi: 10.1038/47528. [DOI] [PubMed] [Google Scholar]
- Ghannam G, Takeda A, Camarata T, Moore MA, Viale A, Yaseen NR. The oncogene Nup98-HOXA9 induces gene transcription in myeloid cells. J Biol Chem. 2004;279:866–875. doi: 10.1074/jbc.M307280200. [DOI] [PubMed] [Google Scholar]
- Gigliotti S, Callaini G, Andone S, Riparbelli MG, Pernas-Alonso R, Hoffmann G, Graziani F, Malva C. Nup154, a new Drosophila gene essential for male and female gametogenesis is related to the nup155 vertebrate nucleoporin gene. J Cell Biol. 1998;142:1195–1207. doi: 10.1083/jcb.142.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavy JS, Krutchinsky AN, Cristea IM, Berke IC, Boehmer T, Blobel G, Chait BT. Cell-cycle-dependent phosphorylation of the nuclear pore Nup107-160 subcomplex. Proc Natl Acad Sci U S A. 2007;104:3811–3816. doi: 10.1073/pnas.0700058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MW, Rutherford SA, Hughes M, Cotter LA, Bagley S, Kiseleva E, Allen TD, Clarke PR. Ran alters nuclear pore complex conformation. J Mol Biol. 2000;300:519–529. doi: 10.1006/jmbi.2000.3891. [DOI] [PubMed] [Google Scholar]
- Goldberg MW, Wiese C, Allen TD, Wilson KL. Dimples, pores, star-rings, and thin rings on growing nuclear envelopes: evidence for structural intermediates in nuclear pore complex assembly. J Cell Sci. 1997;110(Pt 4):409–420. doi: 10.1242/jcs.110.4.409. [DOI] [PubMed] [Google Scholar]
- Gorlich D, Henklein P, Laskey RA, Hartmann E. A 41 amino acid motif in importin-alpha confers binding to importin-beta and hence transit into the nucleus. Embo J. 1996;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- Gorlich D, Seewald MJ, Ribbeck K. Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation. Embo J. 2003;22:1088–1100. doi: 10.1093/emboj/cdg113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Altan N, Lippincott-Schwartz J, Powers MA. Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol Biol Cell. 2002;13:1282–1297. doi: 10.1091/mbc.01-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Craige B, Dimaano C, Ullman KS, Powers MA. Distinct functional domains within nucleoporins Nup153 and Nup98 mediate transcription-dependent mobility. Mol Biol Cell. 2004;15:1991–2002. doi: 10.1091/mbc.E03-10-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Xu S, Powers MA. Nup98 localizes to both nuclear and cytoplasmic sides of the nuclear pore and binds to two distinct nucleoporin subcomplexes. Mol Biol Cell. 2003;14:600–610. doi: 10.1091/mbc.E02-09-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi MR, Cozzolino L, Malva C, Graziani F, Gigliotti S. nup154 genetically interacts with cup and plays a cell-type-specific function during Drosophila melanogaster egg-chamber development. Genetics. 2007;175:1751–1759. doi: 10.1534/genetics.106.062844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin A, Tirrell M, Lodge TP. Tethered Chains in Polymer Microstructures. Advances in Polymer Science. 1992;100:31–71. [Google Scholar]
- Handa N, Kukimoto-Niino M, Akasaka R, Kishishita S, Murayama K, Terada T, Inoue M, Kigawa T, Kose S, Imamoto N, Tanaka A, Hayashizaki Y, Shirouzu M, Yokoyama S. The crystal structure of mouse Nup35 reveals atypical RNP motifs and novel homodimerization of the RRM domain. J Mol Biol. 2006;363:114–124. doi: 10.1016/j.jmb.2006.07.089. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Koujin T, Hayakawa T, Kaneda T, Tsutsumi C, Imamoto N, Akazawa C, Sukegawa J, Yoneda Y, Hiraoka Y. Live fluorescence imaging reveals early recruitment of emerin, LBR, RanBP2, and Nup153 to reforming functional nuclear envelopes. J Cell Sci. 2000;113(Pt 5):779–794. doi: 10.1242/jcs.113.5.779. [DOI] [PubMed] [Google Scholar]
- Harel A, Chan RC, Lachish-Zalait A, Zimmerman E, Elbaum M, Forbes DJ. Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol Biol Cell. 2003a;14:4387–4396. doi: 10.1091/mbc.E03-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A, Orjalo AV, Vincent T, Lachish-Zalait A, Vasu S, Shah S, Zimmerman E, Elbaum M, Forbes DJ. Removal of a single pore subcomplex results in vertebrate nuclei devoid of nuclear pores. Mol Cell. 2003b;11:853–864. doi: 10.1016/s1097-2765(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Hase ME, Cordes VC. Direct interaction with nup153 mediates binding of Tpr to the periphery of the nuclear pore complex. Mol Biol Cell. 2003;14:1923–1940. doi: 10.1091/mbc.E02-09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk-Gara LA, Shibuya EK, Wozniak RW. Vertebrate Nup53 interacts with the nuclear lamina and is required for the assembly of a Nup93-containing complex. Mol Biol Cell. 2005;16:2382–2394. doi: 10.1091/mbc.E04-10-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa MM, Alam SL, Sundquist WI, Ullman KS. Molecular characterization of the ran-binding zinc finger domain of NUP153. J Biol Chem. 2007 doi: 10.1074/jbc.M702715200. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE, Carragher BO, Milligan RA. Architecture and design of the nuclear pore complex. Cell. 1992;69:1133–1141. doi: 10.1016/0092-8674(92)90635-p. [DOI] [PubMed] [Google Scholar]
- Hodel AE, Hodel MR, Griffis ER, Hennig KA, Ratner GA, Xu S, Powers MA. The three-dimensional structure of the autoproteolytic, nuclear pore-targeting domain of the human nucleoporin Nup98. Mol Cell. 2002;10:347–358. doi: 10.1016/s1097-2765(02)00589-0. [DOI] [PubMed] [Google Scholar]
- Hogarth C, Itman C, Jans DA, Loveland KL. Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation? Bioessays. 2005;27:1011–1025. doi: 10.1002/bies.20289. [DOI] [PubMed] [Google Scholar]
- Hutten S, Kehlenbach RH. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Mol Cell Biol. 2006;26:6772–6785. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imreh G, Maksel D, de Monvel JB, Branden L, Hallberg E. ER retention may play a role in sorting of the nuclear pore membrane protein POM121. Exp Cell Res. 2003;284:173–184. doi: 10.1016/s0014-4827(02)00034-4. [DOI] [PubMed] [Google Scholar]
- Isgro TA, Schulten K. Binding dynamics of isolated nucleoporin repeat regions to importin-ß. Structure. 2005;13:1869–1879. doi: 10.1016/j.str.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Isgro TA, Schulten K. Association of nuclear pore FG-repeat domains to NTF2 import and export complexes. J Mol Biol. 2007a;366:330–345. doi: 10.1016/j.jmb.2006.11.048. [DOI] [PubMed] [Google Scholar]
- Isgro TA, Schulten K. Cse1p-binding dynamics reveal a binding pattern for FG-repeat nucleoporins on transport receptors. Structure. 2007b;15:977–991. doi: 10.1016/j.str.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ishii K, Arib G, Lin C, Van Houwe G, Laemmli UK. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell. 2002;109:551–562. doi: 10.1016/s0092-8674(02)00756-0. [DOI] [PubMed] [Google Scholar]
- Jaggi RD, Franco-Obregon A, Ensslin K. Quantitative topographical analysis of nuclear pore complex function using scanning force microscopy. Biophys J. 2003a;85:4093–4098. doi: 10.1016/S0006-3495(03)74821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi RD, Franco-Obregon A, Muhlhausser P, Thomas F, Kutay U, Ensslin K. Modulation of nuclear pore topology by transport modifiers. Biophys J. 2003b;84:665–670. doi: 10.1016/S0006-3495(03)74886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M. The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr Biol. 2004;14:611–617. doi: 10.1016/j.cub.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Joseph J, Tan SH, Karpova TS, McNally JG, Dasso M. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J Cell Biol. 2002;156:595–602. doi: 10.1083/jcb.200110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P, Pralle A, Isacoff EY, Heald R, Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. doi: 10.1038/nature04589. [DOI] [PubMed] [Google Scholar]
- Kalab P, Weis K, Heald R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science. 2002;295:2452–2456. doi: 10.1126/science.1068798. [DOI] [PubMed] [Google Scholar]
- Kasper LH, Brindle PK, Schnabel CA, Pritchard CE, Cleary ML, van Deursen JM. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol Cell Biol. 1999;19:764–776. doi: 10.1128/mcb.19.1.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva E, Allen TD, Rutherford S, Bucci M, Wente SR, Goldberg MW. Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments. J Struct Biol. 2004;145:272–288. doi: 10.1016/j.jsb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Kiseleva E, Goldberg MW, Allen TD, Akey CW. Active nuclear pore complexes in Chironomus: visualization of transporter configurations related to mRNP export. J Cell Sci. 1998;111(Pt 2):223–236. doi: 10.1242/jcs.111.2.223. [DOI] [PubMed] [Google Scholar]
- Kiseleva E, Goldberg MW, Daneholt B, Allen TD. RNP export is mediated by structural reorganization of the nuclear pore basket. J Mol Biol. 1996;260:304–311. doi: 10.1006/jmbi.1996.0401. [DOI] [PubMed] [Google Scholar]
- Kiseleva E, Rutherford S, Cotter LM, Allen TD, Goldberg MW. Steps of nuclear pore complex disassembly and reassembly during mitosis in early Drosophila embryos. J Cell Sci. 2001;114:3607–3618. doi: 10.1242/jcs.114.20.3607. [DOI] [PubMed] [Google Scholar]
- Kitano H. Biological robustness. Nat Rev Genet. 2004;5:826–837. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- Kopito RB, Elbaum M. Reversibility in nucleocytoplasmic transport. Proc Natl Acad Sci U S A. 2007;104:12743–12748. doi: 10.1073/pnas.0702690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull S, Thyberg J, Bjorkroth B, Rackwitz HR, Cordes VC. Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket. Mol Biol Cell. 2004;15:4261–4277. doi: 10.1091/mbc.E04-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitscheck U, Grunwald D, Hoekstra A, Rohleder D, Kues T, Siebrasse JP, Peters R. Nuclear transport of single molecules: dwell times at the nuclear pore complex. J Cell Biol. 2005;168:233–243. doi: 10.1083/jcb.200411005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustanovich T, Rabin Y. Metastable network model of protein transport through nuclear pores. Biophys J. 2004;86:2008–2016. doi: 10.1016/S0006-3495(04)74262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CK, Giddings TH, Jr., Winey M. A novel allele of Saccharomyces cerevisiae NDC1 reveals a potential role for the spindle pole body component Ndc1p in nuclear pore assembly. Eukaryot Cell. 2004;3:447–458. doi: 10.1128/EC.3.2.447-458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart P, Ellenberg J. Monitoring the permeability of the nuclear envelope during the cell cycle. Methods. 2006;38:17–24. doi: 10.1016/j.ymeth.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lenart P, Rabut G, Daigle N, Hand AR, Terasaki M, Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J Cell Biol. 2003;160:1055–1068. doi: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin JM, Lwaleed BA, Cooper AJ, Birch BR. The direct effect of nuclear pores on nuclear chemotherapeutic concentration in multidrug resistant bladder cancer: the nuclear sparing phenomenon. J Urol. 2007;177:1526–1530. doi: 10.1016/j.juro.2006.11.048. [DOI] [PubMed] [Google Scholar]
- Lewis A, Felberbaum R, Hochstrasser M. A nuclear envelope protein linking nuclear pore basket assembly, SUMO protease regulation, and mRNA surveillance. J Cell Biol. 2007;178:813–827. doi: 10.1083/jcb.200702154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Clarke JD, Zhang Y, Dong X. Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol Plant Microbe Interact. 2001;14:1131–1139. doi: 10.1094/MPMI.2001.14.10.1131. [DOI] [PubMed] [Google Scholar]
- Lim RY, Aebi U. In silico access to the nuclear pore complex. Structure. 2005;13:1741–1743. doi: 10.1016/j.str.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Lim RY, Fahrenkrog B. The nuclear pore complex up close. Curr Opin Cell Biol. 2006;18:342–347. doi: 10.1016/j.ceb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Lim RY, Huang NP, Koser J, Deng J, Lau KH, Schwarz-Herion K, Fahrenkrog B, Aebi U. Flexible phenylalanine-glycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proc Natl Acad Sci U S A. 2006;103:9512–9517. doi: 10.1073/pnas.0603521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RY, Koser J, Huang NP, Schwarz-Herion K, Aebi U. Nanomechanical interactions of phenylalanine-glycine nucleoporins studied by single molecule force-volume spectroscopy. J Struct Biol. 2007 doi: 10.1016/j.jsb.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Lim RYH, Aebi U, Stoffler D. From the trap to the basket: getting to the bottom of the nuclear pore complex. Chromosoma. 2006a;115:15–26. doi: 10.1007/s00412-005-0037-1. [DOI] [PubMed] [Google Scholar]
- Lim RYH, Huang NP, Koser J, Deng J, Lau KHA, Schwarz-Herion K, Fahrenkrog B, Aebi U. Flexible phenylalanine-glycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proceedings of the National Academy of Sciences of the United States of America. 2006b;103:9512–9517. doi: 10.1073/pnas.0603521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Prunuske AJ, Fager AM, Ullman KS. The COPI complex functions in nuclear envelope breakdown and is recruited by the nucleoporin Nup153. Dev Cell. 2003;5:487–498. doi: 10.1016/s1534-5807(03)00262-4. [DOI] [PubMed] [Google Scholar]
- Liu Q, Pante N, Misteli T, Elsagga M, Crisp M, Hodzic D, Burke B, Roux KJ. Functional association of Sun1 with nuclear pore complexes. J Cell Biol. 2007;178:785–798. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SM, Stewart M. Structural basis for the high-affinity binding of nucleoporin Nup1p to the Saccharomyces cerevisiae importin-beta homologue, Kap95p. J Mol Biol. 2005;349:515–525. doi: 10.1016/j.jmb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Loiodice I, Alves A, Rabut G, Van Overbeek M, Ellenberg J, Sibarita JB, Doye V. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol Biol Cell. 2004;15:3333–3344. doi: 10.1091/mbc.E03-12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk CP, Makhnevych T, Marelli M, Aitchison JD, Wozniak RW. Karyopherins in nuclear pore biogenesis: a role for Kap121p in the assembly of Nup53p into nuclear pore complexes. J Cell Biol. 2002;159:267–278. doi: 10.1083/jcb.200203079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk CP, Waller DD, Makhnevych T, Dienemann A, Whiteway M, Thomas DY, Wozniak RW. Nup53p is a Target of Two Mitotic Kinases, Cdk1p and Hrr25p. Traffic. 2007 doi: 10.1111/j.1600-0854.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Luthra R, Kerr SC, Harreman MT, Apponi LH, Fasken MB, Ramineni S, Chaurasia S, Valentini SR, Corbett AH. Actively transcribed GAL genes can be physically linked to the nuclear pore by the SAGA chromatin modifying complex. J Biol Chem. 2007;282:3042–3049. doi: 10.1074/jbc.M608741200. [DOI] [PubMed] [Google Scholar]
- Macara IG. Transport into and out of the nucleus. Microbiol Mol Biol Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay C, Forbes DJ. Assembly of the nuclear pore: biochemically distinct steps revealed with NEM, GTP gamma S, and BAPTA. J Cell Biol. 1996;132:5–20. doi: 10.1083/jcb.132.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay C, Meier E, Forbes DJ. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J Biol Chem. 1995;270:254–262. doi: 10.1074/jbc.270.1.254. [DOI] [PubMed] [Google Scholar]
- Madrid AS, Mancuso J, Cande WZ, Weis K. The role of the integral membrane nucleoporins Ndc1p and Pom152p in nuclear pore complex assembly and function. J Cell Biol. 2006;173:361–371. doi: 10.1083/jcb.200506199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima K, Yahata K, Sasaki Y, Nakatomi R, Tachibana T, Hashikawa T, Imamoto F, Imamoto N. Cell-cycle-dependent dynamics of nuclear pores: pore-free islands and lamins. J Cell Sci. 2006;119:4442–4451. doi: 10.1242/jcs.03207. [DOI] [PubMed] [Google Scholar]
- Maison C, Pyrpasopoulou A, Theodoropoulos PA, Georgatos SD. The inner nuclear membrane protein LAP1 forms a native complex with B-type lamins and partitions with spindle-associated mitotic vesicles. Embo J. 1997;16:4839–4850. doi: 10.1093/emboj/16.16.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhnevych T, Lusk CP, Anderson AM, Aitchison JD, Wozniak RW. Cell cycle regulated transport controlled by alterations in the nuclear pore complex. Cell. 2003;115:813–823. doi: 10.1016/s0092-8674(03)00986-3. [DOI] [PubMed] [Google Scholar]
- Makhnevych T, Ptak C, Lusk CP, Aitchison JD, Wozniak RW. The role of karyopherins in the regulated sumoylation of septins. J Cell Biol. 2007;177:39–49. doi: 10.1083/jcb.200608066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Anantharaman V, Aravind L, Koonin EV. Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell Cycle. 2004;3:1612–1637. doi: 10.4161/cc.3.12.1316. [DOI] [PubMed] [Google Scholar]
- Mansfeld J, Guttinger S, Hawryluk-Gara LA, Pante N, Mall M, Galy V, Haselmann U, Muhlhausser P, Wozniak RW, Mattaj IW, Kutay U, Antonin W. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol Cell. 2006;22:93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Marelli M, Aitchison JD, Wozniak RW. Specific binding of the karyopherin Kap121p to a subunit of the nuclear pore complex containing Nup53p, Nup59p, and Nup170p. J Cell Biol. 1998;143:1813–1830. doi: 10.1083/jcb.143.7.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli M, Lusk CP, Chan H, Aitchison JD, Wozniak RW. A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J Cell Biol. 2001;153:709–724. doi: 10.1083/jcb.153.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko JF, Siggia ED. Stretching DNA. Macromolecules. 1995;28:8759–8770. [Google Scholar]
- Marshall IC, Gant TM, Wilson KL. Ionophore-releasable lumenal Ca2+ stores are not required for nuclear envelope assembly or nuclear protein import in Xenopus egg extracts. Cell Calcium. 1997;21:151–161. doi: 10.1016/s0143-4160(97)90039-7. [DOI] [PubMed] [Google Scholar]
- Matunis MJ, Wu J, Blobel G. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J Cell Biol. 1998;140:499–509. doi: 10.1083/jcb.140.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul GG, Deaven LL, Freed JJ, Campbell GL, Becak W. Investigation of the determinants of nuclear pore number. Cytogenet Cell Genet. 1980;26:175–190. doi: 10.1159/000131439. [DOI] [PubMed] [Google Scholar]
- Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev. 2001;81:1–19. doi: 10.1152/physrev.2001.81.1.1. [DOI] [PubMed] [Google Scholar]
- Melcak I, Hoelz A, Blobel G. Structure of Nup58/45 suggests flexible nuclear pore diameter by intermolecular sliding. Science. 2007;315:1729–1732. doi: 10.1126/science.1135730. [DOI] [PubMed] [Google Scholar]
- Mendjan S, Taipale M, Kind J, Holz H, Gebhardt P, Schelder M, Vermeulen M, Buscaino A, Duncan K, Mueller J, Wilm M, Stunnenberg HG, Saumweber H, Akhtar A. Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol Cell. 2006;21:811–823. doi: 10.1016/j.molcel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Menon BB, Sarma NJ, Pasula S, Deminoff SJ, Willis KA, Barbara KE, Andrews B, Santangelo GM. Reverse recruitment: the Nup84 nuclear pore subcomplex mediates Rap1/Gcr1/Gcr2 transcriptional activation. Proc Natl Acad Sci U S A. 2005;102:5749–5754. doi: 10.1073/pnas.0501768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M, Ryan KJ, Wente SR. The integral membrane protein Pom34p functionally links nucleoporin subcomplexes. Genetics. 2006;172:1441–1457. doi: 10.1534/genetics.105.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner ST. Polymer Brushes. Science. 1991;251:905–914. doi: 10.1126/science.251.4996.905. [DOI] [PubMed] [Google Scholar]
- Mooren OL, Erickson ES, Moore-Nichols D, Dunn RC. Nuclear side conformational changes in the nuclear pore complex following calcium release from the nuclear membrane. Phys Biol. 2004;1:125–134. doi: 10.1088/1478-3967/1/2/008. [DOI] [PubMed] [Google Scholar]
- Muhlhausser P, Kutay U. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J Cell Biol. 2007;178:595–610. doi: 10.1083/jcb.200703002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Weis K. The direction of transport through the nuclear pore can be inverted. Proc Natl Acad Sci U S A. 1999;96:9622–9627. doi: 10.1073/pnas.96.17.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim B, Brumfeld V, Kapon R, Kiss V, Nevo R, Reich Z. Passive and facilitated transport in nuclear pore complexes is largely uncoupled. J Biol Chem. 2007;282:3881–3888. doi: 10.1074/jbc.M608329200. [DOI] [PubMed] [Google Scholar]
- Nakielny S, Shaikh S, Burke B, Dreyfuss G. Nup153 is an M9-containing mobile nucleoporin with a novel Ran-binding domain. Embo J. 1999;18:1982–1995. doi: 10.1093/emboj/18.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napetschnig J, Blobel G, Hoelz A. Crystal structure of the N-terminal domain of the human protooncogene Nup214/CAN. Proc Natl Acad Sci U S A. 2007;104:1783–1788. doi: 10.1073/pnas.0610828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemergut ME, Mizzen CA, Stukenberg T, Allis CD, Macara IG. Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B. Science. 2001;292:1540–1543. doi: 10.1126/science.292.5521.1540. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Jeppesen C, Ipsen JH. Managing free-energy barriers in nuclear pore transport. Journal of Biological Physics. 2006;32:465–472. doi: 10.1007/s10867-006-9029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepel M, Strambio-de-Castillia C, Fasolo J, Chait BT, Rout MP. The nuclear pore complex-associated protein, Mlp2p, binds to the yeast spindle pole body and promotes its efficient assembly. J Cell Biol. 2005;170:225–235. doi: 10.1083/jcb.200504140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybakken K, Vokes SA, Lin TY, McMahon AP, Perrimon N. A genome-wide RNA interference screen in Drosophila melanogaster cells for new components of the Hh signaling pathway. Nat Genet. 2005;37:1323–1332. doi: 10.1038/ng1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Scheele S, Ekblom P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp Cell Res. 2004;292:359–370. doi: 10.1016/j.yexcr.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Onischenko EA, Crafoord E, Hallberg E. Phosphomimetic mutation of the mitotically phosphorylated serine 1880 compromises the interaction of the transmembrane nucleoporin gp210 with the nuclear pore complex. Exp Cell Res. 2007 doi: 10.1016/j.yexcr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Onischenko EA, Gubanova NV, Kieselbach T, Kiseleva EV, Hallberg E. Annulate lamellae play only a minor role in the storage of excess nucleoporins in Drosophila embryos. Traffic. 2004;5:152–164. doi: 10.1111/j.1600-0854.2004.0166.x. [DOI] [PubMed] [Google Scholar]
- Onischenko EA, Gubanova NV, Kiseleva EV, Hallberg E. Cdk1 and okadaic acid-sensitive phosphatases control assembly of nuclear pore complexes in Drosophila embryos. Mol Biol Cell. 2005;16:5152–5162. doi: 10.1091/mbc.E05-07-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjalo AV, Arnaoutov A, Shen Z, Boyarchuk Y, Zeitlin SG, Fontoura B, Briggs S, Dasso M, Forbes DJ. The Nup107-160 nucleoporin complex is required for correct bipolar spindle assembly. Mol Biol Cell. 2006;17:3806–3818. doi: 10.1091/mbc.E05-11-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine PL, Moore LC, Horowitz SB. Nuclear-Envelope Permeability. Nature. 1975;254:109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Palancade B, Liu X, Garcia-Rubio M, Aguilera A, Zhao X, Doye V. Nucleoporins Prevent DNA Damage Accumulation by Modulating Ulp1-dependent Sumoylation Processes. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panté N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol Biol Cell. 2002;13:425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise A, Levin MK, Korza G, Carson JH. Significant proportions of nuclear transport proteins with reduced intracellular mobilities resolved by fluorescence correlation spectroscopy. J Mol Biol. 2007;365:50–65. doi: 10.1016/j.jmb.2006.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Ward S, Cernac A, Dharmasiri S, Estelle M. The Arabidopsis SUPPRESSOR OF AUXIN RESISTANCE proteins are nucleoporins with an important role in hormone signaling and development. Plant Cell. 2006;18:1590–1603. doi: 10.1105/tpc.106.041566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SS, Belmont BJ, Sante JM, Rexach MF. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell. 2007;129:83–96. doi: 10.1016/j.cell.2007.01.044. [DOI] [PubMed] [Google Scholar]
- Paulillo SM, Phillips EM, Koser J, Sauder U, Ullman KS, Powers MA, Fahrenkrog B. Nucleoporin domain topology is linked to the transport status of the nuclear pore complex. J Mol Biol. 2005;351:784–798. doi: 10.1016/j.jmb.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Paulillo SM, Powers MA, Ullman KS, Fahrenkrog B. Changes in nucleoporin domain topology in response to chemical effectors. J Mol Biol. 2006;363:39–50. doi: 10.1016/j.jmb.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C, Behfar A, Mery A, van Deursen JM, Terzic A, Puceat M. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ Res. 2003;92:444–452. doi: 10.1161/01.RES.0000059415.25070.54. [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C, Faustino RS, Boorsma BJ, Arrell DK, Niederlander NJ, Behfar A, Terzic A. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat Clin Pract Cardiovasc Med 4 Suppl. 2007;1:S68–76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- Peters R. Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality. Traffic. 2005;6:421–427. doi: 10.1111/j.1600-0854.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–120. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- Prunuske AJ, Liu J, Elgort S, Joseph J, Dasso M, Ullman KS. Nuclear envelope breakdown is coordinated by both Nup358/RanBP2 and Nup153, two nucleoporins with zinc finger modules. Mol Biol Cell. 2006;17:760–769. doi: 10.1091/mbc.E05-06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut G, Doye V, Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol. 2004;6:1114–1121. doi: 10.1038/ncb1184. [DOI] [PubMed] [Google Scholar]
- Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc Natl Acad Sci U S A. 2006;103:17801–17806. doi: 10.1073/pnas.0608484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K, Gorlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. Embo J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MA, Park S, Sun ZY, Silver PA, Wagner G, Hogle JM. Multiple conformations in the ligand-binding site of the yeast nuclear pore-targeting domain of Nup116p. J Biol Chem. 2005;280:35723–35732. doi: 10.1074/jbc.M505068200. [DOI] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Magnasco MO, Chait BT. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol. 2003;13:622–628. doi: 10.1016/j.tcb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol. 2000;148:635–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KJ, McCaffery JM, Wente SR. The Ran GTPase cycle is required for yeast nuclear pore complex assembly. J Cell Biol. 2003;160:1041–1053. doi: 10.1083/jcb.200209116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KJ, Zhou Y, Wente SR. The karyopherin Kap95 regulates nuclear pore complex assembly into intact nuclear envelopes in vivo. Mol Biol Cell. 2007;18:886–898. doi: 10.1091/mbc.E06-06-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri N, Roth P, Xylourgidis N, Sadeghifar F, Adler J, Samakovlis C. Distinct functions of the Drosophila Nup153 and Nup214 FG domains in nuclear protein transport. J Cell Biol. 2007;178:557–565. doi: 10.1083/jcb.200612135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina D, Enarson P, Rattner JB, Burke B. Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J Cell Biol. 2003;162:991–1001. doi: 10.1083/jcb.200304080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli JJ, Hodge CA, Cole CN. The yeast integral membrane protein Apq12 potentially links membrane dynamics to assembly of nuclear pore complexes. J Cell Biol. 2007;178:799–812. doi: 10.1083/jcb.200702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Arib G, Laemmli C, Nishikawa J, Durussel T, Laemmli UK. Nup-PI: the nucleopore-promoter interaction of genes in yeast. Mol Cell. 2006;21:379–391. doi: 10.1016/j.molcel.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Schwartz TU. Modularity within the architecture of the nuclear pore complex. Curr Opin Struct Biol. 2005;15:221–226. doi: 10.1016/j.sbi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Schwarz-Herion K, Maco B, Sauder U, Fahrenkrog B. Domain Topology of the p62 Complex Within the 3-D Architecture of the Nuclear Pore Complex. J Mol Biol. 2007;370:796–806. doi: 10.1016/j.jmb.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Shahin V, Albermann L, Schillers H, Kastrup L, Schafer C, Ludwig Y, Stock C, Oberleithner H. Steroids dilate nuclear pores imaged with atomic force microscopy. J Cell Physiol. 2005;202:591–601. doi: 10.1002/jcp.20152. [DOI] [PubMed] [Google Scholar]
- Shahin V, Danker T, Enss K, Ossig R, Oberleithner H. Evidence for Ca2+- and ATP-sensitive peripheral channels in nuclear pore complexes. Faseb J. 2001;15:1895–1901. doi: 10.1096/fj.00-0838com. [DOI] [PubMed] [Google Scholar]
- Sheehan MA, Mills AD, Sleeman AM, Laskey RA, Blow JJ. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988;106:1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Matsuoka Y, Yoneda Y. Nucleocytoplasmic transport of proteins and poly(A)+ RNA in reconstituted Tpr-less nuclei in living mammalian cells. Genes Cells. 2002;7:421–434. doi: 10.1046/j.1365-2443.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- Shulga N, Goldfarb DS. Binding dynamics of structural nucleoporins govern nuclear pore complex permeability and may mediate channel gating. Mol Cell Biol. 2003;23:534–542. doi: 10.1128/MCB.23.2.534-542.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol. 1995;130:15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F, Hulsmann BB, Spang A, Hartmann E, Cordes VC, Gorlich D. NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes. J Cell Biol. 2006a;173:509–519. doi: 10.1083/jcb.200601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F, Nautrup-Pedersen G, Cordes VC, Gorlich D. Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells. J Cell Biol. 2006b;173:477–483. doi: 10.1083/jcb.200601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- Stoffler D, Feja B, Fahrenkrog B, Walz J, Typke D, Aebi U. Cryo-electron tomography provides novel insights into nuclear pore architecture: implications for nucleocytoplasmic transport. J Mol Biol. 2003;328:119–130. doi: 10.1016/s0022-2836(03)00266-3. [DOI] [PubMed] [Google Scholar]
- Stoffler D, Goldie KN, Feja B, Aebi U. Calcium-mediated structural changes of native nuclear pore complexes monitored by time-lapse atomic force microscopy. J Mol Biol. 1999;287:741–752. doi: 10.1006/jmbi.1999.2637. [DOI] [PubMed] [Google Scholar]
- Strambio-de-Castillia C, Blobel G, Rout MP. Proteins connecting the nuclear pore complex with the nuclear interior. J Cell Biol. 1999;144:839–855. doi: 10.1083/jcb.144.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn LA, Shen T, Shulga N, Goldfarb DS, Wente SR. Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat Cell Biol. 2004;6:197–206. doi: 10.1038/ncb1097. [DOI] [PubMed] [Google Scholar]
- Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell. 2003;4:775–789. doi: 10.1016/s1534-5807(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Therizols P, Fairhead C, Cabal GG, Genovesio A, Olivo-Marin JC, Dujon B, Fabre E. Telomere tethering at the nuclear periphery is essential for efficient DNA double strand break repair in subtelomeric region. J Cell Biol. 2006;172:189–199. doi: 10.1083/jcb.200505159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timney BL, Tetenbaum-Novatt J, Agate DS, Williams R, Zhang W, Chait BT, Rout MP. Simple kinetic relationships and nonspecific competition govern nuclear import rates in vivo. J Cell Biol. 2006;175:579–593. doi: 10.1083/jcb.200608141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Um JW, Min DS, Rhim H, Kim J, Paik SR, Chung KC. Parkin ubiquitinates and promotes the degradation of RanBP2. J Biol Chem. 2006;281:3595–3603. doi: 10.1074/jbc.M504994200. [DOI] [PubMed] [Google Scholar]
- van Deursen J, Boer J, Kasper L, Grosveld G. G2 arrest and impaired nucleocytoplasmic transport in mouse embryos lacking the proto-oncogene CAN/Nup214. Embo J. 1996;15:5574–5583. [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Alves A, Pickersgill H, Loiodice I, Hetzer M, Galy V, Hulsmann BB, Kocher T, Wilm M, Allen T, Mattaj IW, Doye V. The conserved Nup107-160 complex is critical for nuclear pore complex assembly. Cell. 2003a;113:195–206. doi: 10.1016/s0092-8674(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Walther TC, Askjaer P, Gentzel M, Habermann A, Griffiths G, Wilm M, Mattaj IW, Hetzer M. RanGTP mediates nuclear pore complex assembly. Nature. 2003b;424:689–694. doi: 10.1038/nature01898. [DOI] [PubMed] [Google Scholar]
- Walther TC, Pickersgill HS, Cordes VC, Goldberg MW, Allen TD, Mattaj IW, Fornerod M. The cytoplasmic filaments of the nuclear pore complex are dispensable for selective nuclear protein import. J Cell Biol. 2002;158:63–77. doi: 10.1083/jcb.200202088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Clapham DE. Conformational changes of the in situ nuclear pore complex. Biophys J. 1999;77:241–247. doi: 10.1016/S0006-3495(99)76885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich CS, Erzberger JP, Berger JM, Weis K. The N-terminal domain of Nup159 forms a beta-propeller that functions in mRNA export by tethering the helicase Dbp5 to the nuclear pore. Mol Cell. 2004;16:749–760. doi: 10.1016/j.molcel.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Weis K. Nucleocytoplasmic transport: cargo trafficking across the border. Curr Opin Cell Biol. 2002;14:328–335. doi: 10.1016/s0955-0674(02)00337-x. [DOI] [PubMed] [Google Scholar]
- Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- Winey M, Yarar D, Giddings TH, Jr., Mastronarde DN. Nuclear pore complex number and distribution throughout the Saccharomyces cerevisiae cell cycle by three-dimensional reconstruction from electron micrographs of nuclear envelopes. Mol Biol Cell. 1997;8:2119–2132. doi: 10.1091/mbc.8.11.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Guan T, Gerace L. Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis. J Cell Biol. 1997;137:1199–1210. doi: 10.1083/jcb.137.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol Cell. 1998;1:223–234. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- Yang W, Gelles J, Musser SM. Imaging of single-molecule translocation through nuclear pore complexes. Proc Natl Acad Sci U S A. 2004;101:12887–12892. doi: 10.1073/pnas.0403675101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Musser SM. Nuclear import time and transport efficiency depend on importin beta concentration. J Cell Biol. 2006;174:951–961. doi: 10.1083/jcb.200605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U, et al. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]
- Zeitler B, Weis K. The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo. J Cell Biol. 2004;167:583–590. doi: 10.1083/jcb.200407156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goritschnig S, Dong X, Li X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell. 2003;15:2636–2646. doi: 10.1105/tpc.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X. A putative nucleoporin 96 Is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1,constitutive 1. Plant Cell. 2005;17:1306–1316. doi: 10.1105/tpc.104.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Brittain WJ. Polymer brushes: surface-immobilized macromolecules. Progress in Polymer Science. 2000;25:677–710. [Google Scholar]
- Zhong H, Takeda A, Nazari R, Shio H, Blobel G, Yaseen NR. Carrier-independent nuclear import of the transcription factor PU.1 via RanGTP-stimulated binding to Nup153. J Biol Chem. 2005;280:10675–10682. doi: 10.1074/jbc.M412878200. [DOI] [PubMed] [Google Scholar]
- Zilman A, Di Talia S, Chait BT, Rout MP, Magnasco MO. Efficiency, Selectivity, and Robustness of Nucleocytoplasmic Transport. PLoS Comput Biol. 2007;3:e125. doi: 10.1371/journal.pcbi.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimowska G, Aris JP, Paddy MR. A Drosophila Tpr protein homolog is localized both in the extrachromosomal channel network and to nuclear pore complexes. J Cell Sci. 1997;110(Pt 8):927–944. doi: 10.1242/jcs.110.8.927. [DOI] [PubMed] [Google Scholar]
- Zuccolo M, Alves A, Galy V, Bolhy S, Formstecher E, Racine V, Sibarita JB, Fukagawa T, Shiekhattar R, Yen T, Doye V. The human Nup107-160 nuclear pore subcomplex contributes to proper kinetochore functions. Embo J. 2007;26:1853–1864. doi: 10.1038/sj.emboj.7601642. [DOI] [PMC free article] [PubMed] [Google Scholar]