s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue (original) (raw)
Abstract
Mammary stem cells (MaSCs) play critical roles in normal development and perhaps tumorigenesis of the mammary gland. Using combined cell markers, adult MaSCs have been enriched in a basal cell population, but the exact identity of MaSCs remains unknown. We used the s-SHIP promoter to tag presumptive stem cells with GFP in the embryos of a transgenic mouse model. Here we show, in postnatal mammary gland development, that GFP+ cap cells in puberty and basal alveolar bud cells in pregnancy each exhibit self-renewal and regenerative capabilities for all mammary epithelial cells of a new functional mammary gland upon transplantation. Single GFP+ cells can regenerate the mammary epithelial network. GFP+ mammary epithelial cells are p63+, CD24mod, CD49fhigh, and CD29high; are actively proliferating; and express s-SHIP mRNA. Overall, our results identify the activated MaSC population in vivo at the forefront of rapidly developing terminal end buds (puberty) and alveolar buds (pregnancy) in the mammary gland. In addition, GFP+ basal cells are expanded in MMTV-Wnt1 breast tumors but not in ErbB2 tumors. These results enable MaSC in situ identification and isolation via a consistent single parameter using a new mouse model with applications for further analyses of normal and potential cancer stem cells.
Keywords: Stem cell, mammary gland development, cap cells, alveolar bud cells, s-SHIP, breast tumors
The female mammary gland is a fascinating model for studying adult mammary stem cells (MaSCs), because complex stage-specific development occurs in puberty and pregnancy (Wiseman and Werb 2002; Hennighausen and Robinson 2005). After birth, only a few small rudimentary mammary ducts are present in the fat pad. At the onset of puberty, ductal elongation begins with the formation of bulb-like terminal end buds (TEBs) at the ends of primitive ducts to generate the main trunks of the mammary epithelial tree (Daniel and Silberstein 2000). Mammary epithelium is comprised of an outer layer of myoepithelial and inner luminal cells. TEBs contain outer cap cells and inner multiple layers of body cells. From morphology and immunostaining analyses, it is thought that cap cells are progenitors of myoepithelial cells, and body cells represent progenitors of luminal cells of the subtending ducts (Daniel and Silberstein 2000; Smalley and Ashworth 2003). The fact that some cap cells appear in body cell layers has led to the hypothesis that cap cells might be MaSCs (Williams and Daniel 1983; Daniel and Silberstein 2000; Smalley and Ashworth 2003; Srinivasan et al. 2003); however, no functional evidence has been presented to address this notion. In the post-pubertal virgin mammary gland, dormant MaSCs are believed to be scattered throughout the epithelial ductwork (Welm et al. 2002; Smith and Medina 2008), while pregnancy lobular development starts with the generation of alveolar buds that grow and terminally differentiate into secretory alveoli to produce milk during lactation (Daniel and Silberstein 2000; Hennighausen and Robinson 2005; Deugnier et al. 2006). Following weaning, the lobulo–alveoli degrade, and the gland remodels to a virgin-like state in preparation for the next pregnancy (Smalley and Ashworth 2003; Hennighausen and Robinson 2005). Although alveolar progenitors may be committed to the alveolar cell fates (Hennighausen and Robinson 2005; Smith and Medina 2008), little is known about MaSCs at pregnancy.
The existence of MaSCs has been demonstrated by cleared mammary fat pad transplantation assays (DeOme et al. 1959; Daniel et al. 1968). MaSCs from adult virgin mouse mammary tissue have been enriched in the CD49fhi, CD29hi, CD24+/mod, and Sca-1−/low subset of basal mammary epithelial cells (Shackleton et al. 2006; Sleeman et al. 2006; Stingl et al. 2006; Lindeman et al. 2008), which are negative for steroid hormone receptors (Asselin-Labat et al. 2006; Sleeman et al. 2007). However, the precise identity of adult MaSCs remains a fundamental question.
The product of the novel s-ship gene was initially identified in embryonic and hematopoietic stem cells, but not in differentiated cells (Tu et al. 2001). We therefore generated a transgenic mouse model (Tg11.5kb-GFP), and found that the 11.5-kb s-SHIP promoter specifically expressed GFP in many stem cell populations, including mammary bud cells, in embryonic development (Rohrschneider et al. 2005). Here we show (Supplemental Fig. 1A) in the postnatal mammary gland that GFP labels puberty cap cells and pregnancy basal alveolar bud cells, and both in vivo and in vitro experiments demonstrate they are activated MaSCs. Similar GFP+ cells are expressed in MMTV-Wnt1 but not ErbB2 mammary tumors. Identification of exact stem cell types and their in situ localization is an essential step toward understanding and using stem cells in medical applications.
Results
GFP is expressed in cap cells at puberty
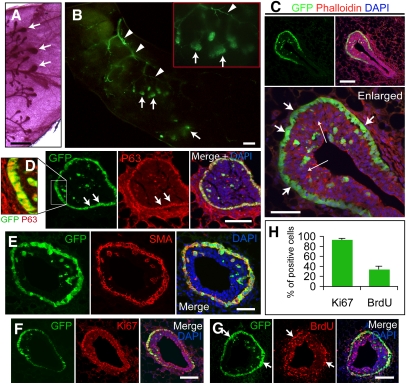
At the beginning of puberty (4 wk of age), GFP expression was detected in TEBs at the distal tips of the growing ducts (Fig. 1A,B). The majority of GFP+ cells were located in the peripheral cap cell layer, and a minor population (16%–18% of total GFP+ cells; n = 20 TEBs) was seen within the inner body cell compartment of the TEBs (Fig. 1C). During ductal elongation, GFP expression remained in the cap cells, but was not detectable in epithelial cells of mature ducts (Fig. 1C; Supplemental Fig. 1C). GFP expression was present neither before puberty in the primitive ducts measured in tissue sections and flow cytometry (Supplemental Figs. 1B, 6C), nor after puberty in the mature ducts (Supplemental Figs. 1D, 6B). Throughout mammary development, a distinct GFP expression pattern was observed in angiogenic blood vessels (Fig. 1B), which we are studying separately. These findings indicate that the 11.5-kb s-SHIP promoter drives GFP expression specifically in cap cells in the mammary gland of puberty Tg11.5kb-GFP female mice. Because cap cells are the putative stem cells (Williams and Daniel 1983; Srinivasan et al. 2003), we characterized these GFP+ cells in more detail.
Figure 1.
GFP expression occurs in cap cells of the TEBs at puberty. (A) TEBs (arrows) in carmine whole-mount-stained 5-wk mammary tissue. (B) GFP+ TEBs (green, arrows) and blood vessels (green, arrowheads) in 4-wk transgenic whole-mount mammary tissue. (C) One section of a 4-wk TEB. Cap cells are GFP+ (short arrows), and some GFP+ cap cells are among the GFP− body cells (long arrows). (Red) Phalloidin-Alexa 594; (blue) DAPI. (D) GFP+ cap cells (green) are also p63+ (red); shown in enlargement (left panel; GFP and p63 only), and separately (middle two panels). Arrows mark the cap cell layer. (E) Cap cells (green) are SMA+ (red). (F) Most GFP+ cap cells are Ki67+ (red). (G) Some GFP+ cap cells were labeled with BrdU (red) within 4 h of BrdU injection. Bars: A,B, 1 mm; C,D,F,G, 100 μm; C (enlarged), E, 50 μm. (H) Histogram shows that 93.8% ± 2.4% of GFP+ cells in TEBs are Ki67+, and 34.6% ± 5.9% of GFP+ cells were labeled with BrdU during a 4-h exposure. Data represent mean ± SD; n = 20 TEBs.
GFP+ cap cells expressed basal/myoepithelial cell markers, including p63 (Fig. 1D) (which is essential for mammary gland development [Mills et al. 1999; Yang et al. 1999] and epithelial stem cell proliferation [Senoo et al. 2007]), α-smooth muscle actin (SMA) (Fig. 1E), low levels of Keratin 14 (K14), and basement membrane component laminin (Supplemental Fig. 2A,B). GFP+ cells were negative for E-cadherin (E-cad), progesterone receptor (PR), and keratin 8 (K8) (Supplemental Fig. 2C–E)—markers prevalent in body/luminal cells (Srinivasan et al. 2003; Asselin-Labat et al. 2006). K8 staining of the body cells was similar to E-cad staining with GFP+ cells surrounded by K8+ and E-cad+ body cells. (Supplemental Fig. 2E). GFP+ cells in the body cell layers shared common markers with those in the outer cap cell layer, supporting previous views that some cap cells migrate into the body cell compartment (Williams and Daniel 1983; Daniel and Silberstein 2000). GFP+ cells were 93.8% ± 2.4% (n = 20 TEBs) positive for proliferation marker Ki67 (Fig. 1F,H), and 34.6% ± 5.9% (n = 20 TEBs) positive for 5-bromo-2′-deoxyuridine (BrdU) within 4 h of labeling (Fig. 1G,H). Many body cells in TEBs were also Ki67+ and BrdU+ (Fig. 1F,G). These data indicate that GFP+ cap cells exhibit a basal cell phenotype and are actively dividing.
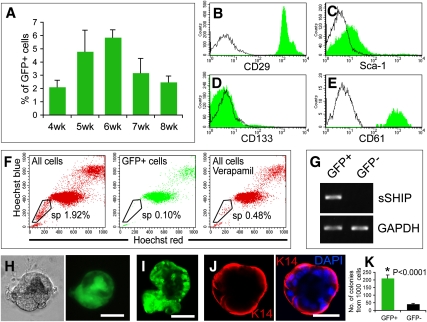
We next examined GFP+ cap cells for markers historically associated with stem/progenitor cells in various tissues. Using the integrin α6/CD49f marker of stem cells (Iwashita et al. 2003; Stingl et al. 2006; Lawson et al. 2007), we first determined that GFP+ cap cells (CD49fhigh) were separable from GFP+ vascular cells (CD49f−/low) (Supplemental Fig. 3A,B). Analyzing lin− mammary cells (excluding CD31+ endothelial and CD45+TER119+ hematopoietic cells) from puberty and prepuberty by flow cytometry, we then identified and isolated GFP+ cap cells as the distinct GFP+CD49fhigh population, whereas the GFP+CD49f−/low cell group corresponded to the GFP+ vascular cells (Supplemental Fig. 3C–E). GFP+ cap cells accounted for 2%–6% of lin− mammary cells in puberty glands (Fig. 2A). GFP+ cap cells were CD29high (integrin β1, a stem cell marker in skin [Jones et al. 1995] and mammary gland [Shackleton et al. 2006]) (Fig. 2B); Sca-1−/low (Fig. 2C); negative for prominin1/CD133 (Fig. 2D), a potential cancer stem cell marker (Singh et al. 2004; Zhu et al. 2009); and positive for integrin β3/CD61 (Fig. 2E), expressed in mammary stem/progenitor cells (Asselin-Labat et al. 2007). GFP+ cells were not in the side population (Fig. 2F) used to enrich mammary progenitors (Welm et al. 2002; Liu et al. 2004).
Figure 2.
Characterization of GFP+ cap cells in puberty. (A) Histogram shows the percentage of lin−GFP+CD49fhigh cap cells in total lin− puberty mammary cells. Results represent mean ± SD of four mice for each group. (B–E) Flow cytometric analysis shows that GFP+CD49fhigh cap cells are CD29high (B), Sca-1−/low (C), CD133− (D), and CD61+ (E). (F) Side population analysis shows that puberty GFP+ cells are depleted in the side population. (G) RT–PCR shows s-SHIP mRNA in sorted GFP+ cells but not in GFP− cells. (H,I) Single GFP+CD49fhigh cap cells form alveolar-like colonies in Matrigel; photographed with a Nikon microscope in situ in Martrigel (phase and GFP) (H), or removed from Matrigel and examined for GFP by confocal microscopy (I). (J) K14+ cells (red) comprise the outer layer of the colony (DAPI-stained for nuclei; blue). Bars: H,J, 20 μm; I, 50 mm. (K) Histogram shows the colony-forming capacity of lin−GFP+CD49fhigh and lin−GFP− cells in Matrigel. Data represent mean ± SD of five independent experiments.
To examine whether GFP+ cells express s-SHIP, RT–PCR was performed on sorted GFP+ and GFP− cells. The results demonstrate that the s-SHIP promoter-driven GFP expression correlates with the endogenous s-SHIP mRNA expression (Fig. 2G). To determine the in vitro growth and differentiation potential of GFP+ cap cells, GFP+CD49fhigh and GFP− cells were sorted from the lin− population and cultured in Matrigel for 12 d. Single lin−GFP+CD49fhigh cells were able to generate colonies, and ∼10%–15% of the colonies expressed GFP (Fig. 2H,I). In most of the GFP+ colonies, only a subfraction of cells were GFP+, as shown in Figure 2, H and I. K14 was strongly expressed in the outer layer of cells, but was not expressed in the inner layer of cells of the colonies (Fig. 2J). Compared with lin−GFP− cells, lin−GFP+CD49fhigh cells exhibited a fivefold higher colony-forming ability in Matrigel (42.0 ± 4.2 vs. 211.2 ± 21.0 colonies per 1000 cells; mean ± SD; P < 0.0001) (Fig. 2K).
Multipotential and self-renewal capability of GFP+ cap cells
To determine whether GFP+ cap cells can regenerate new mammary gland epithelial tissue, freshly sorted lin−GFP+CD49fhigh cells (Supplemental Fig. 3C,D) were transplanted at various dilutions into cleared fat pads of 3-wk-old wild-type C57BL/6J female mice. Lin−GFP− cells were collected from the same tissues and transplanted as a control. Donor cells were harvested from Tg11.5kb-GFP mice or bitransgenic Tg11.5kb-GFP;Rosa26 mice, allowing confirmation of donor origin by staining for constitutive lacZ expression (Friedrich and Soriano 1991). Results of transplantation experiments (Supplemental Table 1A) showed that GFP+ cap cells displayed much higher regenerative ability (10 outgrowths out of 10 injected fat pads when 400 cells were injected), compared with GFP− cells (one out of 10; 400 cells injected); injection of 200 GFP+ cells repopulated 11 out of 12 fat pads, while GFP− cells were unable to produce an outgrowth. Limiting dilution analysis (Shackleton et al. 2006) indicated that the frequency of MaSCs in the GFP+ population was one out of 48, compared with one out of 7570 in GFP− cells (P < 0.0001). Since low numbers of GFP+ cells (three to eight cells) could regenerate outgrowths (Supplemental Table 1A), we performed single GFP+ cell transplantation and obtained two outgrowths from 28 transplants (Supplemental Table 1B).
The frequency of MaSCs in lin− unfractionated cells was one out of 3523 (Supplemental Table 2). Regenerative activity was present in GFP+CD49fhigh cap cells, but not in the GFP+CD49f−/low vascular cells (Supplemental Fig. 4).
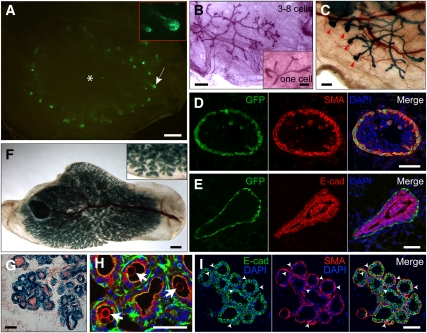
We next investigated the ability of GFP+ cells to form cells and structures of the outgrowths in puberty. Outgrowths from GFP+ cap cells (recipients in puberty) exhibited GFP expression in TEBs as observed in Tg11.5kb-GFP females, except the outgrowth pattern radiated from the injection site (Fig. 3A). An outgrowth from three to eight GFP+ cells and from one single cell are shown in Figure 3B and its inset, respectively. Outgrowths from approximately eight GFP+LacZ+ cells could be transplanted serially, and regenerated outgrowths with TEBs at the tips of ducts in virgin recipients (Fig. 3C). TEBs in the GFP+ cap cell-derived outgrowths were composed of GFP+ SMA+ cap cells (Fig. 3D) and E-cad+ body cells (Fig. 3E). Recipient mice bred for pregnancy exhibited typical outgrowths (GFP+LacZ+ cell-derived) at pregnancy and early lactation stages (Fig. 3F,G). Milk protein was apparent inside alveolar lumen (Fig. 3H). Outgrowth lobulo–alveoli were composed of SMA+ myoepithelial cells and E-cad+ alveolar luminal cells (Fig. 3I). Flow cytometric analysis showed that GFP+ cells in the outgrowths were CD49fhigh and CD24mod, and were present in relative numbers comparable with those seen in Tg11.5kb-GFP female mice (Supplemental Fig. 5). These results indicated that GFP+ cap cells give rise to all structures and cell lineages of the new mammary gland in puberty and pregnancy.
Figure 3.
Characterization of transplantation outgrowths from puberty GFP+ cap cells. (A) GFP+ TEBs (green, arrow) in an outgrowth from 40 GFP+ cap cells; enlarged TEBs are shown in the inset. (*) Approximate injection site. (B) Carmine alum-stained ductal outgrowths from four (three to eight) cells, or one single GFP+ cap cell (inset), respectively. (C) X-gal staining shows the TEBs (arrowheads) in a second serial outgrowth originated from eight (six to 10) primary GFP+LacZ+ cap cells. (D,E) Outgrowth TEBs consist of GFP+ SMA+ cap cells (D) and E-cad+ body cells (E). (F–I) Pregnancy-induced lobulo–alveolar formation in lactating outgrowths (lactation day 0.5) derived from 40 GFP+LacZ+ cap cells. (F) X-gal whole-mount staining shows lobulo–alveolar structures of outgrowth. (G) Frozen section of outgrowth lobulo–alveoli stained with X-gal (blue) and nuclear fast red (red). (H) Antibody staining for milk protein (arrows) in alveolar lumen. (Red) Milk; (green) phalloidin; (blue) DAPI. (I) Outgrowth lobulo–alveoli are composed of SMA+ myoepithelial cells (arrowheads) and E-cad+ alveolar luminal cells. Bars: A,B,F, 1 mm; B (inset), C, 0.25 mm; D,E,G–I, 50 μm.
Interestingly, the few outgrowths obtained from GFP− cells also exhibited GFP+ cap cells in TEBs (data not shown), suggesting the existence of GFP− dormant MaSCs, which turn on GFP expression upon activation. We tested the possibility that GFP+ cells were contained in the GFP− population by resorting GFP− cells freshly isolated by FACS. The results in Supplemental Figure 6A demonstrate that GFP+ cells were not detectable in 104 sorted GFP− cells. Derivation of GFP+ outgrowths from GFP− cells was further confirmed by transplantation of lin−CD24+CD49fhiGFP− mammary cells from adult virgin mice (Supplemental Fig. 6B). This indicates that GFP− cells transplanted into cleared fat pads must have turned on GFP expression in forming an epithelial outgrowth.
To assess the self-renewal ability of GFP+ cap cells, serial transplantations of 100 and approximately eight GFP+ cap cells were performed. Supplemental Table 1C shows that all injected fat pads were repopulated with epithelial outgrowths in the second, third, and fourth serial transplants. These data demonstrate that GFP+ cap cells possess not only the activity of regeneration and differentiation, but also the capacity of self-renewal.
GFP+ cells in alveolar buds at pregnancy
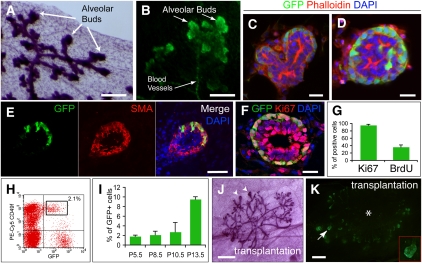
After puberty, GFP expression was not detected in mammary epithelial cells of adult virgin transgenic mice examined in tissue sections and flow cytometry (Supplemental Figs. 1D, 6B). However, in pregnancy, GFP expression was observed in apparent alveolar buds along the ductal network (Fig. 4A,B), and was restricted mainly to the outer layer of cells and the tips of alveolar buds (Fig. 4C,D). A few GFP+ cells were also present in the luminal cell layers (Fig. 4D). Expression of GFP was detected in the alveolar buds as early as pregnancy day 2.5 (P2.5) (Fig. 4E) and was retained until P15.5, when GFP turned off in all mammary epithelial cells for the duration of late pregnancy. Expression was seen in neither terminally differentiated lobulo–alveolar cells at P17.5, nor lactation, nor involution stages of mammary tissues (Supplemental Fig. 1E–G).
Figure 4.
Identification and characterization of GFP+ cells in alveolar buds at pregnancy. (A–D) Alveolar buds in P11.5 transgenic mammary tissues. Carmine whole-mount staining shows alveolar buds along the ductal network (A), GFP expression (green) in the apical alveolar buds, and some blood vessels (B). Frozen sections show that GFP is expressed mainly in the peripheral cells, especially the tips of the alveolar buds (C,D), and some GFP+ cells are seen in the inner layer of the alveolar buds (D). (E) GFP+ cells in P2.5 mammary tissue are SMA+ (red). (F) Most GFP+ cells in P7.5 alveolar bud are Ki67+ (red). (G) Histogram shows that 91.5% ± 5.9% of GFP+ cells at pregnancy are Ki67+, and 27.7% ± 10.9% of GFP+ cells were labeled with BrdU within 4 h of injection. Data represent mean ± SD; n = 20 alveolar buds. (H) Flow cytometric analysis of lin− mammary cells from P5.5 mammary tissues. GFP+ cells consist of CD49fhigh alveolar bud cells (gate) and CD49f −/low blood vessel cells. (I) Histogram shows the percentage of GFP+CD49fhigh alveolar bud cells in the total lin− population during pregnancy. Results represent mean ± SD of four mice for each group. (J) Carmine alum staining of an outgrowth from 100 pregnancy GFP+ cells shows the TEBs (arrowheads) at the ductal ends. (K) GFP+ TEBs in the outgrowth of 50 pregnancy GFP+ cells. (*) Approximate injection site. Bars: A,B, 200 μm; C,D,F, 20 μm; E, 50 μm; J,K, 1 mm.
Pregnancy GFP+ epithelial cells were SMA+ (Fig. 4E), p63+, laminin+, K14low, CD49fhigh, and E-cad− (Supplemental Fig. 7A–E). Most GFP+ cells (91.5% ± 5.9%; n = 20 alveolar buds) were stained with Ki67 (Fig. 4F,G), and 27.7% ± 10.9% (n = 20 alveolar buds) of GFP+ cells were labeled with BrdU within 4 h (Fig. 4G; Supplemental Fig. 7F), suggesting these cells are highly proliferative. GFP+CD49fhigh alveolar bud cells from early pregnancy comprised ∼2% of the lin− population, and the number increased to ∼9.5% in midpregnancy (Fig. 4H,I). Pregnancy GFP+CD49fhigh cells were CD24mod (Supplemental Fig. 8), CD29high, Sca-1−/low, CD133−, and CD61+, and not side population cells (Supplemental Fig. 9A–E). s-SHIP mRNA was expressed only in GFP+ cells (Supplemental Fig. 9F). Single pregnancy GFP+ cells in Matrigel formed alveolar-like colonies, composed of an outer layer of integrin α6+ and inner integrin α6− cells. GFP+ cells showed a more than threefold higher colony-forming ability than GFP− cells (194.2 ± 15.5 vs. 52.6 ± 4.0 colonies per 1000 cells; mean ± SD; P < 0.0001) (Supplemental Fig. 9G–I).
Taken together, GFP+ alveolar bud cells in pregnancy displayed properties similar to GFP+ cap cells in puberty.
GFP+ alveolar bud cells possess MaSC activity
Limiting dilution studies showed that GFP+ alveolar bud cells exhibited marked regenerative capacity, and low numbers of cells (four to six cells) could reconstitute the ductal network (Supplemental Table 3A; Supplemental Fig. 10A). The frequency of MaSCs in pregnancy GFP+ cells was one out of 79, compared with one out of 1948 in GFP− cells (P < 0.0001) (Supplemental Table 3A), and one out of 1750 in lin− unfractionated cells (Supplemental Table 4), indicating that MaSCs are much more abundant in the GFP+ population compared with GFP− (24-fold) or unfractionated cells (22-fold).
Transplanted into pubertal fat pads, pregnancy GFP+ cells regenerated ductal epithelial trees with TEBs, which expressed GFP (Fig. 4J,K). Outgrowth TEBs contained GFP+ SMA+ cap cells and E-cad+ body cells (Supplemental Fig. 10B,C). Lobulo–alveoli were formed in lactating outgrowths derived from GFP+lacZ+ pregnancy cells (Supplemental Fig. 10D–G), and alveoli produced milk (Supplemental Fig. 10H). Outgrowth alveoli consisted of SMA+ myoepithelial cells and E-cad+ alveolar luminal cells (Supplemental Fig. 10I). These data demonstrate that pregnancy GFP+ cells can regenerate a fully functional mammary gland and contribute to all cell types during puberty and pregnancy development. Finally, serial transplantation indicated complete capabilities for self-renewal of GFP+ alveolar bud cells (Supplemental Table 3B).
Direct comparison of GFP+ MaSCs with isolates characterized previously
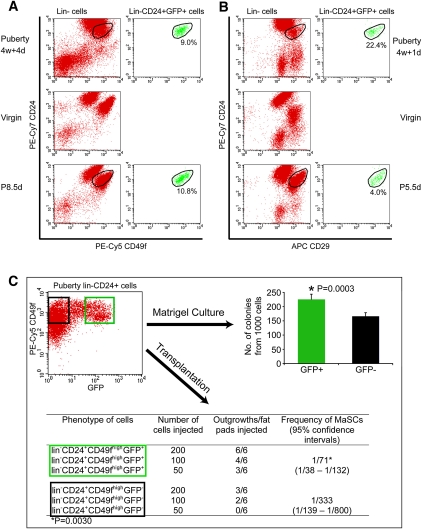
The GFP+ cap cell population was compared directly with the MaSCs isolated by the Eaves (Stingl et al. 2006) and Visvader (Shackleton et al. 2006) groups. In each case, the results (Fig. 5A,B) demonstrate that our lin−GFP+CD49f+ mammary cap cell fraction represents a precise subpopulation of the MaSCs identified with Lin−CD24modCD49fhigh and lin−CD24modCD29high phenotypes from the respective laboratories. In puberty, the GFP+ fraction comprised 9% of the former population and 22% of the latter. GFP expression is not yet known to occur in the virgin phase of mammary development, and therefore a full comparison cannot be made between the GFP+ cells and those of the other groups, which obtained MaSCs from virgin epithelial ducts. In addition, the colony-forming capability of lin−CD24+CD49fhighGFP+ cells was also significantly higher than that of lin−CD24+CD49fhighGFP− cells (P = 0.0003) (Fig. 5C). The characteristics of GFP+ mammary epithelial cells are consistent with the phenotype and stem cell nature of mammary epithelial cell fractions isolated by others, but represents a subfraction of the MaSCs isolated by multiple parameters in other laboratories.
Figure 5.
Comparison of GFP+ mammary epithelial cells with previously identified lin−CD24modCD49fhigh and lin−CD24modCD29high MaSC phenotypes. (A) Mammary cells from puberty (4w + 4d), adult virgin (6m), and pregnancy (P8.5d, 6m) were stained and analyzed simultaneously. The left panels show that the distributions of lin− mammary cells from different developmental stages based on CD24 and CD49f expression level are quite different. Unlike in virgin mammary cells, there are no clearly distinguishable CD24highCD49flow and CD24modCD49fhigh populations in puberty (4w + 4d) lin− mammary cells. The right panels show that GFP+ epithelial cells account for 9.0% and 10.8% of the overlapped lin−CD24modCD49fhigh cells in puberty (4w + 4d) and in pregnancy (P8.5d), respectively. Lin−CD24modCD49fhighGFP+ cells are not present in virgin transgenic mammary cells. (B) Mammary cells from puberty (4w + 1d), adult virgin (6m), and pregnancy (P8.5d, 6m) were stained and analyzed simultaneously. The left panels show the differences among the distribution of lin− mammary cells from different stages according to CD24 and CD29 expression. Clearly distinguishable CD24highCD29low and CD24modCD29high populations do not exist in puberty (4w + 4d) lin− mammary cells. The right panels show that GFP+ epithelial cells represent 22.4% and 4.0% of the overlapped lin−CD24modCD29high cells in puberty (4w + 1d) and pregnancy (P5.5d), respectively. (C) lin−CD24+CD49fhighGFP+ and lin−CD24+CD49fhighGFP− cells were sorted from pubertal mammary glands of 5-w + 1-d-old transgenic mice (shown in the dot plot), and were cultured in Matrigel or transplanted into cleared fat pads. The histogram shows the colony-forming capability of lin−CD24+CD49fhighGFP+ cells is significantly higher than that of lin−CD24+CD49fhighGFP− cells. Data represent mean ± SD of five independent experiments. The table shows lin−CD24+CD49fhighGFP+ cells possess significantly higher regenerative potential than lin−CD24+CD49fhighGFP− cells upon transplantation. The latter GFP− population may contain dormant MaSCs.
We also examined transplantations of lin−GFP+ mammary epithelial cells contained within CD49fhigh and CD24+ fractions (Shackleton et al. 2006; Sleeman et al. 2006; Stingl et al. 2006). These experiment showed that lin−CD24+CD49fhighGFP+ cap cells possessed a significantly higher regenerative potential than lin−CD24+CD49fhighGFP− cells (one out of 71 vs. one out of 333 MaSC frequency; P = 0.003) (Fig. 5C).
Comparing our GFP+ cells and lin−CD24modCD49fhigh or lin−CD24modCD29high populations, we observed large differences in the distribution of cell populations between puberty, virgin, and pregnancy mammary cells. Regardless, although GFP was not present in the virgin mammary cells, GFP+ cells exhibited a relatively consistent distribution, and represented 11% and 4% of the lin−CD24modCD49fhigh and lin−CD24modCD29high populations, respectively (Fig. 5A,B).
These results demonstrate that the s-SHIP promoter expression-marked GFP+ MaSC populations have properties similar to MaSCs defined in other laboratories. The single parameter isolation of activated MaSCs in puberty and pregnancy offers a facile one-step method for isolation of these cells, and, importantly, GFP expression enables identification of MaSCs in vivo.
GFP+ cells in breast tumors
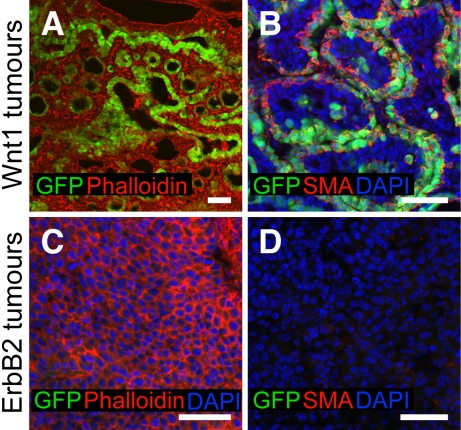
We investigated GFP expression in different breast tumors from bitransgenic 11.5kb-GFP;MMTV-Wnt1 and 11.5kb-GFP;MMTV-ErbB2/neu/HER2 female mice. Wnt1 was reported to expand the MaSC-enriched population, and Wnt1-induced tumors consist of both myoepithelial and luminal cells (Li et al. 2003; Liu et al. 2004; Shackleton et al. 2006), whereas ErbB2 does not increase the number of MaSCs, and ErbB2-driven tumors contain primarily luminal cells (Li et al. 2003; Shackleton et al. 2006; Stingl and Caldas 2007). Our results showed that a large number (8%–20%) of GFP+SMA+ basal cells were detectable in Wnt1-inducted tumors (Fig. 6A,B). In contrast, ErbB2-induced tumors appeared devoid of GFP+ cells (Fig. 6C,D). The expansion of GFP+ basal cells in Wnt1 tumors supports previous results that Wnt1 specifically stimulates MaSCs growth (Li et al. 2003; Liu et al. 2004; Shackleton et al. 2006; Stingl and Caldas 2007), and illustrates the potential utility of the Tg11.5kb-GFP mouse model in addressing critical questions on the roles of MaSCs in mammary tumor formation.
Figure 6.
GFP expression in breast tumors. (A,B) GFP is expressed in Wnt1 tumors (A) and GFP+ cells are SMA+ (B). (C,D) No GFP expression was detected in ErbB2 tumors (C), and no SMA+ cells were detected in ErbB2 tumors (D). Bars, 50 μm.
Discussion
Our data demonstrate that the cap cells at puberty and basal alveolar bud cells at early–midpregnancy are activated MaSCs that self-renew and contribute to all cell lineages of the mammary gland during development. GFP expression occurs in mammary buds at embryonic development (Rohrschneider et al. 2005), TEBs at puberty, and alveolar buds in pregnancy mammary glands of the Tg11.5kb-GFP mice, but not in mature cells of the mammary gland or at other developmental stages such as virgin, lactation, involution, and post-involution, in which MaSCs are relatively quiescent (Welm et al. 2002; Smith and Medina 2008). Therefore, we propose that s-SHIP promoter expression labels the actively functioning MaSCs in the developing mammary gland, but not dormant MaSCs or differentiated cells. Our results suggest that MaSCs, dormant in prepubertal and virginal epithelial ducts, become activated when required for development in puberty and pregnancy. Dormant (i.e., GFP−) MaSCs are also present in puberty, probably along the completed portions of the epithelial ductwork, and in the pregnancy gland as well. Similar active (proliferating) stem cells have also been reported in the mouse intestine (Barker et al. 2007), the growth phase (anagen) of hair follicles (Blanpain et al. 2007; Jaks et al. 2008), and stimulated/injured bone marrow (Wilson et al. 2008).
Although transplantation is used routinely to identify MaSCs, it is not a quantitative assay due to technical limitations. Cell survival and functional activity of MaSCs may be compromised, owing to loss of cell viability caused by digestion and FACS-sorting stress (Vaillant et al. 2008), unavoidable cell loss during transplantation (Shackleton et al. 2006), and absence of the original stem cell niche. In addition, the GFP+ cell population may contain some heterogeneity, composed of GFP+ MaSCs and some relatively differentiated GFP+ progenitor cells, which may have recently turned off GFP expression but yet remain GFP+ due to the long half-life of GFP (24 h). This suggests that the actual MaSC activity may be higher than observed by the results of transplantation.
An hierarchy of MaSCs comprised of ductal stem cells for puberty morphogenesis and alveolar stem/progenitor cells for lobulo–alveolar formation during pregnancy has been proposed (Smalley and Ashworth 2003; Hennighausen and Robinson 2005; Smith and Medina 2008). Our results, however, support the idea that MaSCs from different developmental stages have identical multipotential capabilities for producing all mammary cell types, including new MaSCs, committed progenitors (Smith 1996; Kordon and Smith 1998), and mature differentiated cells in puberty and pregnancy. For instance, GFP+ alveolar bud cells, in the process of forming lobulo–alveoli, can switch to the pubertal developmental program and generate branched ductal networks along with TEBs when placed into pubertal fat pads (results in Fig. 4J,K). This suggests that MaSC fate determination is regulated by distinct niche/hormonal signals within each developmental stage, and is consistent with stromal cell signals providing instructive roles in mammary cell fate determination and morphogenesis during development (Hennighausen and Robinson 2001; Wiseman and Werb 2002). That mammary microenvironment reprograms testicular and neural stem cells also reflects the inductive function of niche signals (Boulanger et al. 2007; Booth et al. 2008). In addition, the niche is a critical factor in cancer development (Bissell and Labarge 2005). Thus, in both normal and aberrant development, the mammary niche/hormonal microenvironment plays a dominant role in lineage determination.
The isolation of adult stem cells in general—and MaSCs in particular—using multichannel flow cytometry is subject to variability from limited antibody specificity and technical variance (Alexander et al. 2009). In addition, the distribution of mammary tissue cells, based on cell surface marker expression, changes with the developmental phase (see Fig. 5A,B). Identification of MaSCs by GFP expression, however, is remarkably reproducible, and, although dormant MaSCs have not yet been detected in virgin ducts by GFP expression, we are working on methods to overcome this limitation. In addition, the coexpression of s-SHIP mRNA in activated GFP+ MaSCs suggests that s-SHIP may play a critical role in stem cell function. Therefore, isolation of MaSCs by GFP expression offers clear advantages of facile, reproducible isolation, along with the opportunity for functional analysis of s-SHIP in stem cells.
The Tg11.5kb-GFP mouse has enabled us to visualize the MaSCs and the compartments in which they reside. Basal-like human breast cancers are thought to develop from MaSCs (Yehiely et al. 2006; Stingl and Caldas 2007; Liu et al. 2008). Therefore, analyses of stem cells in mammary gland development and tumor formation in the Tg11.5kb-GFP mouse will provide significant insights into the regulatory mechanisms of MaSCs, and has major implications in developing new strategies to treat breast cancers.
Materials and methods
Mice
Tg11.5kb-GFP mice were backcrossed >10 generations onto a C57BL/6J background and genotyped by PCR (Rohrschneider et al. 2005). MMTV-Wnt1 mice were from Dr. C. Alexander, and MMTV-ErbB2 mice were from Dr. W. Muller. Animals were handled in accordance with the Guidelines for Animal Experiments at the Fred Hutchinson Cancer Research Center (FHCRC).
Mammary cell preparation
No. 3 and No. 4 mammary glands were dissected from 4- to 6-wk-old female mice for puberty, and P5.5–P8.5 at 10–24 wk of age for pregnancy experiments. The lymph node was removed from the No. 4 gland. Fresh tissue was minced to a paste with scissors and placed into culture medium (DMEM/F12 [Sigma] containing 1 mM glutamine [GIBCO-BRL], 5 μg/mL insulin [Sigma], 1 μg/mL hydrocortisone [StemCell Technologies, Inc.], 10 ng/mL epidermal growth factor [Sigma], and 20 ng/mL cholera toxin [Sigma]) supplemented with 5% fetal bovine serum (FBS), and incubated with 300 U/mL collagenase IA (Sigma) and 100 U/mL hyaluronidase (Sigma) for 1.5 h at 37°C. The resultant cells/organoids were dissociated sequentially in 0.25% trypsin-EDTA for 2–3 min, and 5 mg/mL dispase (StemCell Technologies, Inc.) and 0.1 mg/mL DNase I (StemCell Technologies, Inc.) for 1 min, then 4 vol 0.8% NH4Cl (StemCell Technologies, Inc.) was added for 3 min, followed by filtration through a 40-μm cell strainer. For serial transplantation of unsorted cells, the cells/organoids of one lactating outgrowth were resuspended in 160 μL of Hanks' Balanced Salt Solution (HBSS) with 2% FBS.
Flow cytometry, antibodies, and cell sorting
Single cells were preincubated with mouse Fc block-purified rat anti-CD16/CD32 Fcγ III/II receptor antibody (1:50; BD Pharmingen) for 5 min at 4°C, stained with antibodies for 20 min at 4°C, washed, and resuspended in HBSS containing 10 mM HEPES, 2% FBS, and 1 μg/mL propidium iodide (PI) or 1 μg/mL DAPI before analysis. Antibodies used are as follows: PE-Cy5 rat anti-human CD49f (1:100; BD Pharmingen), PE rat anti-mouse CD24 (1:100; BD Pharmingen), PE-Cy7 rat anti-mouse CD24 (1:200; BD Pharmingen), PE Armenian hamster anti-mouse CD29 (1:200; BioLegend), APC Armenian hamster anti-mouse CD29 (1:100; eBioscience), PE rat anti-mouse Sca-1 (1:100; eBioscience), PE Armenian hamster anti-mouse CD61 (1:100; eBioscience), and PE rat anti-mouse prominin 1/CD133 (1:100; eBioscience); and lineage markers PE rat anti-mouse CD31 (1:100; eBioscience), PE rat anti-mouse CD45 (1:200; BD Pharmingen), PE rat anti-mouse TER-119 (1:200; eBioscience); and isotype control PE rat IgG (1:100; BD Pharmingen), PE Armenian hamster IgG (1:200; BioLegend), PE-Cy5 Rat IgG (1:100; BD Pharmingen), PE-Cy7 rat IgG2b (1:200; BD Pharmingen), and APC Armenian hamster IgG (1:100; eBioscience). The single live cells were gated and analyzed or sorted on FACSVantage or FACSAria flow cytometers. The purity of sorted populations was ≥95%. Mammary cells from age-matched or pregnancy day-matched wild-type C57BL/6J mice were always collected and stained as control. Side population analysis was performed as described previously (Goodell et al. 1996).
Cleared fat pad transplantation
Cells were sorted into the wells of 96-well round-bottom ultralow binding plates (Nunc) containing HBSS with 2% FBS, recounted in 0.2% Trypan blue solution (Sigma), and diluted. The inguinal (No. 4) glands of 3-wk-old female C57BL/6J mice were cleared of endogenous epithelium as described previously (DeOme et al. 1959). Cells in 10 μL of HBSS with 2% FBS were injected into each cleared fat pad.
Single-cell transplantation
Three-hundred microliters of cell suspension (1 × 102 cells per milliliter) was placed in a 10-cm bacterial dish, spread to an ∼3.0-cm-diameter area, and set on ice with the dish covered for ≥40 min to let cells settle down. Using a dissection microscopy, single sorted cells were identified and picked using Micro Pipettes (Fisher), and injected. In control experiments, one cell in each drop of the Micro Pipettes was confirmed in 50 out of 50 cases.
Transplantation analysis
Recipient glands were collected at 5–10 wk after surgery, and at ≥12 wk after transplantation of 10 or fewer cells. Most recipients were mated to induce lobulo–alveolar development. A positive outgrowth was defined as a complete epithelial structure containing ducts radiating from the central injection site with elaborated branches and/or TEBs (at puberty) or lobulo–alveoli (at pregnancy and lactation). Outgrowths were detected by GFP expression and carmine alum whole-mount (Rasmussen et al. 2000) or X-gal staining. Frozen sections were analyzed by immunostaining or X-gal staining.
Matrigel cell culture
Cells were resuspended in 100% Matrigel (Becton Dickinson), and a 50-μL suspension of Matrigel per cells was placed into each well of eight-well coverslip bottom chambers (Nunc) and allowed to settle for 20 min at 37°C before covering with culture medium supplemented with 5% FBS. After 48 h, the medium was changed to DMEM/F12 containing 1 mg/mL fatty acid-free bovine serum albumin (BSA) (Sigma), 5 μg/mL insulin, 5 μg/mL prolactin (Sigma), 1 μg/mL progesterone (Sigma), 1 μg/mL hydrocortisone, and 10 ng/mL epidermal growth factor. Cells were cultured for another 10 d, and then fixed in 4% paraformaldehyde and stained with antibodies as described (Debnath et al. 2003).
BrdU incorporation
Mice were injected i.p. with 100 μg/g BrdU (Sigma) 4 h prior to sample collection (Barker et al. 2007).
Immunofluorescence and antibodies
Tissues were fixed in 2% paraformaldehyde for 4 h, put into 30% sucrose overnight, embedded in O.C.T., frozen on dry ice, and cut into 14-μm sections. Tissue sections were permeabilized with 0.5% Triton X-100, blocked with 5% FBS, and then incubated sequentially with primary antibodies for 45 min at room temperature, and with secondary antibodies for 45 min at room temperature. Slides were washed three times in phosphate-buffered saline (PBS) after each antibody incubation, and sections were mounted in Prolong Gold (Invitrogen). For BrdU staining, sections were treated with 2 N HCl for 30 min to denature DNA before permeabilization. M.O.M kit (Vector) was used for detecting mouse primary antibodies. Primary antibodies used for this study were Alexa Fluor 488-conjugated rabbit anti-GFP (1:800; Invitrogen), Alexa Fluor 594 phalloidin (1:100; Molecular Probes), Alexa Fluor 488 phalloidin (1:100; Molecular Probes), mouse monoclonal anti-p63 (1:100; Santa Cruz Biotechnology), mouse monoclonal Cy3-conjugated anti-α-SMA (1:800; Sigma), rabbit anti-laminin general (1:10,000; a kind gift of Dr. William Carter), rabbit anti-mouse K14 (1:1000; Covance), affinity-purified rat anti-human/mouse integrin α6 (1:100; eBioscience), rabbit polyclonal anti-Ki67 (1:400; Abcam), Alexa Fluor 594-conjugated mouse anti-BrdU (1:400; Molecular Probes), rat anti-E-cad (1:200; Zymed), rabbit polyclonal anti-human PR (1:100; DakoCytomation), and rabbit polyclonal antiserum to mouse milk-specific proteins (1:400; Nordic Immunological Laboratories). Fluorochrome-conjugated secondary antibodies included Alexa Fluor 594-conjugated goat anti-rabbit IgG, Alexa Fluor 488-conjugated goat anti-rabbit IgG, Alexa Fluor 594-conjugated goat anti-rat IgG, Alexa Fluor 488-conjugated goat anti-rat IgG, and Alexa Fluor 594-conjugated goat anti-mouse IgG (all 1:1000; Molecular Probes). Sections were counterstained with DAPI (1 μg/mL; Sigma). Pictures were taken on a Zeiss LSM 510 META Confocal Microscope (Carl Zeiss MicroImaging, Inc.)
X-gal staining
Whole-mount mammary tissue was fixed in 2% formaldehyde, 0.25% glutaraldehyde, and 0.01% Nonidet P-40 for 1 h at 4°C, stained with 1 mg/mL X-gal overnight at 37°C for outgrowth of lacZ+ cell transplantation, then dehydrated and cleared in xylene before imaging. Frozen sections from fixed tissues were stained with X-gal and counterstained with nuclear fast red.
RT–PCR analyses
RT–PCR was performed using gene-specific primers with the One-Step RT–PCR kit (Qiagen) according to the manufacturer's instructions with some modifications. Twenty cells were sorted into the well of a 96-well low-binding plate containing 20 μL of RNA sample buffer (0.5% Nonidet P-40, 1× one-step RT buffer, 1 U/μL RNase inhibitor in RNase free H2O). Eight microliters of RNA sample was used for s-SHIP gene amplification, and 2 μL of RNA sample was used for GAPDH. A second round of PCR was performed with the same primers of each gene using the first round of PCR product as a template, 6 μL for s-SHIP, and 0.5 μL for GAPDH, respectively. Primer sequences were as follows: s-SHIP forward, 5′-GTTCCCACTAGTTGTTGAACTTTACCTT-3′; s-SHIP reverse, 5′-CAACGTCCACTTTGAGATGCAT-3′; GAPDH forward, 5′-AAGGTCATCCCAGAGCTGAACGG-3′; GAPDH reverse, 5′-TGAGGGAGATGCAGTGTTGGG-3′. All primers spanned more than one intron, and positive and negative control amplifications were performed in parallel to ensure no contaminating genomic DNA was present.
Statistics
MaSC frequency was calculated using a complementary log–log binomial generalized linear model with 95% confidence intervals, using limdil software as described previously (Shackleton et al. 2006; Quintana et al. 2008). The _P_-value was determined by likelihood ratio test. The single-hit assumption was tested with standard χ2 statistics, and was not rejected for any dilution series (P > 0.05). Colony-forming capability in Matrigel was compared with two-tailed Student's _t_-test.
Acknowledgments
We thank C. Alexander for the MMTV-Wnt1 mice, W. Miller for the MMTV-ErbB2 mice, William Carter for the laminin general antibody, and Cecelia Moens and Bruce Edgar for use of their confocal microscope and assorted digital microscopes, respectively. Members of the Rohrschneider laboratory offered valuable discussions, and the Shared Resources at the Center (Image Analysis, Flow Cytometry, Animal Health Resources) provided essential, expert, and helpful assistance throughout this project. We thank Yan Liu (FHCRC) for the statistics assistance. We give special thanks to Mark Shackleton, Bryan Welm, and Matthew Smalley for helpful advice. We thank Roland Bourette, Jim Roberts, and Jon Cooper for critical review and helpful suggestions on the manuscript. This work was supported by interim funds from the FHCRC, NIH/NCI grants R56 CA123360 and R01 CA40987, and financial support from anonymous donors.
Footnotes
References
- Alexander CM, Puchalski J, Klos KS, Badders N, Ailles L, Kim CF, Dirks P, Smalley MJ 2009. Separating stem cells by flow cytometry: Reducing variability for solid tissues. Cell Stem Cell 5: 579–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ 2006. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 98: 1011–1014 [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. 2007. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9: 201–209 [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Labarge MA 2005. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell 7: 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E 2007. Epithelial stem cells: Turning over new leaves. Cell 128: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH 2008. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci 105: 14891–14896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Mack DL, Booth BW, Smith GH 2007. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci 104: 3871–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB 2000. Working with the mouse mammary end bud. In Methods in mammary gland biology and breast cancer research (ed. Ip MM, Asch BB), pp. 155–162 Kluwer Academic/Plenum, New York [Google Scholar]
- Daniel CW, DeOme KB, Young JT, Blair PB, Faulkin LJ Jr 1968. The in vivo life span of normal and preneoplastic mouse mammary glands: A serial transplantation study. Proc Natl Acad Sci 61: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS 2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30: 256–268 [DOI] [PubMed] [Google Scholar]
- DeOme KB, Faulkin LJ Jr, Bern HA, Blair PB 1959. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 19: 515–520 [PubMed] [Google Scholar]
- Deugnier MA, Faraldo MM, Teuliere J, Thiery JP, Medina D, Glukhova MA 2006. Isolation of mouse mammary epithelial progenitor cells with basal characteristics from the Comma-Dβ cell line. Dev Biol 293: 414–425 [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P 1991. Promoter traps in embryonic stem cells: A genetic screen to identify and mutate developmental genes in mice. Genes Dev 5: 1513–1523 [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC 1996. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW 2001. Signaling pathways in mammary gland development. Dev Cell 1: 467–475 [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW 2005. Information networks in the mammary gland. Nat Rev Mol Cell Biol 6: 715–725 [DOI] [PubMed] [Google Scholar]
- Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ 2003. Hirschsprung disease is linked to defects in neural crest stem cell function. Science 301: 972–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R 2008. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM 1995. Stem cell patterning and fate in human epidermis. Cell 80: 83–93 [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH 1998. An entire functional mammary gland may comprise the progeny from a single cell. Development 125: 1921–1930 [DOI] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON 2007. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci 104: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. 2003. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci 100: 15853–15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman GJ, Visvader JE, Smalley MJ, Eaves CJ 2008. The future of mammary stem cell biology: The power of in vivo transplants. Breast Cancer Res 10: 402 doi: 10.1186/bcr.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, Alexander CM 2004. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci 101: 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS 2008. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci 105: 1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A 1999. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398: 708–713 [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ 2008. Efficient tumour formation by single human melanoma cells. Nature 456: 593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SB, Young LJ, Smith GH 2000. Preparing mammary gland whole mounts from mice. In Methods in mammary gland biology and breast cancer research (ed. Ip MM, Asch BB), pp. 82–83 Kluwer Academic/Plenum, New York [Google Scholar]
- Rohrschneider LR, Custodio JM, Anderson TA, Miller CP, Gu H 2005. The intron 5/6 promoter region of the ship1 gene regulates expression in stem/progenitor cells of the mouse embryo. Dev Biol 283: 503–521 [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F 2007. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129: 523–536 [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE 2006. Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB 2004. Identification of human brain tumour initiating cells. Nature 432: 396–401 [DOI] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ 2006. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res 8: R7 doi: 10.1186/bcr1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ 2007. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol 176: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley M, Ashworth A 2003. Stem cells and breast cancer: A field in transit. Nat Rev Cancer 3: 832–844 [DOI] [PubMed] [Google Scholar]
- Smith GH 1996. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat 39: 21–31 [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D 2008. Re-evaluation of mammary stem cell biology based on in vivo transplantation. Breast Cancer Res 10: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L 2003. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell 4: 371–382 [DOI] [PubMed] [Google Scholar]
- Stingl J, Caldas C 2007. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer 7: 791–799 [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ 2006. Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997 [DOI] [PubMed] [Google Scholar]
- Tu Z, Ninos JM, Ma Z, Wang JW, Lemos MP, Desponts C, Ghansah T, Howson JM, Kerr WG 2001. Embryonic and hematopoietic stem cells express a novel SH2-containing inositol 5′-phosphatase isoform that partners with the Grb2 adapter protein. Blood 98: 2028–2038 [DOI] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE 2008. The mammary progenitor marker CD61/β3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res 68: 7711–7717 [DOI] [PubMed] [Google Scholar]
- Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA 2002. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol 245: 42–56 [DOI] [PubMed] [Google Scholar]
- Williams JM, Daniel CW 1983. Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol 97: 274–290 [DOI] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. 2008. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129 [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z 2002. Stromal effects on mammary gland development and breast cancer. Science 296: 1046–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. 1999. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398: 714–718 [DOI] [PubMed] [Google Scholar]
- Yehiely F, Moyano JV, Evans JR, Nielsen TO, Cryns VL 2006. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med 12: 537–544 [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ 2009. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]