CD25+CD4+ Regulatory T Cells from the Peripheral Blood of Asymptomatic HIV-infected Individuals Regulate CD4+ and CD8+ HIV-specific T Cell Immune Responses In Vitro and Are Associated with Favorable Clinical Markers of Disease Status (original) (raw)
Abstract
Human immunodeficiency virus (HIV) disease is associated with loss of CD4+ T cells, chronic immune activation, and progressive immune dysfunction. HIV-specific responses, particularly those of CD4+ T cells, become impaired early after infection, before the loss of responses directed against other antigens; the basis for this diminution has not been elucidated fully. The potential role of CD25+CD4+ regulatory T cells (T reg cells), previously shown to inhibit immune responses directed against numerous pathogens, as suppressors of HIV-specific T cell responses was investigated. In the majority of healthy HIV-infected individuals, CD25+CD4+ T cells significantly suppressed cellular proliferation and cytokine production by CD4+ and CD8+ T cells in response to HIV antigens/peptides in vitro; these effects were cell contact dependent and IL-10 and TGF-β independent. Individuals with strong HIV-specific CD25+ T reg cell function in vitro had significantly lower levels of plasma viremia and higher CD4+: CD8+ T cell ratios than did those individuals in whom this activity could not be detected. These in vitro data suggest that CD25+CD4+ T reg cells may contribute to the diminution of HIV-specific T cell immune responses in vivo in the early stages of HIV disease.
Keywords: cytokine, proliferation, human, suppression, FoxP3
Introduction
HIV-1 infection is characterized by a progressive loss of CD4+ T cells, chronic immune activation, and an increasing array of immune dysfunctions (1–3). HIV-specific T cell immune responses are particularly impaired, and defects appear earlier in disease as compared with responses directed at other microbial antigens (4–6). The basis of HIV-specific T cell immune dysfunction has not been elucidated fully, and it is likely that both HIV-specific and nonspecific mechanisms are involved (7–12). In this regard, one avenue that has not been explored fully in relation to HIV-specific immune responses is the potential role of host-mediated immunosuppressive mechanisms that might be activated in the face of persistent antigenic exposure.
CD25+CD4+ suppressor/regulatory T cells (T reg cells), a cellular subset with constitutive immunosuppressive activity first described in the context of autoimmune disorders (13, 14), are one of several cellular subsets involved in controlling inappropriate or excessive immune activation (15–18). CD25+CD4+ T reg cells have been demonstrated to suppress antigen-specific CD4+ and CD8+ T cell responses directed against tumors (19, 20) and allografts (21, 22) as well as against parasitic (23–25), bacterial (26, 27), fungal (28, 29), and viral (30–32) antigens or infections in vivo in mice. Furthermore, the immunosuppressive activity of CD25+CD4+ T reg cells has been implicated in the inability of mice to clear infection with certain pathogens (23, 29–32) or to mount effective responses to vaccination (26). Although studies on CD25+CD4+ T reg cells have been conducted primarily in murine systems, a population with a virtually identical phenotypic and functional profile has been described in humans (33). The present paper presents evidence supporting the potential role of CD25+CD4+ T reg–mediated immunosuppression in the diminution of HIV-specific CD4+ and CD8+ T responses in asymptomatic HIV-infected individuals.
Materials and Methods
Purification of Cellular Populations.
Phlebotomy (National Institutes of Health [NIH] protocol no. 91-I-0140) or apheresis (NIH protocol no. 81-I-164) was performed on uninfected control individuals (n = 20) or on HIV-infected, untreated, or antiretroviral therapy-treated individuals (n = 52; see Table I). PBMCs, obtained by Ficoll-Hypaque density gradient centrifugation, were either depleted of CD25+ cells using anti-CD25–coupled immunomagentic beads (Miltenyi Biotec) to obtain CD25-depleted PBMCs or exposed to a cocktail of immunomagnetic beads (StemCell Technologies Inc.) to obtain CD4+ T cells (>95% purity) by negative selection. For lymphocyte proliferation assays (LPAs), CD4+ memory T cells were obtained by depleting CD45RA+ cells from CD4+ T cells using anti-CD45RA mAb (BD Biosciences) coupled to goat anti–mouse immunomagnetic beads (Dynal); subsequently, CD25+ and CD25−CD4+ memory T cell subsets were obtained using anti-CD25–coupled immunomagentic beads (Miltenyi Biotec). CD25 expression in CD25-depleted and CD25+CD4+ subpopulations was <5% and ≥87%, respectively (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20032069/DC1). Autologous APCs were γ-irradiated (5,000 R) CD2− or CD25− PBMCs. CD8+ cells were isolated by positive selection using anti-CD8–coupled immunomagnetic beads (Dynal); beads were removed using DETACHaBEAD (Dynal). Autologous CD4+CD25− T cells were used as the HIV-producing target cell population in assays to determine HIV-induced proliferation of CD8+ effector T cells. Flow cytometric quantification of cellular subsets was performed using fresh PBMCs surfaced stained with mAbs recognizing CD3, CD4, CD25, CD45RO, CD103, or CD122, or stained for intracellular CTLA-4 (BD Biosciences).
Table I.
Clinical and Functional Characteristics of HIV-infected Subjects
| Patient ID | CD4+ Tc | CD8+ Tc | CD4:CD8 ratio | Viral load | Change in p24 SIa | Percent CD25hi b |
|---|---|---|---|---|---|---|
| cells/μL | cells/μL | HIV RNA copies/ml | ||||
| All HIV+ subjectsc | ||||||
| Mean | 543 | 1,001 | 0.6 | 14,275 | 3.2 | 6.0 |
| SD | 259 | 474 | 0.4 | 21,845 | 3.2 | 2.7 |
| Geometric mean | 481 | 907 | 0.5 | 2,031 | 2.2 | 5.4 |
| [A] LPA suppressorsd | ||||||
| Mean | 619 | 956 | 0.8 | 5,839 | 4.2 | 6.2 |
| SD | 270 | 478 | 0.4 | 9,977 | 3.3 | 2.8 |
| Geometric mean | 565 | 844 | 0.7 | 821 | 3.5 | 5.6 |
| Range | 252–1,380 | 218–2,400 | 0.3–1.8 | <50–29,000 | 1.6–18.5 | 2.1–11.9 |
| [B] LPA nonsuppressorse | ||||||
| Mean | 476 | 1,186 | 0.4 | 20,112 | 0.9 | 5.5 |
| SD | 211 | 448 | 0.2 | 25,950 | 0.4 | 2.4 |
| Geometric mean | 417 | 1,121 | 0.4 | 6,175 | 0.8 | 5.0 |
| Range | 250–741 | 750–2,344 | 0.2–0.7 | <50–86,888 | 0.3–1.4 | 2.3–9.2 |
| [C] Nonrespondersf | ||||||
| Mean | 361 | 1,117 | 0.4 | 34,319 | NA | 4.9 |
| SD | 248 | 638 | 0.3 | 11,909 | NA | 1.7 |
| Geometric mean | 308 | 969 | 0.4 | 29,867 | NA | 4.5 |
| Range | 160–781 | 461–2,093 | 0.1–1.1 | <50–79,000 | NA | 2.2–7.3 |
| p-value | ||||||
| A vs. B | 0.110 | 0.161 | 0.006 | 0.012 | NA | 0.45 |
| A vs. C | 0.026 | 0.990 | 0.133 | 0.0001 | NA | 0.19 |
| B vs. C | 0.370 | 0.272 | 0.593 | 0.260 | NA | 0.51 |
LPA.
PBMCs or memory CD4+ T cell subsets were plated at 1–2 × 105 cells/well in media (RPMI 1640 supplemented with 1 mM glutamine, antibiotics, and Hepes buffer) plus 10% human AB serum. CD25− and CD25+CD45RA−CD4+ T cells were cultured alone or at a 1:1 ratio. Autologous CD25− PBMCs (APCs) were γ-irradiated (4,000 R) and added at a ratio of 1:1 (APCs:total T cells) to wells containing CD4+ memory T cell populations. Cells were either untreated or exposed to 5–10 μg/ml HIV-1 p24 (Protein Sciences). In certain experiments neutralizing 0.5 μg/ml of mouse anti–human IL-10 (R&D Systems), 10 μg/ml of chicken anti–human TGF-β (R&D Systems) or isotype control antibodies were added to cultures; in addition, transwell inserts were used to separate CD25+ and CD25−CD4+ T cells to assess the requirement for cell–cell contact. Supernatant was harvested and frozen for later assessment of cytokine production, and at day 6, cells were exposed to 0.5 μCi/well [3H]thymidine (PerkinElmer) for 16 h to assess cellular proliferation. In a subset of subjects, CD25+ or CD25−CD45RA−CD4+ T cells (104–105) were added to 105 CD25−CD45RA−CD4+ T cells/well and stimulated with a pool of allogeneic γ-irradiated PBMCs (2 × 105), and proliferation was assessed at day 5. Proliferation stimulation indexes (SI) were calculated by dividing the cpm obtained in antigen-stimulated conditions by the cpm obtained in unstimulated (background) conditions.
Intracellular or Secreted Cytokine Production.
For assessment of HIV p24-induced intracellular cytokine (ICC) expression in CD4+ T cells, 106 PBMCs, CD25− PBMCs, or CD45RA− CD4+ subpopulations (plus 20% CD2− PBMCs) were untreated or exposed to 10 μg/ml HIV-1 p24 for 4–5 h. 1 μL/ml Golgi Plug (BD Biosciences) was added for an additional 9–10 h, and cells were harvested and stained for surface markers and intracellular IL-2 or IFN-γ as per manufacturer's recommendations (BD Biosciences). CD8+ and CD4+ T cells present in unfractionated or CD25− PBMCs were tested for rapid cytokine production (6 h) in response to a pool of overlapping 15-mer Gag peptides (AIDS Reagent Repository) as described previously (5). Assessment of antigen or anti-CD3 (immobilized 10 μg/ml)–induced supernatant-associated levels of cytokines and chemokines was performed using Multiplex assay systems (Biosource International or Upstate Biotechnology); assays were read using a Luminex reader (Mirabio). TGF-β levels were assessed in supernatants from cells cultured in media plus 1% FCS and stimulated with 10 μg/ml of immobilized anti-CD3 using a TGF-β ELISA (R&D Systems).
Proliferation of Effector (Perforin+) CD8+T Cells in Response to Autologous HIV Super-infected Target Cells.
CD25+ or CD25− CD4+ T cells, CD8+ T cells, and autologous CD2-depleted PBMCs (APCs) were isolated as aforementioned. A portion of CD25−CD4+ T cells to serve as targets were exposed to VSV env-pseudotyped (Δ nef-luciferase) HIV virus (single round replication competent) at a multiplicity of infection of 0.5 for 6–14 h and washed three times. Purified CD8+ T cells were labeled with the fluorescent dye carboxyfluorscein diacetate succinimidyl ester (CFSE) by standard methods. Uninfected or VSV env-pseudotyped HIV super-infected target cells were cultured with CFSE-labeled CD8+ T cells alone or together with CD25+ or CD25− CD4+ T cells at a ratio of 0.05–0.2:1:1, respectively, with 20% CD2− PBMCs in media plus 10% human AB serum. Cells were stained for CD69, CD3, or CD8 and intracellular perforin 5–11 d after coculture as described previously (34).
Assessment of FoxP3 Expression by Western Blot.
Total cellular lysate from 5 × 105 freshly isolated PBMC subsets were run on 10% Tris/Glycine polyacrylamide gels. Proteins were transferred to nitrocellulose membranes (Pierce Chemical Co.), and membranes were probed with polyclonal rabbit anti–human FoxP3 antisera followed by horseradish peroxidase–conjugated goat anti–rabbit secondary Ab (Cell Signaling); signal detection was achieved with SuperSignal West Pico chemiluminescent substrate (Pierce Chemical Co.).
Online Supplemental Material.
Fig. S1 shows the purity of isolated CD25− and CD25+CD4+ T cell subsets; cells were gated on CD3+ T cells (>97%) and assessed for surface CD4 and CD25 expression. Fig. S2 depicts the mean net cpm ± SD obtained in LPAs after exposure of CD4+ T cell subsets from p24 LPA suppressors and nonsuppressors to 10 μg/ml HIV p24. Fig. S3 shows the mean net cpm ± SD obtained in LPAs after exposure of CD4+ T cell subsets from p24 LPA suppressors, nonsuppressors, and nonresponders to Candida albicans. Proliferative responses of CD4+ memory T cell subsets to C. albicans were performed using 5 μg/ml of C. albicans (Sigma Aldrich). Fig. S4 depicts the mean ± SD copies of HIV DNA present in fresh CD25+ versus CD25−CD4+ T cells isolated from seven HIV-infected donors. HIV DNA levels were determined by quantitative real-time PCR. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20032069/DC1.
Results
Characterization of CD25+CD4+ T Cells in the Peripheral Blood of HIV-infected and Uninfected Individuals.
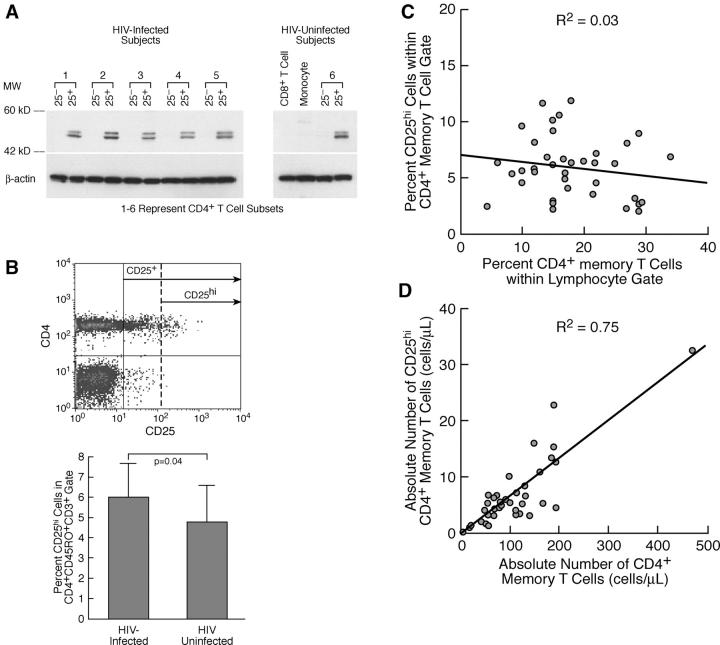
Human CD25+CD4+ T cells have been shown to be a heterogeneous population that includes immunosuppressive T reg cells, anergic but nonsuppressive T cells, and normal activated T cells (35, 36). Until very recently (37), no surface markers had been reported to be uniquely expressed on suppressive CD25+CD4+ T reg cells, making identification and quantification of this population difficult. However, the cellular transcription factor FoxP3 has been demonstrated to be functionally relevant to immunosuppressive activity of CD25+CD4+ T cells in both mice and humans (38–42). In this regard, fresh CD25+, but not CD25−, CD4+ T cells from both HIV-infected and uninfected individuals expressed FoxP3, and there were no clear qualitative differences in FoxP3 expression between these two groups of individuals (Fig. 1 A).
Figure 1.
CD25+CD4+ T cells in PBMCs from HIV-infected individuals express phenotypic markers consistent with CD25+ T reg cells: quantification of total CD25+CD4+ T reg cells in PBMCs of HIV-infected subjects by surface expression of CD25+hi. (A) FoxP3 protein expression, detected by Western blot, is comparable in fresh CD25+CD4+ T cells isolated from HIV-infected versus HIV-uninfected individuals Fresh CD25−CD4+ T cells, CD8+ T cells, and monocytes do not express FoxP3. (B, top) Representative example of FACS® profile and gates used to quantify CD25+hiCD4+CD45RO+ T cells. Freshly isolated PBMCs were gated on CD45RO+CD3+ and analyzed for CD4 and CD25 expression. CD25+hi gates were set so that ≤0.05% of all PBMC subsets, other than CD4+ T cells, fell to the right of the CD25+hi gate (dashed line). (bottom) Comparison of the frequency of CD25+hi cells within the CD4+CD45RO+ T cell subset of PBMCs from HIV-infected (n = 38) versus uninfected (n = 20) subjects. (C) The frequency of and the (D) absolute number of CD25+hi versus total CD4+ memory T cells in PBMCs isolated from 38 HIV-infected individuals representing a broad percentage range of CD4+ memory T cells.
In addition, the expression of high-density CD25 has been correlated, in numerous studies, with immunosuppressive T reg cell activity in human CD4+CD25+ T cells (35, 36, 43–45). Using this marker, we attempted to quantify total CD25+CD4+ T reg cells in PBMCs from HIV-infected (n = 38) and uninfected (n = 20) individuals. PBMCs from HIV-infected and uninfected individuals contained similar frequencies of total CD25+ cells in the memory (CD45RO+) CD4+ T cell population (P > 0.05; unpublished data); however, the frequency of cells expressing high density CD25 (Fig. 1 B, top) was modestly but significantly elevated in PBMCs of HIV-infected individuals (Fig. 1 B, bottom). The frequency of CD25hi cells in PBMCs of HIV+ subjects remained relatively constant over a broad range of memory CD4+ T cell percentages (Fig. 1 C), and the absolute numbers of this population declined proportionately with the loss of CD4+ T (memory) cells and disease progression (Fig. 1 D). In both HIV-infected and uninfected subjects, CD25+CD45RO+CD4+ T cells expressed significantly (P < 0.002) higher levels, as compared with their CD25− counterparts, of surface markers (CD103, CD122, and intracellular CD152 [CTLA-4]) shown previously to be preferentially expressed on human CD25+CD4+ T cells that exhibit T reg cell–like suppressive activity (references 35, 43, 45–48 and unpublished data).
Functional Characterization of CD25+CD45RA−CD4+ T Cells Isolated from HIV-infected Individuals: Suppression of HIV-specific and HIV-nonspecific CD4+ T Cell Proliferation In Vitro.
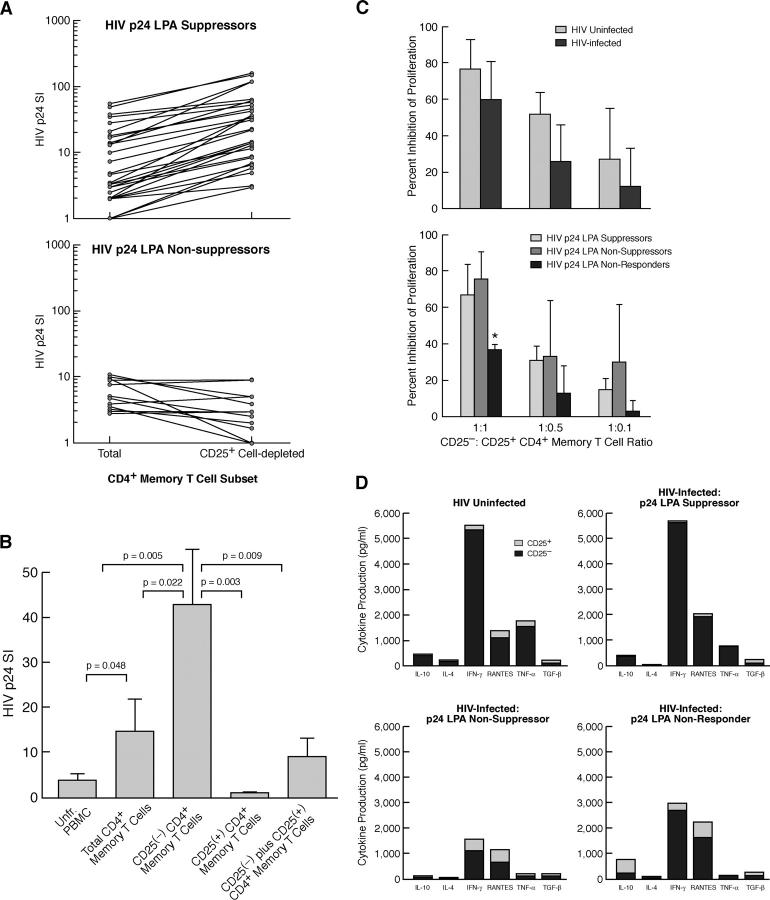
HIV p24 LPAs were performed using PBMCs and CD4+CD45RA−/RO+ (memory) T cell subsets (total vs. CD25+ subset depleted) from 52 HIV-infected individuals (Table I and Fig. 2 A). In experiments with PBMCs isolated from a portion of individuals (10/52; referred to as HIV p24 LPA nonresponders) the SI to HIV p24 failed to exceed a value of three under any cellular subset condition (unpublished data). In experiments with cellular subsets from the remaining individuals (n = 42) that did proliferate in response to HIV p24, CD25+ subset depletion of memory CD4+ T cells led to an average increase in the p24 SI of 3.2-fold (P = 0.0003; Table I). However, within this group, we observed two distinct patterns concerning the suppressive effect of the CD25+ subset on HIV p24 proliferative responses. Using a cut-off index of >1.5-fold enhancement of the p24 SI after CD25+ subset depletion from the total of memory CD4+ T cell population, individuals were separated into two groups as follows: those with strong (HIV p24 LPA suppressors) or those with no or weak (HIV p24 LPA nonsuppressors) CD25+ subset suppressor effect. Approximately 31% of the individuals tested (13/42) fell under the category of HIV p24 LPA nonsuppressors (mean ± SD HIV p24 SI of total memory CD4+ T cells vs. CD25− memory CD4+ T cells = 5.3 ± 3.4 vs. 4.6 ± 3.3, respectively; P = NS; Table I and Fig. 2 A, bottom). The majority of p24 responsive individuals (69%; 29/42) were HIV p24 LPA suppressors (mean ± SD HIV p24 SI of total memory CD4+ T cells vs. CD25− memory CD4+ T cells = 10.6 ± 16 vs. 44.7 ± 44, respectively; P = 0.0001; Table I and Fig. 2 A, top). No significant difference was found in the HIV p24 (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20032069/DC1) or the C. albicans (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20032069/DC1) proliferative response (mean net cpm) of total CD4+ memory T cells from HIV p24 LPA nonsuppressors as compared with HIV p24 LPA suppressors. In more extensive HIV p24 LPA analyses conducted with cells from 19 HIV p24 LPA suppressors, CD25+CD45RA−CD4+ cells were found to be anergic to stimulation with HIV p24 and the addition of this population to the CD25− memory population significantly suppressed HIV p24-induced proliferation of the latter memory subset (Fig. 2 B).
Figure 2.
Functional analyses of the CD25+ memory CD4+ T cell subset from HIV-infected individuals. (A) Comparison of the HIV p24 proliferation SI of total versus CD25+ cell subset-depleted CD4+ memory T cells isolated from HIV-infected individuals with strong (top, HIV p24 LPA Suppressors) or no/weak (bottom, HIV p24 LPA Non-suppressors) HIV-specific CD25+ T reg cell suppressor activity. The geometric mean HIV p24 SI of total and CD25+ cellular subset-depleted populations from LPA suppressors versus LPA nonsuppressors was 5.6 and 20.4 versus 5.3 and 3.6, respectively. (B) HIV p24 SI in more extensive LPA assays using PBMCs and CD4+ memory T cell subsets from LPA suppressors (n = 19). Data are represented as mean HIV p24 SI ± SD. (C) Comparison of the suppressive activity of CD25+CD4+ memory T cells isolated from (top) HIV-uninfected individuals (n = 6) versus all HIV+ subjects (n = 9) and (bottom) from HIV+ subjects comparing HIV p24 LPA suppressors, nonsuppressors, and nonresponders (n = 3 for each category) under polyclonal (pool of allogeneic PBMCs) stimulation conditions. Only CD25+ memory CD4+ T cells from HIV p24 LPA nonresponders exhibited significantly (P ≤ 0.04) weaker polyclonal T reg cell activity as compared with HIV-uninfected subjects or other groups of HIV+ subjects. Data are represented as mean ± SD percent inhibition of control (CD25− subset) SI. (D) Comparison of cytokine/chemokine production by anti-CD3–stimulated CD25+ and CD25− subsets of CD4+ memory cells isolated from HIV-uninfected individuals (n = 6) and HIV p24 LPA suppressors, nonsuppressors, and nonresponders (n = 4 for each category).
Individuals within the different HIV p24 LPA response categories were found to differ significantly in regard to several clinical parameters of HIV disease progression. Those individuals who displayed strong HIV-specific CD25+ CD4+CD45RA− T cell suppressor activity in vitro (HIV p24 LPA suppressors) had significantly lower viral loads and higher CD4+:CD8+ T cell ratios or CD4+ T cell counts than HIV p24 nonsuppressors and nonresponders (Table I). These observations support the hypothesis that HIV-specific CD25+ T reg cell activity is associated with immune competency and that HIV disease–related factors may reduce the activity of and/or the sensitivity to HIV-specific CD25+CD4+ T reg cells.
To assess whether the suppressive activity of CD25+ CD4+ T cells isolated from HIV-infected donors was also variable in the context of HIV nonspecific stimulation, CD25+CD45RA−CD4+ T cells isolated from a subset of individuals (n = 9 HIV+ and n = 6 HIV− subjects) were tested for their ability to suppress proliferation under polyclonal stimulation conditions (allogeneic PBMCs). No significant differences (P > 0.05) were observed in the suppressive activity of CD25+CD45RA−CD4+ T cells from uninfected versus HIV-infected subjects (as a group) (Fig. 2 C, top). However, upon segregation of HIV+ subjects based on their p24 LPA designation, CD25+ cells from HIV p24 LPA nonresponders exhibited significantly lower polyclonal suppressive activity than each of the other groups (P < 0.05; Fig. 2 C, bottom).
Cytokine Production Profiles of CD25+ and CD25− CD45RA−CD4+ T Cells.
Consistent with previous papers (35, 46, 47, 49), anti-CD3–stimulated CD25+ CD45RA−CD4+ T cells from HIV p24 LPA suppressors and uninfected subjects produced dramatically lower levels of cytokines/chemokines, but similar levels of TGF-β, as compared with the CD25− subset (Fig. 2 D). Although not statistically significant (P = 0.07), there was a trend for higher cytokine production by the CD25+ subset from HIV p24 LPA nonsuppressors and nonresponders as compared with LPA suppressors and HIV− subjects (Fig. 2 D). Of interest, the CD25− subset isolated from HIV p24 LPA nonsuppressors produced significantly lower levels of IL-10, TNF-α, and IFN-γ as compared with CD25− cells isolated from HIV p24 LPA suppressors, suggesting that the function of the normal (CD25−) memory CD4+ T cell population may differ between these groups.
CD25+CD45RA−CD4+ T Cells Suppress HIV p24 Proliferative Responses Via a Cell Contact–dependent and IL-10/TGF-β–independent Mechanism.
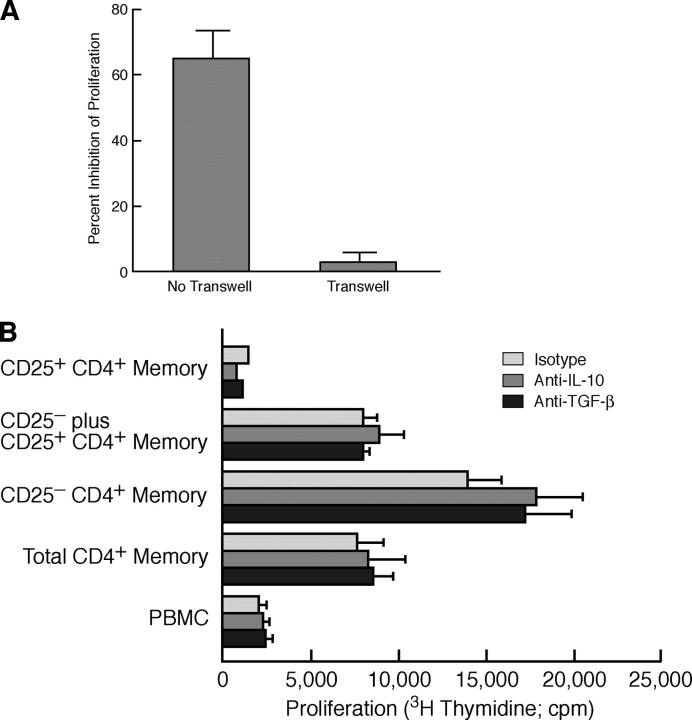
Immunosuppression mediated by CD25+CD4+ T reg cells has been reported to be cell contact dependent, IL-10 independent and, depending on the experimental system, independent or partially dependent on TGF-β (50–53). To determine whether CD25+CD45RA−CD4+ cell-mediated suppression of HIV p24-induced proliferation occurred via classic T reg cell mechanisms, HIV p24 LPA assays using cells from LPA suppressors were performed either in the context of transwells or in the presence of neutralizing anti–human IL-10 or anti–human TGF-β antibodies. Separation of CD25+ and CD25−CD45RA−CD4+ T cells with transwells completely eliminated the suppressive effects of the CD25+ subset (Fig. 3 A), but neither anti–IL-10 nor anti–TGF-β neutralizing antibodies significantly abrogated suppression (Fig. 3 B).
Figure 3.
CD25+CD4+ memory T cell–mediated suppression of HIV p24-stimulated proliferation is cell contact dependent and IL-10 and TGF-β independent. (A) CD25− ± CD25+ memory CD4+ T cells from four p24 LPA suppressors were stimulated with HIV p24 (plus 10% CD2− PBMCs) either together (no transwell) or separated by a 0.4 μM transwell insert to prevent cell–cell contact. Data are presented as mean ± SD percent inhibition of HIV p24-stimulated proliferation achieved in the presence, as compared with the absence, of the CD25+ subset. (B) A representative example of an HIV p24 LPA assay of PBMC subsets isolated from an HIV p24 LPA Suppressor conducted in the presence or absence of neutralizing anti–human IL-10 or TGF-β antibodies; data are presented as mean cpm ± SD of triplicate wells.
CD25+ PBMCs Suppress HIV Gag Protein or Peptide-induced Cytokine Production by CD4+ and CD8+ T Cells.
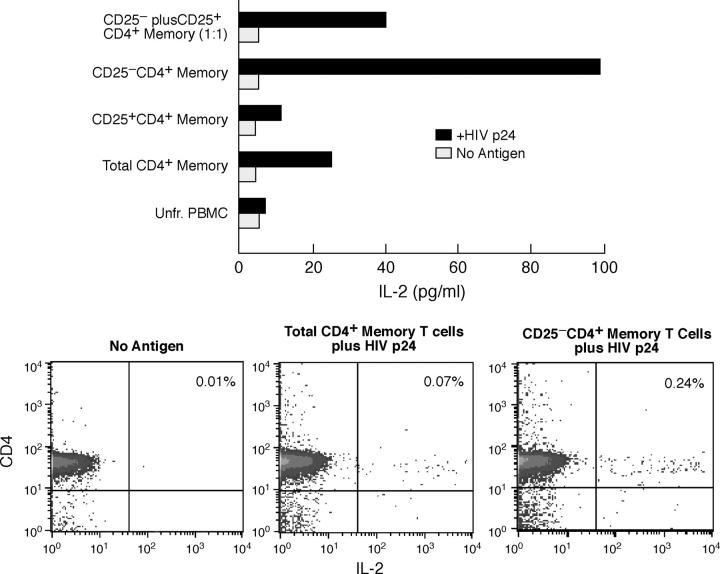
The ability to inhibit IL-2 production by CD4+ T cells is one of the first activities ascribed to CD25+CD4+ T reg cells (54). As expected, inhibition of HIV p24-stimulated memory CD4+ T cell proliferation by CD25+CD45RA− CD4+ T cells from HIV p24 LPA suppressors was associated with reduction in antigen-induced IL-2 secretion; furthermore, the CD25+ subset itself did not produce IL-2 in response to HIV p24 (Fig. 4 A). To rule out the possibility of IL-2 absorption by CD25+ cells, ICC flow cytometry was performed (Fig. 4 B). Sufficient numbers of cellular subsets were obtained in eight p24 LPA suppressor and five p24 LPA nonsuppressor subjects to perform parallel LPA and IL-2 ICC assays using p24 protein. Considering all HIV+ subjects, depletion of the CD25+CD4+ T cell subset resulted in a mean fold increase in the frequency of HIV antigen-induced IL-2+ cells of 2.6 ± 2.0 (Table II). Although the frequency of total CD4+ memory T cells producing IL-2 in response to HIV antigen was not significantly lower in HIV p24 LPA nonsuppressors as compared with suppressors (unpublished data), there was a significant difference between these two groups (P = 0.04) in regard to the enhancing effect of CD25+ cellular subset depletion on this activity (Table II). A similar enhancement was observed in regard to HIV antigen-induced IFN-γ production by CD4+ T cells after CD25+ cellular subset depletion, however, statistical significance was not achieved (Table II). No significant IL-2 or IFN-γ production was observed in the CD25+ memory CD4+ T cell subset in any experiments (unpublished data).
Figure 4.
Suppression of HIV p24-stimulated proliferation by CD25+CD4+ memory T cells is associated with inhibition of IL-2 production. (A) IL-2 levels present in supernatants of an HIV p24 LPA using CD4+ memory subsets isolated from an HIV p24 LPA suppressor. Data are representative of 19 independent experiments; the limit of detection for this assay was 15 pg/ml. (B) IL-2 ICC assay of total versus CD25+ cell-depleted memory CD4+ T cells (plus 10% CD2− PBMCs as APCs) isolated from an HIV p24 LPA suppressor stimulated with p24 protein for 13 h. Data are representative of eight independent experiments.
Table II.
Effect of CD25+ Cell Depletion on HIV Gag-induced Cytokine Production by CD4+ and CD8+ T Cells
| Change in frequency of ICC+ cellsa | |||
|---|---|---|---|
| CD8+ IFN-γ+ | CD4+ IFN-γ+ | CD4+ IL-2+ | |
| All HIV+ subjects | |||
| Mean ± SD | 2.1 ± 1.4 | 2.1 ± 1.3 | 2.6 ± 2.0 |
| Frequency of subjects w/ increased ICC+ cells | 10/17 | 6/11 | 9/13 |
| LPA suppressors | |||
| Mean ± SD | 2.5 ± 1.6 | 2.1 ± 1.5 | 3.5 ± 2.1 |
| Frequency of subjects w/ increased ICC+ cells | 9/12 | 5/7 | 8/8 |
| LPA nonsuppressors | |||
| Mean ± SD | 1.2 ± 0.4 | 1.9 | 1.4 ± 0.7 |
| Frequency of subjects w/ increased ICC+ cells | 1/5 | 1/4 | 1/5 |
| p-valueb | 0.020 | NS | 0.042 |
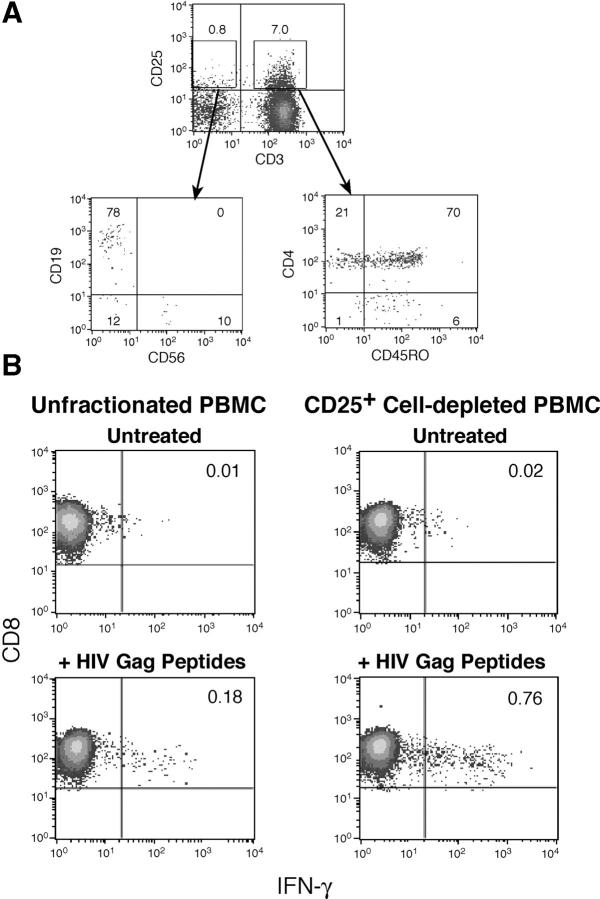
CD25+ CD4+ T reg cells have also been found to potently inhibit antigen-specific CD8+ T cell functions such as cytokine production, proliferation, and cytolysis (26, 31, 32, 55, 56). In the present paper, the effect of CD25+ cell depletion on HIV Gag peptide-induced IFN-γ production was assessed in PBMCs and CD25+ cellular subset-depleted PBMCs isolated from 17 HIV-infected individuals. As the CD25+ PBMC subset is composed primarily of CD45RO+ CD4+ T cells (Fig. 5 A), depletion of PBMCs based solely on CD25 expression closely reflects the removal of CD25+ memory CD4+ T cells as seen when more purified populations are used. As reported previously (56), depletion of the CD25+ cellular subset from PBMCs of HIV-infected individuals (analyzed as a group) resulted in an increase in the percentage of CD8+ T cells producing IFN-γ in response to a pool of HIV Gag peptides (Table II). However, similar to data obtained in CD4+ T cell functional assays, enhancement was observed almost exclusively with cells from HIV p24 LPA suppressors (Fig. 5 B), and there was a significant difference between HIV p24 LPA suppressors and nonsuppressors for this effect (P = 0.02; Table II).
Figure 5.
Depletion of CD25+ cells enhances the frequency of CD8+ T cells producing IFN-γ in response to HIV Gag peptides. (A) CD25+ PBMCs are primarily CD4+ memory T cells. PBMCs were stained for CD25 and CD3; CD3+ and CD3−CD25+ populations (top) were analyzed (bottom) for the expression of other markers identifying the major PBMC cellular subsets. Approximately 90% of CD25+ PBMCs are CD3+ and the majority of these are CD4+CD45RO+ cells (bottom right); the remainder of the CD25+ PBMCs are primarily CD19+ B cells (bottom left). Data are representative of 25 independent experiments. (B) The frequency of CD8+ T cells producing IFN-γ after 6 h of exposure to HIV Gag peptides in unfractionated versus CD25+ cell-depleted PBMCs isolated from an HIV p24 LPA suppressor. Data are representative of nine independent experiments.
CD25 + CD4 + T Cells Suppress HIV-induced Proliferation of Perforin-expressing CD8 + T Cells. One of the most distinctive qualitative features of CD8+ T cells from HIV-infected individuals who effectively control viral replication in vivo (long-term nonprogressors [LTNPs]), as compared with those with progressive disease, is the potent proliferative capacity of the HIV-specific CD8+ effector (perforin+) T cell population after exposure to autologous HIV-infected cells (34). In the present paper, the effect of the CD25+ CD4+ T cell subset on this activity was tested in cells isolated from six HIV p24 LPA suppressors and two LTNPs. CD8+ T cells proliferating (CFSElo) in response to HIV super-infected target cells were largely confined to the CD69+ population (Fig. 6 A, left) and, as reported previously (34), were exclusively associated with perforin expression (Fig. 6 A, right). The percentage of perforin+ CD8+ T cells that had undergone proliferation in response to HIV super-infected autologous target cells was significantly (P = 0.03) reduced in cultures containing CD25+, as compared with CD25−CD4+ T cells or CD8+ T cells alone (Fig. 6, A, right, and B, left). As expected, a greater proportion of CD8+ T cells from HIV-infected LTNPs proliferated in response to HIV-infected target cells than did CD8+ T cells from HIV p24 LPA suppressors with normal disease progression (Fig. 6 B, right); however, statistical analyses of the suppressive effect of CD25+CD4+ T cells in experiments with cells isolated from LTNPs was not possible due to the low number of subjects tested.
Figure 6.
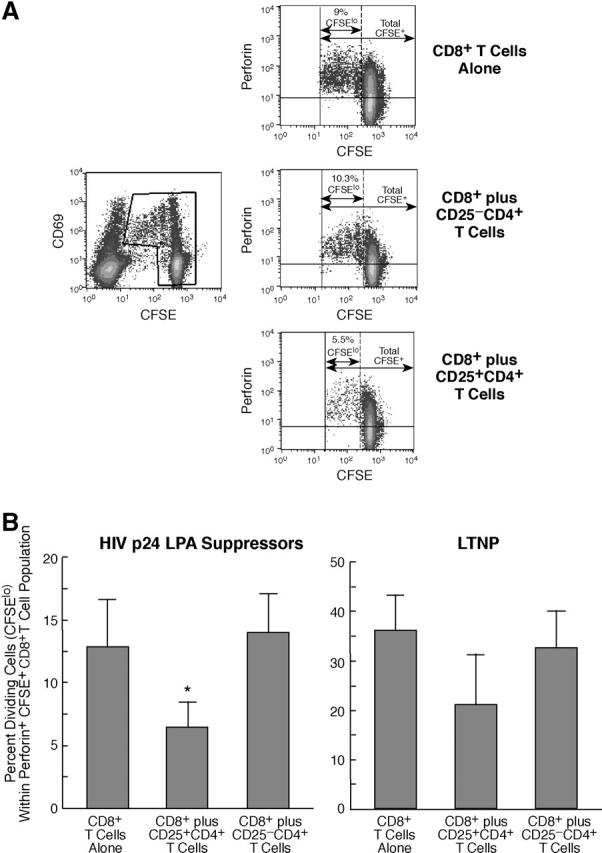
CD25+, but not CD25−, CD4+ T cells suppress the proliferation of perforin-expressing effector CD8+ T cells cultured in the presence of autologous HIV super-infected target cells. (A) HIV super-infected target cells were added to CFSE-labeled CD8+ T cells alone, CFSE-labeled CD8+ T cells plus CD25−CD4+ T cells, or CFSE-labeled CD8+ T cells plus CD25+CD4+ T cells. Cultures were stained periodically for CD69 and CD3, and CFSE intensity was assessed over the course of 3–10 d after coculture (left). At the time by which at least four division cycles could be visualized (6–10 d), CD8 + cells from each culture condition were analyzed for perforin expression and for cellular division by CFSE (right). Values representing the percent CFSElo (dividing cells) perforin+ cells within the total CFSE+ perforin+ cellular population are indicated. Data are representative of six independent experiments conducted using cells from HIV p24 LPA suppressors. (B) Mean ± SD percent dividing cells (CFSElo) within the perforin+ CFSE+ (CD8+) T cell population in experiments with cells isolated from HIV p24 LPA suppressors with normal HIV disease progression (left, n = 6) or from long-term nonprogressors (LTNPs; right, n = 2). In experiments with cells from HIV p24 LPA suppressors, perforin+ CD8+ T cells cultured with CD25+CD4+ T cells proliferated to a significantly lesser degree in response to HIV-infected target cells than did those with target cells alone or those cultured with CD25−CD4+ T cells (*, P < 0.03).
Discussion
The present paper presents evidence supporting the potential role of host-mediated immunosuppressive mechanisms, specifically CD25+CD4+ regulatory T cells, in the diminution of HIV-specific CD4+ and CD8+ T responses in asymptomatic HIV-infected individuals. HIV-infected individuals whose CD25+CD4+ T cells did not demonstrate HIV-specific T reg cell activity in vitro had significantly higher levels of plasma viremia and lower CD4+ T cell counts or CD4+:CD8+ T cell ratios than did individuals with strong HIV-specific T reg cell activity. These data suggest that CD25+CD4+ T reg cell–mediated immunosuppression may play a role in the diminution of HIV-specific CD4+ and CD8+ T cell responses early in disease but that other factors associated with HIV infection obscure or reduce this activity in individuals with more progressed disease.
The primary purpose of the present paper was to determine whether HIV-specific CD25+ T reg cells are present in infected individuals and, if so, to characterize their suppressive activity in regard to CD4+ and CD8+ HIV-specific T cell immune responses. Due to the low frequency of CD25+CD4+ T reg cells and the weak cytokine production by this population, quantifying antigen-specific subsets by classical MHC-peptide tetramers or ICC assays has not been feasible. As CD25+ T reg cells must be activated via classic antigen-specific TCR triggering to exert suppressive activity (15, 18, 33, 57), the most effective method of assessing HIV-specific CD25+ T reg cells is to determine the ability of CD25+CD4+ T cells to inhibit T cell responses after stimulation with HIV antigens. Our data demonstrate that HIV-specific T reg cells, capable of suppressing both CD4+ and CD8+ HIV-specific T cell function in vitro, are present in the majority of healthy HIV+ individuals tested. However, the quality and/or quantity of HIV-specific CD25+CD4+ T reg cells appears to be diminished in certain individuals (HIV p24 LPA nonsuppressors; Tables I and II) before the loss of polyclonal T reg cell activity (Fig. 2 C) and the loss of normal (CD25−) HIV-specific CD4+ or CD8+ T cell responses (Fig. 2 A). In HIV p24 LPA nonresponders, both polyclonal and HIV-specific T reg cell activity, as well as normal HIV-specific CD4+ T cell function, is impaired.
Although variability in human CD25+ T reg cell–mediated suppression of T cell responses can be seen depending on the in vitro systems used (33), HIV disease–associated dysfunction of both CD25− (normal) CD4+ T cells and CD25+CD4+ T reg cells might be expected. In addition to the potential cytopathic effects of direct HIV infection of cells expressing CD4, particularly HIV-specific memory T cells (7), the interaction between HIV or envelope proteins and CD4 or chemokine receptors (HIV coreceptors) has been shown to significantly alter normal T cell function and sensitivity to apoptosis (9, 58–61). In this regard, loss of both HIV-specific and nonspecific CD25−CD4+ memory T cell and CD25+ T reg cell responses were associated with significantly higher levels of plasma HIV viremia (Table I). Of interest, among individuals with intact HIV-specific CD25−CD4+ T cell function, those lacking HIV-specific CD25+ T reg cell activity in vitro had significantly lower CD4+:CD8+ T cell ratios, a strong prognostic indicator in HIV disease progression (62), as compared with those who maintained this activity. These data suggest that, rather than playing an increasingly important role in HIV-associated immune dysfunction as disease progresses, HIV-specific CD25+ T reg cell–mediated immunosuppression is most relevant in healthy HIV-infected individuals that are relatively immunocompetent. Furthermore, HIV-specific CD25+ T reg cell function appears to be particularly sensitive to HIV disease–associated immune dysregulation and may be compromised even before normal (CD25−) HIV-specific CD4+ T cell responses.
Persistent antigens/pathogens, such as HIV, are believed to promote the expansion and activation of antigen-specific CD25+CD4+ T reg cells (16, 17, 63–66) and, under certain conditions, to induce normal CD4+ T cells to gain CD25+ T reg cell phenotype and function (15, 24). However, numerous factors associated with HIV infection, such as diminished capacity to produce IL-2 (1, 2, 67, 68), a cytokine critical for the development and the expansion of CD25+ T reg cells (69); HIV-mediated disruption of CD4+ T cell signaling (9, 58, 60); or alterations in Toll-like receptor signaling that can regulate T reg cell activity (29, 70–72), could limit the ability of HIV-specific T reg cells to become activated and proliferate in vivo. In addition, abnormal immune activation associated with HIV disease may alter the relative frequency of T reg cells, anergic cells, and normal activated T cells within the total CD25+CD4+ memory T cell population in the peripheral blood, as suggested by our cytokine data (Fig. 2 D). In this regard, the peripheral blood may not be the most appropriate compartment in which to accurately assess HIV-specific CD25+ T reg cell activity as antigen-specific CD25+CD4+ T reg cells have been shown to accumulate or expand at tissue sites of antigen expression or pathogen replication, where they exert site-localized immunosuppression (44, 63, 65, 73). With this in mind, we are currently assessing whether, in individuals with more active viral replication, HIV-specific CD25+ CD4+ T reg cells reside largely in the lymphoid tissue, the primary site of HIV replication in vivo (74).
The data of the present paper suggest that HIV-specific CD25+ T reg cell function may be compromised relatively early in HIV disease. Therefore, the question is raised whether or not this is reflective of a reduction in the numbers or function of the total CD25+ T reg cell population. Several lines of evidence obtained in the present paper suggest that the total CD25+CD4+ T reg cell population is relatively intact in the majority of healthy HIV-infected individuals. First, the frequency of the CD25+hi subset within the CD45RO+CD4+ T cell population of HIV-infected individuals is stable across a broad percentage range of CD4+ memory T cells (Fig. 1 C) and is, in fact, slightly elevated as compared with HIV-uninfected individuals (Fig. 1 B). Second, Fox P3 expression, a functionally relevant marker for immunosuppressive CD25+CD4+ T cells (38–42), was strongly expressed in CD25+CD4+ T cells from all HIV+ subjects tested and did not appear significantly altered as compared with HIV uninfected donors (Fig. 1 A). Third, polyclonal CD25+ T reg cell activity was not significantly different in the majority of healthy HIV+ subjects tested as compared with HIV-uninfected individuals (Fig. 2 C). However, it should be noted that polyclonal CD25+ T reg cell function was impaired in the subset of individuals (HIV LPA nonresponders) who, on average, had the lowest CD4+ T cell counts and highest levels of plasma viremia (Table I). Additional studies that include a larger number of individuals with advanced disease will be necessary to confirm this observation. Finally, the levels of HIV provirus (DNA) detected in CD25+ CD4+ T cells were not significantly different than those detected in the CD25− subset (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20032069/DC1), indicating that there is not preferential infection of CD25+ T reg cells. Together, these data support the hypothesis that there is a preferential alteration in the quantity, function, or tissue distribution of the HIV-specific, as compared with the total, CD25+ T reg cell population with HIV disease progression.
Definitive data regarding the role of CD25+CD4+ T reg cells in pathogenic infections in humans is difficult to obtain at this point in time; however, the possibility that CD25+ CD4+ T reg cells, and perhaps other cellular populations, suppress HIV-specific CD4+ and CD8+ T cell responses in infected individuals in vivo is intriguing. CD25+CD4+ T reg cell–mediated suppression of antimicrobial immune responses clearly could have deleterious effects, but in certain infections, may also have potentially beneficial ramifications. In this regard, it has been proposed that the function of CD25+CD4+ T reg cells in the context of pathogenic infections is to control excessive, potentially dangerous, cellular immune responses and/or allow low level pathogen persistence as an effective strategy to prevent reinfection with more virulent strains (15, 16, 18, 23, 28, 75, 76). HIV antigen-stimulated CD25+CD4+ T reg cell–mediated suppression of HIV-specific and nonspecific T cell responses may be one mechanisms by which several detrimental processes, such as cellular activation-associated apoptosis/anergy, immune-mediated destruction, and, for CD4+ T cells, susceptibility to productive HIV infection, are kept under control (10, 12, 77, 78). In this scenario, loss of HIV-specific CD25+ T reg cell activity could prove to have an overall detrimental effect on the health of HIV-infected individuals. Additional studies will be needed determined whether HIV-specific CD25+ T reg cell activity, if operative in vivo, hastens or delays HIV disease progression.
Acknowledgments
We would like to thank Dr. C. Kovacs at the University of Toronto and his patients for participation in these research studies. We would also like to thank P. Walsh and M. Rust for editorial assistance.
This paper was partially supported by National Institutes of Health grant AI48779 (to S. Ziegler).
The authors have no conflicting financial interests.
Abbreviations used in this paper: CFSE, carboxyfluorscein diacetate succinimidyl ester; ICC, intracellular cytokine; LPA, lymphocyte proliferation assay; LTNP, long-term non progressor; SI, stimulation indexes; T reg cell, regulatory T cell.
References
- 1.Antonen, J., A. Ranki, S.L. Valle, E. Seppala, H. Vapaatalo, J. Suni, and K. Krohn. 1987. The validity of immunological studies in human immunodeficiency virus infection: a three-year follow-up of 235 homo- or bisexual persons. Acta Pathol. Microbiol. Immunol. Scand. [C]. 95:275–282. [DOI] [PubMed] [Google Scholar]
- 2.Clerici, M., N.I. Stocks, R.A. Zajac, R.N. Boswell, D.R. Lucey, C.S. Via, and G.M. Shearer. 1989. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Invest. 84:1892–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miedema, F. 1992. Immunological abnormalities in the natural history of HIV infection: mechanisms and clinical relevance. Immunodefic. Rev. 3:173–193. [PubMed] [Google Scholar]
- 4.Pontesilli, O., M. Carlesimo, A.R. Varani, R. Ferrara, E.C. Guerra, M.L. Bernardi, G. Ricci, A.M. Mazzone, G. D'Offizi, and F. Aiuti. 1995. HIV-specific lymphoproliferative responses in asymptomatic HIV-infected individuals. Clin. Exp. Immunol. 100:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalod, M., M. Dupuis, J.C. Deschemin, C. Goujard, C. Deveau, L. Meyer, N. Ngo, C. Rouzioux, J.G. Guillet, J.F. Delfraissy, et al. 1999. Weak anti-HIV CD8(+) T-cell effector activity in HIV primary infection. J. Clin. Invest. 104:1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris, P.J., and E.S. Rosenberg. 2001. Cellular immune response to human immunodeficiency virus. AIDS. 15:S16–S21. [DOI] [PubMed] [Google Scholar]
- 7.Douek, D.C., J.M. Brenchley, M.R. Betts, D.R. Ambrozak, B.J. Hill, Y. Okamoto, J.P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 417:95–98. [DOI] [PubMed] [Google Scholar]
- 8.Champagne, P., G.S. Ogg, A.S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G.P. Rizzardi, S. Fleury, M. Lipp, et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106–111. [DOI] [PubMed] [Google Scholar]
- 9.Popik, W., and P.M. Pitha. 2000. Exploitation of cellular signaling by HIV-1: unwelcome guests with master keys that signal their entry. Virology. 276:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Appay, V., and S.L. Rowland-Jones. 2002. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 23:580–585. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 98:1667–1677. [DOI] [PubMed] [Google Scholar]
- 12.Lawn, S.D., S.T. Butera, and T.M. Folks. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14:753–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 14.Suri-Payer, E., A.Z. Amar, A.M. Thornton, and E.M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212–1218. [PubMed] [Google Scholar]
- 15.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi, S. 2003. Regulatory T cells: mediating compromises between host and parasite. Nat. Immunol. 4:10–11. [DOI] [PubMed] [Google Scholar]
- 17.Mills, K.H., and P. McGuirk. 2004. Antigen-specific regulatory T cells–their induction and role in infection. Semin. Immunol. 16:107–117. [DOI] [PubMed] [Google Scholar]
- 18.Powrie, F., and K.J. Maloy. 2003. Immunology. Regulating the regulators. Science. 299:1030–1031. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu, J., S. Yamazaki, and S. Sakaguchi. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211–5218. [PubMed] [Google Scholar]
- 20.Onizuka, S., I. Tawara, J. Shimizu, S. Sakaguchi, T. Fujita, and E. Nakayama. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 59:3128–3133. [PubMed] [Google Scholar]
- 21.Kingsley, C.I., M. Karim, A.R. Bushell, and K.J. Wood. 2002. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 168:1080–1086. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Fueyo, A., M. Weber, C. Domenig, T.B. Strom, and X.X. Zheng. 2002. Tracking the immunoregulatory mechanisms active during allograft tolerance. J. Immunol. 168:2274–2281. [DOI] [PubMed] [Google Scholar]
- 23.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 24.Hisaeda, H., Y. Maekawa, D. Iwakawa, H. Okada, K. Himeno, K. Kishihara, S. Tsukumo, and K. Yasutomo. 2004. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat. Med. 10:29–30. [DOI] [PubMed] [Google Scholar]
- 25.Long, T.T., S. Nakazawa, S. Onizuka, M.C. Huaman, and H. Kanbara. 2003. Influence of CD4+CD25+ T cells on Plasmodium berghei NK65 infection in BALB/c mice. Int. J. Parasitol. 33:175–183. [DOI] [PubMed] [Google Scholar]
- 26.Kursar, M., K. Bonhagen, J. Fensterle, A. Kohler, R. Hurwitz, T. Kamradt, S.H. Kaufmann, and H.W. Mittrucker. 2002. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 196:1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori, S., T.L. Carvalho, and J. Demengeot. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282–1291. [DOI] [PubMed] [Google Scholar]
- 28.Montagnoli, C., A. Bacci, S. Bozza, R. Gaziano, P. Mosci, A.H. Sharpe, and L. Romani. 2002. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 169:6298–6308. [DOI] [PubMed] [Google Scholar]
- 29.Netea, M.G., R. Sutmuller, C. Hermann, C.A. Van der Graaf, J.W. Van der Meer, J.H. van Krieken, T. Hartung, G. Adema, and B.J. Kullberg. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172:3712–3718. [DOI] [PubMed] [Google Scholar]
- 30.Iwashiro, M., R.J. Messer, K.E. Peterson, I.M. Stromnes, T. Sugie, and K.J. Hasenkrug. 2001. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. USA. 98:9226–9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dittmer, U., H. He, R.J. Messer, S. Schimmer, A.R. Olbrich, C. Ohlen, P.D. Greenberg, I.M. Stromnes, M. Iwashiro, S. Sakaguchi, et al. 2004. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 20:293–303. [DOI] [PubMed] [Google Scholar]
- 32.Suvas, S., U. Kumaraguru, C.D. Pack, S. Lee, and B.T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baecher-Allan, C., V. Viglietta, and D.A. Hafler. 2004. Human CD4+CD25+ regulatory T cells. Semin. Immunol. 16:89–98. [DOI] [PubMed] [Google Scholar]
- 34.Migueles, S.A., A.C. Laborico, W.L. Shupert, M.S. Sabbaghian, R. Rabin, C.W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068. [DOI] [PubMed] [Google Scholar]
- 35.Levings, M.K., R. Sangregorio, C. Sartirana, A.L. Moschin, M. Battaglia, P.C. Orban, and M.G. Roncarolo. 2002. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baecher-Allan, C., J.A. Brown, G.J. Freeman, and D.A. Hafler. 2003. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp. 252:67–88. [DOI] [PubMed] [Google Scholar]
- 37.Bruder, D., M. Probst-Kepper, A.M. Westendorf, R. Geffers, S. Beissert, K. Loser, H. von Boehmer, J. Buer, and W. Hansen. 2004. Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 34:623–630. [DOI] [PubMed] [Google Scholar]
- 38.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 39.O'Garra, A., and P. Vieira. 2003. Twenty-first century Foxp3. Nat. Immunol. 4:304–306. [DOI] [PubMed] [Google Scholar]
- 40.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 41.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 42.Walker, M.R., D.J. Kasprowicz, V.H. Gersuk, A. Benard, M.V. Landeghen, J.H. Buckner, and S.F. Zeigler. 2003. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J. Clin. Invest. 112:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wing, K., A. Ekmark, H. Karlsson, A. Rudin, and E. Suri-Payer. 2002. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 106:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao, D., V. Malmstrom, C. Baecher-Allan, D. Hafler, L. Klareskog, and C. Trollmo. 2003. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 33:215–223. [DOI] [PubMed] [Google Scholar]
- 45.Baecher-Allan, C., J.A. Brown, G.J. Freeman, and D.A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253. [DOI] [PubMed] [Google Scholar]
- 46.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levings, M.K., R. Sangregorio, and M.G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehmann, J., J. Huehn, M. de la Rosa, F. Maszyna, U. Kretschmer, V. Krenn, M. Brunner, A. Scheffold, and A. Hamann. 2002. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc. Natl. Acad. Sci. USA. 99:13031–13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A.H. Enk. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stassen, M., E. Schmitt, and H. Jonuleit. 2004. Human CD(4+)CD(25+) regulatory T cells and infectious tolerance. Transplantation. 77:S23–S25. [DOI] [PubMed] [Google Scholar]
- 51.Piccirillo, C.A., J.J. Letterio, A.M. Thornton, R.S. McHugh, M. Mamura, H. Mizuhara, and E.M. Shevach. 2002. CD4+ CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J. Exp. Med. 196:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact–dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface–bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levings, M.K., R. Bacchetta, U. Schulz, and M.G. Roncarolo. 2002. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int. Arch. Allergy Immunol. 129:263–276. [DOI] [PubMed] [Google Scholar]
- 54.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piccirillo, C.A., and E.M. Shevach. 2001. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 167:1137–1140. [DOI] [PubMed] [Google Scholar]
- 56.Aandahl, E.M., J. Michaelsson, W.J. Moretto, F.M. Hecht, and D.F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taams, L.S., M. Vukmanovic-Stejic, J. Smith, P.J. Dunne, J.M. Fletcher, F.J. Plunkett, S.B. Ebeling, G. Lombardi, M.H. Rustin, J.W. Bijlsma, et al. 2002. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 32:1621–1630. [DOI] [PubMed] [Google Scholar]
- 58.Cicala, C., J. Arthos, S.M. Selig, G. Dennis, Jr., D.A. Hosack, D. Van Ryk, M.L. Spangler, T.D. Steenbeke, P. Khazanie, N. Gupta, et al. 2002. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc. Natl. Acad. Sci. USA. 99:9380–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maddon, P.J., A.G. Dalgleish, J.S. McDougal, P.R. Clapham, R.A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 47:333–348. [DOI] [PubMed] [Google Scholar]
- 60.Arthos, J., C. Cicala, S.M. Selig, A.A. White, H.M. Ravindranath, D. Van Ryk, T.D. Steenbeke, E. Machado, P. Khazanie, M.S. Hanback, et al. 2002. The role of the CD4 receptor versus HIV coreceptors in envelope-mediated apoptosis in peripheral blood mononuclear cells. Virology. 292:98–106. [DOI] [PubMed] [Google Scholar]
- 61.Siliciano, R.F. 1996. The role of CD4 in HIV envelope-mediated pathogenesis. Curr. Top. Microbiol. Immunol. 205:159–179. [DOI] [PubMed] [Google Scholar]
- 62.Fahey, J.L., J.M. Taylor, R. Detels, B. Hofmann, R. Melmed, P. Nishanian, and J.V. Giorgi. 1990. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med. 322:166–172. [DOI] [PubMed] [Google Scholar]
- 63.Walker, L.S., A. Chodos, M. Eggena, H. Dooms, and A.K. Abbas. 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vahlenkamp, T.W., M.B. Tompkins, and W.A. Tompkins. 2004. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4(+)CD25(+) T regulatory cells. J. Immunol. 172:4752–4761. [DOI] [PubMed] [Google Scholar]
- 65.Yamazaki, S., T. Iyoda, K. Tarbell, K. Olson, K. Velinzon, K. Inaba, and R.M. Steinman. 2003. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugimoto, K., F. Ikeda, J. Stadanlick, F.A. Nunes, H.J. Alter, and K.M. Chang. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 38:1437–1448. [DOI] [PubMed] [Google Scholar]
- 67.Iyasere, C., J.C. Tilton, A.J. Johnson, S. Younes, B. Yassine-Diab, R.P. Sekaly, W.W. Kwok, S.A. Migueles, A.C. Laborico, W.L. Shupert, et al. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900–10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Younes, S.A., B. Yassine-Diab, A.R. Dumont, M.R. Boulassel, Z. Grossman, J.P. Routy, and R.P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2–producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson, B.H. 2004. IL-2, regulatory T cells, and tolerance. J. Immunol. 172:3983–3988. [DOI] [PubMed] [Google Scholar]
- 70.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]
- 71.Yang, Y., C.T. Huang, X. Huang, and D.M. Pardoll. 2004. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 5:508–515. [DOI] [PubMed] [Google Scholar]
- 72.Caramalho, I., T. Lopes-Carvalho, D. Ostler, S. Zelenay, M. Haury, and J. Demengeot. 2003. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oldenhove, G., M. de Heusch, G. Urbain-Vansanten, J. Urbain, C. Maliszewski, O. Leo, and M. Moser. 2003. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J. Exp. Med. 198:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pantaleo, G., C. Graziosi, L. Butini, P.A. Pizzo, S.M. Schnittman, D.P. Kotler, and A.S. Fauci. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA. 88:9838–9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu, D., H. Liu, M. Komai-Koma, C. Campbell, C. McSharry, J. Alexander, and F.Y. Liew. 2003. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 170:394–399. [DOI] [PubMed] [Google Scholar]
- 76.Suvas, S., A.K. Azkur, B.S. Kim, U. Kumaraguru, and B.T. Rouse. 2004. CD4(+)CD25(+) regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:4123–4132. [DOI] [PubMed] [Google Scholar]
- 77.Deeks, S.G., and B.D. Walker. 2004. The immune response to AIDS virus infection: good, bad, or both? J. Clin. Invest. 113:808–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leng, Q., G. Borkow, and Z. Bentwich. 2002. Attenuated signaling associated with immune activation in HIV-1-infected individuals. Biochem. Biophys. Res. Commun. 298:464–467. [DOI] [PubMed] [Google Scholar]