Mechanics of Dynamin-Mediated Membrane Fission (original) (raw)
. Author manuscript; available in PMC: 2015 Jan 10.
Abstract
In eukaryotic cells, membrane compartments are split into two by membrane fission. This ensures discontinuity of membrane containers and thus proper compartmentalization. The first proteic machinery implicated in catalyzing membrane fission was dynamin. Dynamin forms helical collars at the neck of endocytic buds. This structural feature suggested that the helix of dynamin could constrict in order to promote fission of the enclosed membrane. However, verifying this hypothesis revealed itself to be a challenge, which inspired many in vitro and in vivo studies. The primary goal of this review is to discuss recent structural and physical data from biophysical studies that have refined our understanding of the dynamin mechanism. In addition to the constriction hypothesis, other models have been proposed to explain how dynamin induces membrane fission. We present experimental data supporting these various models and assess which model is the most probable.
Keywords: constriction, endocytosis, torque, membrane elasticity, clathrin
INTRODUCTION
Living cells are separated from their environment—the surrounding fluid and other cells if they are part of a multicellular organism—by a lipid membrane. The cell membrane is a lipid bilayer, a structure that relies essentially on the ability of cylindrical phospholipids to self-assemble into a bilayer. The bilayer is a fluid, two-dimensional, deformable sheet. The low permeability of lipid bilayers to most solutes (e.g., small hydrophilic molecules, ions) is good enough to maintain a constant cellular composition. Thus, the first role of lipid membranes in cells is to serve as concentration barriers. In eukaryotic cells, cellular compartments (e.g., reticulum, Golgi apparatus, mitochondria, endosomes) are also surrounded by lipid membranes, allowing the compartments a specific proteolipidic and ionic composition adapted to their function.
However, the cell must incorporate material from its environment or exchange material between compartments. This is done through budding of membrane carriers from a donor compartment and fusion with an acceptor compartment. The formation of all membrane carriers in cells follows a simple three-step process: (a) deformation of the membrane into a spherical or tubular bud, (b) sorting of lipids and proteins that need to be exported, and (c) separation of the bud from the donor membrane. This last step is called membrane fission, and it is critical to cell function as compartments may leak or end up fusing together if fission does not occur properly.
Although critical to membrane traffic, membrane fission is still only partly characterized. Proteins and lipids essential for this reaction are unknown for many routes of membrane traffic. There are two reasons for this: (a) As in the case of fusion, the reverse reaction of fission, fission is a rapid reaction, and intermediates are difficult to isolate with any techniques; (b) contrary to fusion, there is no fission machinery common to all fission reactions. In fusion, the core of the fusion machinery is the SNARE complex. It is present in most fusion reactions in membrane traffic, and its discovery led to a detailed understanding of the pathway to membrane fusion.
The first machinery discovered to be involved in membrane fission was dynamin. Dynamin is a 100-kDa GTPase that forms helical polymers around the membrane neck of nascent endocytic buds at the plasma membrane (Figure 1_a_). This peculiar structure immediately suggested a mechanism by which dynamin could drive fission: By constriction of the helix, the radius of the neck could be reduced to the point at which the membrane would fuse onto itself and break. Because early evidence of the role of dynamin in membrane fission came from mutants affecting the GTPase activity of dynamin (16, 36, 82), GTP hydrolysis was thought to trigger this constriction. This mechanism implies that dynamin generates enough force to curve the membrane to radii close to the thickness of the bilayer (3–4 nm) and even further. Even though lipid membranes are highly flexible, attaining such high curvatures requires huge forces. Dynamin works against membrane elasticity, and as discussed below, the role of membrane elasticity in dynamin-mediated fission was recently shown to be essential (51).
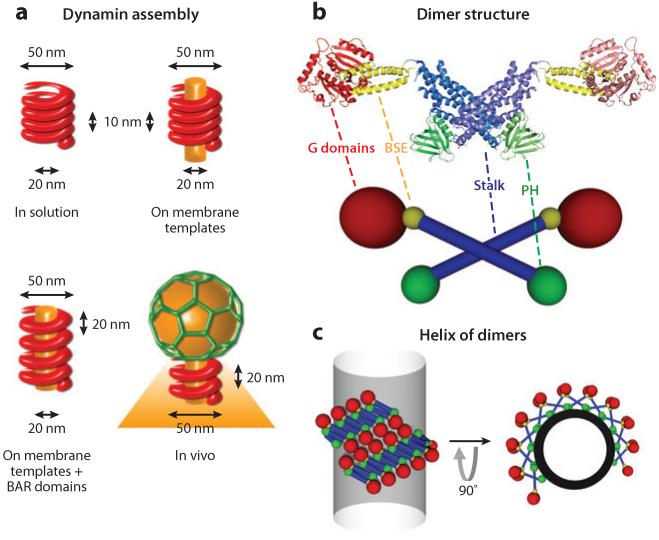
Figure 1.
Dynamin assembly. (a) Schematic representations of dynamin helix in four different conditions: in solution, on membrane templates, on membrane templates with BAR domains, and in vivo. The helical diameter is identical in all conditions in the absence of GTP. However, the pitch is longer in the presence of BAR domains and in vivo. (b) Crystal (top) and schematic (bottom) structures of a dynamin dimer (PDB ID: 3SHN). The proline-rich domain (PRD) is not represented. Monomers interact in a cross shape via their stalk. (c) Schematic representations of a dynamin helix around a lipid tube ( gray). Radial (right) and transverse (left) views show how dimers assemble into a helix. Abbreviation: BSE, bundling signal element.
Because lipid bilayers are fluid two-dimensional sheets, they can be stretched (area expansion causing tension) or bent (shape changes perpendicular to the membrane, causing curvature). It is difficult to stretch lipids apart, and lipid bilayers are usually considered as incompressible fluids within their plane (the maximum area expansion before rupture is between 5% and 8%). However, they are fairly easy to bend, easy enough such that Brownian movement of the surrounding fluid creates fluctuations, undulations (up to microns) of the bilayers that can be observed by light microscopy. These undulations generate low membrane tension; basically, all free lipid membranes in a water environment are under tension. Lipid bilayers are auto-sealable. If a pore opens, it spontaneously closes, and only tension higher than 10−3–10−2 N/m can rupture the membrane. This makes lipid membranes as resistant to stress as rubber, taking into account the extremely small thickness of lipid bilayers. Obviously, the auto-sealable property of lipid bilayers makes them difficult to break; thus, seen from a membrane mechanics perspective, membrane fission is far from being a spontaneous process.
The goal of this review is to evaluate, after twenty years of accumulated data on the role of dynamin in membrane fission, the validity of the early, so-called constriction model. Over the course of verifying this model, alternative models have been proposed. We examine the pros and the cons of each model, with tentative explanations for inconsistencies between the different models and interpretations found in the literature. We use the constriction model as a guideline. To validate the constriction model, scientists have focused on several problems: (a) the ability of dynamin to self-assemble into a helical polymer around a membrane tube, (b) the ability of the dynamin helical polymer to constrict upon GTP hydrolysis, and (c) the ability of dynamin constriction to lead to fission.
We first detail the evidence for the assembled structure of dynamin and for parameters that can influence nucleation and polymerization. We then present results converging on the idea that the dynamin polymer can constrict upon GTP hydrolysis, and that this constriction is driven by a conformational change at the level of each dynamin dimer in the polymer. Last, we present results showing how this conformational change can lead to fission. Because the dynamin fission reaction combines protein and membrane mechanics, we emphasize the physical understanding of membrane fission.
ASSEMBLY AND POLYMERIZATION OF DYNAMIN
Dynamin was initially found by its association with microtubules (67). It is copurified with microtubule-associated proteins. It forms helices around microtubules, which can trigger formation of bundles. In this section, we review data showing that formation of a helical polymer is an intrinsic property of dynamin that is critical for its function. Recent structural data show how the protein polymer is built at the monomeric level and how it is probably conserved for many members of the dynamin-like proteins.
Proof of Assembly and Basic Structure of the Polymer
The fact that dynamin forms helical rings on microtubules suggests oligomerization ability (67, 68). In solution, dynamin was found to exist as a tetramer (53), and higher-molecular-weight complexes were found by gel filtration, proving the ability to self-assemble. But the most striking finding was that purified dynamin in low-salt, low-pH buffer forms long oligomers with a clear helical structure visible by electron microscopy (8, 31). This process does not require any nucleotide load, even if GTP-bound dynamin polymerizes better (8). These helices, approximately 50 nm wide and a few tens of nanometers long, were found to be the same size as the helical collars of clathrin-coated pits (CCPs) seen in the Shibire mutant at the blocking temperature (35) and in synaptosomes treated with GTPγS (77). The oligomeric properties of dynamin are thought to generate these structures, as the Shibire gene encodes dynamin (12), and they contain dynamin as shown by immunostaining (77). Thus, structures found in vivo when dynamin function is blocked have the same inner diameter (20 nm) as structures formed in vitro with purified dynamin in absence of nucleotides (Figure 1_a_) (8, 31). An important difference between in vivo helical structures formed by dynamin and in vitro structures is the pitch. In vitro, with dynamin only, the pitch is between 10 and 13 nm (8, 31), whereas in vivo, the pitch is close to 18–20 nm (Figure 1_a_) (34). As discussed below, this difference is due likely to the association with BAR proteins in vivo, which increases the pitch. Also, when bound to GTP or to nonhydrolyzable forms of GTP (GTPγS or GMP-PCP), dynamin has an enhanced polymerization activity (8, 84). Polymerization and nucleation, contrary to tubulin or actin, are not strictly dependent on the nucleotide load, as nucleotide-free dynamin is able to assemble in vitro under low-salt and low-pH conditions (31) and on negatively charged membranes (75; see below).
Genetic Structure of Different Isoforms and Domain Structure of the Protein
Dynamin is the primary member of a large family of proteins called the dynamin-related proteins or dynamin-like proteins (57). The canonical dynamin domain structure is composed of (from N terminus to C terminus) the GTPase domain; the middle domain; the pleckstrin homology (PH) domain, which binds phosphatidylinositol (4,5)-bisphosphate (PIP2) at the membrane (65); the GTPase-enhancing domain (GED), which binds to the GTPase domain (53) and enhances its intrinsic GTPase activity; and the proline-rich domain (PRD), which is unfolded and binds most of the partners of dynamin (61). The PH domain and PRD can be absent in some of the members of the DRP (dynamin-related protein) family, especially in DRPs working in organelle fission/fusion (57). But domains involved in assembly and GTPase activity, namely the GTPase domain, the middle domain, and the GED, are present in the vast majority of DRPs. Also, the conserved domains share a very high percentage of similarity (≈80%; 24). Thus, it is thought that the polymeric nature of dynamin is common to all members of the dynamin family, as it was experimentally established for highly divergent members (3, 18, 19, 32).
Dynamin has a single gene but several isoforms in invertebrates (12, 15); in mammals it has three genes (DYN1, DYN2, and DYN3) with multiple splicing variants. Dynamin1 is neuron specific and is involved in the rapid formation of synaptic vesicles (23). Dynamin2 is ubiquitous and is responsible for clathrin-mediated endocytosis in any cell type (83), whereas Dynamin3 is the least characterized isoform and is thought to be involved in long-term potentiation regulation at the postsynaptic button (29, 46), but it also has a redundant presynaptic function with Dynamin1 (59). Dynamin3 is expressed in the testis, where it participates in the formation of a tubulobulbar structure involved in the release of sperm cells from nurse Sertoli cells (81). However, the three isoforms share more than 80% homology, in support of a common molecular mechanism. Moreover, most of the variability is actually found in the PRDs, which explains why different isoforms bind different partners (59), but it is not part of the core structural domains.
Owing to its polymerization activity, dynamin was not easily crystallized, and two groups have recently made the tour de force of solving the crystal structure of ΔPRD dynamin by use of polymerization-deficient mutants (21, 25). These data partially confirmed the position of GTPase, PH, and GED domains in the helical polymer (see Figure 1_b_). Moreover, the groups confirmed the existence of the structural domain BSE (bundling signal element) (Figure 1_b_), which is essential for polymeric assembly (9, 10, 27) and is composed of three α-helices encoded by noncontiguous parts of the amino acid sequence. These structures also showed that the middle and GED domains fold together in a long, rigid coiled-coil structure called the stalk (Figure 1_b_). The BSE domain forms the lever arm between the stalk and GTPase domain.
The diffraction data indicated that dynamin is a dimer in the crystal, with a very peculiar structure. The stalk domains interact in a cross-shape essential for the oligomerization process (9, 27), with GTPase domains pointing out on one side of the cross (Figure 1_b_) and PH domains on the other side. Amazingly, these crosses also aligned in the crystal, forming long strings of dimers (21). Each dimer interacts with the next one through the upper and lower parts of the stalk, but with a shift between the axes of neighboring dimers. This shift is critical in the helical assembly, as it creates the necessary shift between dimers to form a helix instead of a ring. Also, because interactions between dimers are weak, they must be flexible to allow the linear strings seen in the crystal to curve into a helix (9, 10, 21, 25). Another finding of the structural data (25), in accordance with earlier biochemical findings (70), is strong interactions between GTPase domains of coaligned strings of dimers. These interactions in the linear form of the polymer are fully compatible with strong interactions between GTPase domains of adjacent helical turns in the helicoidal polymer (Figure 1_c_).
In conclusion, the helical structure of the dynamin polymer arises from an unusual assembly of cross-shaped dimers, allowing flexibility for curving: The cross shape allows the angle between the two stalk domains to change. Also, as dimers interact through limited zones of interaction, their exact positioning is flexible. These structural data explain how the protein polymer is organized, and identify the parts for potential conformational change.
Assembly at the Membrane
One striking feature of dynamin is its ability to polymerize and deform a lipid membrane. It was the first protein shown to form decorated tubules out of protein-free liposomes (75). When purified dynamin is added to negatively charged liposomes (containing 100% phosphatidylserine) (75) or to liposomes made of brain purified lipids, supplemented with PIP2, dynamin is able to polymerize into helical polymers circling membrane tubules connected to liposomes (78). The helical structure is even clearer when dynamin is mixed with lipid nanorods (lipid cylindrical semicrystalline structures approximately 20 nm in diameter) onto which dynamin can also polymerize (48, 73). The obtained final structures resemble the helices seen at the neck of CCPs in dynamin-blocking conditions (35, 54, 77) (see Figure 1_a_). Dynamin-coated tubules have an inner diameter (diameter of the membrane tube inside the dynamin helix) of 20 nm and a dynamin coat that is 15 nm thick, which makes the outer diameter close to 50 nm.
The dimensions of the dynamin helical polymer are known, as the whole structure has been reconstructed in three dimensions from cryo-electron microscopy (cryo-EM) images. In the nonconstricted state, it is a right-handed helix, with 14.3 dimers of dynamin per helical turn (13, 49). By fitting the crystal structure of the full dynamin (25), one can see that the same, but curved, alignment of dimers seen in the crystal is respected (21, 25). The PH domain is oriented toward the membrane tube, the GTPase domains are arranged on the outer layer of the helical ring, and the cross structures of the stalk domains make an intermediate layer, thickening the coat. Crystal structures of the full dynamin and three-dimensional reconstruction of the polymer together give a high definition of the organization of dynamin dimers in the polymer. A striking difference between dynamin polymers and cytoskeletal polymers (such as actin and microtubules) is that dynamin polymers are nonpolarized structures, as helices are made of homodimers. This difference has important implications for the constriction mechanism (see below).
The dimensions of the tubes and of the surrounding helix are the same when dynamin is polymerized in solution on its own (Figure 1_a_). This observation suggests that dynamin deforms the membrane through its polymerization activity: It binds PIP2 through its PH domain, and negatively charged lipids through positive residues of its membrane-bound surface. This would drive assembly, which is most favorable in the shape of a 50-nm-wide helix. Because dynamin is bound to the membrane, and because the PH domain is oriented toward the inner part of the helix, polymerization drives membrane flow inside the growing helix, forming a membrane tubule constrained by the dynamin coat. Dimensions of the dynamin helix are the same with or without membrane support; thus, the dynamin polymer is thought to be rigid enough to constrain a membrane. When constricted to a diameter of 20 nm, the elasticity of the membrane, which tends to widen the tube, competes with the rigidity of the dynamin coat. Dynamin is indeed quite rigid, as the persistence length of the dynamin-coated membrane tubule is close to 40 μm (26). The persistence length measures the length below which the polymer stays more or less straight when subjected to thermal fluctuations. For example, DNA has a persistence length in the range of 50 nm (5), actin in the range of 16–17 μm (40), and microtubules in the range of more than 1 mm (28).
Although the stiffness of the polymer allows us to understand how dynamin can maintain the membrane in a 20-nm-constricted state, it still does not explain how it can deform the membrane. The polymerization force of dynamin drives deformation. The average energy gained by each dimer joining the polymer is given by the chemical potential of polymerizing dynamin. This chemical potential can be seen as the maximal amount of mechanical work that can be generated by the addition of a single dimer, pulling the membrane tube inside the dynamin helix. The mechanical work is the force applied by distance unit, and in this case the work exerted by the addition of a single dimer happens on a distance that can be deduced from the dynamin pitch. The dynamin pitch is between 10 and 13 nm (13), and there are 14 dimers per helix turn (see above). Thus, the addition of a single dimer increases the length of a tube by approximately 1 nm. The polymerization force of dynamin is thus its mechanical work, i.e., its chemical potential, divided by 1 nm.
The polymerization force of dynamin has been measured (63). By pulling a single membrane tubule out of giant liposomes aspirated into a micropipette, by means of optical tweezers, the force needed to maintain the tube can be recorded without dynamin or when dynamin is polymerized. From these data, the polymerization force of dynamin is estimated to be ~20 pN for concentrations between 10 and 15 μM (63). The value of the polymerization force is sufficient to form tubules from a flat membrane, confirming that dynamin in vitro (several micromolar) can form membrane tubules by scaffolding.
But a prediction of the force dependence on the dynamin concentration is that, at lower concentrations, dynamin would no longer be able to form membrane tubules. It would especially be the case at physiological concentrations, thought to be less than 1 μM, even though the exact intracellular concentration of dynamin is unknown. In vivo, dynamin comes late at the end of CCP formation (20, 79), suggesting that dynamin is recruited once most of the curvature has been generated, rather than actively participating in membrane deformation. However, this statement must be nuanced: Mutations (71) and chemical inhibition (47) of dynamin block the CCPs at stages earlier than the dynamin-assembled stage, arguing in favor of dynamin’s role in CCP closure.
These data raise an essential question: How are dynamin scaffolds formed specifically at the neck of endocytic buds? There is an apparent discrepancy between in vitro data, showing that dynamin is strong enough to curve membranes, and the late arrival of dynamin during CCP formation in vivo, suggesting that dynamin is recruited by curvature. Many curvature-sensing proteins containing the crescent-shaped BAR (Bin-amphiphysin-Rvs) domain have been proposed to help the specific recruitment of dynamin to CCP necks. Amphiphysin and endophilin have a BAR domain that binds to the membrane and an SH3 domain that binds to the PRD of dynamin. BAR domains are dual structures that have amphipathic helices to promote membrane binding and deformation (22, 78), and they form a crescent-like dimer, which has been involved in curvature sensing (56). The ability to sense or generate curvature depends on the density of the membrane-bound protein (72), and the low membrane-bound density of these proteins argues in favor of curvature-sensing behavior in vivo.
Dynamin nucleation itself is curvature dependent at concentrations lower than micromolar (63). When membrane tubes of a controlled radius are used as dynamin polymerization templates, nucleation is seen for radii below a threshold radius. As dynamin squeezes tubes to a radius of 10 nm by assembly, it uses polymerization energy to curve the membrane (see above). If the membrane is already curved in the shape of a tube, it thus requires less energy to be squeezed by dynamin. There is a competition between the energy needed to squeeze the membrane, which depends on the initial radius of curvature, and the polymerization energy of dynamin, which depends on its bulk concentration. When the tube radius is too large, dynamin cannot polymerize. As the threshold radius depends on dynamin polymerization energy, it depends on the bulk concentration of dynamin. At high concentration of dynamin (above 1 μM) the threshold radius is large (few hundreds of nanometers), and at low concentration the threshold radius is close to the inner radius of dynamin. These results show how dynamin, in different conditions, can tubulate membranes and have a curvature-dependent nucleation. In addition, they explain how dynamin, without the need for other proteins, could be recruited specifically to the neck of CCPs because of their high curvature.
In conclusion, dynamin is a large GTPase that has the ability to polymerize into a wide and rigid helical structure that can deform membranes. This structure is made of antiparallel homodimers, forming a nonpolar, right-handed helix of 14 dimers per turn. Its assembly nucleation depends on various factors, such as negatively charged membranes, PIP2, initial curvature of the membrane, pH level, and salt concentration. The GTP-bound state of dynamin has an increased propensity to polymerize, but nucleotides are not required for polymerization. Although dynamin and the cytoskeletal proteins tubulin and actin share a similar structure (i.e., long helicoidal polymers), their nucleation properties are very different, as dynamin assembles in a nucleotide-independent manner. The biochemistry data on dynamin GTPase activity and its associated conformational change at the monomeric level make dynamin appear more similar to a molecular motor, such as myosin, than to tubulin or actin.
HYDROLYSIS OF GTP AND CONFORMATIONAL CHANGE OF DYNAMIN
In this section, we focus on the peculiar aspects of the dynamin GTPase activity by comparing them to those of other small regulatory GTPases and ATPase motors. We describe the associated conformational change at both the dimeric level and the entire helical polymer level. With a powerstroke similar to that of myosins, dynamin is able to constrict its helix. We then describe how this conformational change generates torque, the constriction force, and how torque depends on GTPase activity.
Basic Biochemistry
The GTPase domain of dynamin binds and hydrolyzes GTP molecules. It is structurally similar to the canonical Ras GTPase domain, but the nucleotide-free state does not correspond to the classical inactive (GDP or nucleotide-free) state of small GTPases (60). Moreover, the GTPase domain shares some structural features with the ATPase domains of kinesins, at least at the amino acid sequence level (55). It was actually first thought to be an ATPase (67), as basal ATPase activity was found. Even if the _k_cat is much higher for GTP, ATPase activity is not negligible, and proteins of the EHD family that share many structural and assembly features with dynamin are clearly ATPases (18). It has thus been proposed that dynamin has evolved from ATPases, rather than from small GTPases, even though the signature at the structural level is not clear.
When looking at the enzymatic activity, dynamin seems even more divergent from small regulatory GTPases and more similar to ATPases involved in force generation such as myosins or kinesins. In the absence of any lipid templates or protein partners and at physiological ionic strength, the basal GTPase activity of dynamin is relatively high: _k_cat = 0.015–0.3 s−1 (84). Dynamin self-assembly stimulates its own GTPase activity. At the low ionic strength at which self-assembly occurs, the GTPase rate increases tenfold (84). On lipid templates as well, dynamin GTPase activity is observed to increase up to tenfold (from 0.3 to 4.3 s−1) (48). In comparison, the small G protein Ras has a basal GTPase rate _k_cat = 1.3 10−4 s−1 (6). Dynamin also has a low affinity for GTP: Km = 10–150 μM (80, 84). For Ras proteins, Km = 0.2–0.5 μM (1). Small regulatory GTPases are molecular switches that strongly bind nucleotides (low Km), and they rarely change their nucleotide state (low intrinsic GTPase activity) unless triggered by partners to do so. Dynamin exchanges nucleotides very fast (high Km) and burns them even faster (high GTPase activity when assembled). In comparison, the ATPase activity of muscular myosin has a _k_cat value of 25 s−1 when bound to actin, and a Km value of 10 μM (2). These values are very similar to those for dynamin GTPase activity, indicating that when dynamin is assembled, its GTPase activity is similar to that of a molecular motor and far from that of a regulatory GTPase. Thus, the GTPase activity of assembled dynamin is dedicated to gaining as much energy from GTP as it can, by burning GTPs one after the other.
Interactions between dimers of dynamin are responsible for stimulating GTPase activity (84). Kinetics measurements revealed positive cooperativity in GTPase activity depending on dynamin concentration; a Hill coefficient of 2.3 was determined, implying that at least two dynamin dimers must interact to reach maximal GTPase activity (80). This cooperativity in GTPase activity was further shown to be linked to the assembly process (73). Structurally, this effect is due to the GTP-binding motif called switch 1 in the GTPase domain, which remains in a GTP-bound state conformation even if no nucleotide is present in the active site (60). Switch 1 positioning allows the GTPase domain to directly position itself in the most favorable hydrolytic conformation, and its positioning depends on interactions with other GTPase domains. No cooperativity toward GTP is found (80), meaning that each monomer burns its GTP independently of other monomers. It is a surprising result, as one would expect that the highly coordinated constriction of a helix requires a high degree of cooperativity between dynamin dimers. However, this result is confirmed by the fact that the time it takes for dynamin to break a single tube shows no cooperativity in GTP as well (51).
Taken together, structural and enzymatic analyses indicate the GTPase activity of dynamin mostly resembles motor-like activity. In other mechanoenzymes, such as molecular motors associated with the cytoskeleton, important conformational changes and a complex cycle of nucleotide/filament binding and unbinding are associated with the hydrolysis of ATP or GTP and the generation of force (Figure 2_a_). In the case of dynamin, the conformational change associated with GTP hydrolysis has been partially elucidated only recently. Also, a full understanding of the GTP hydrolysis cycle and associated conformational changes has not yet been reached, but the broad picture, as discussed below, allows us to draw further the analogy to other motors.
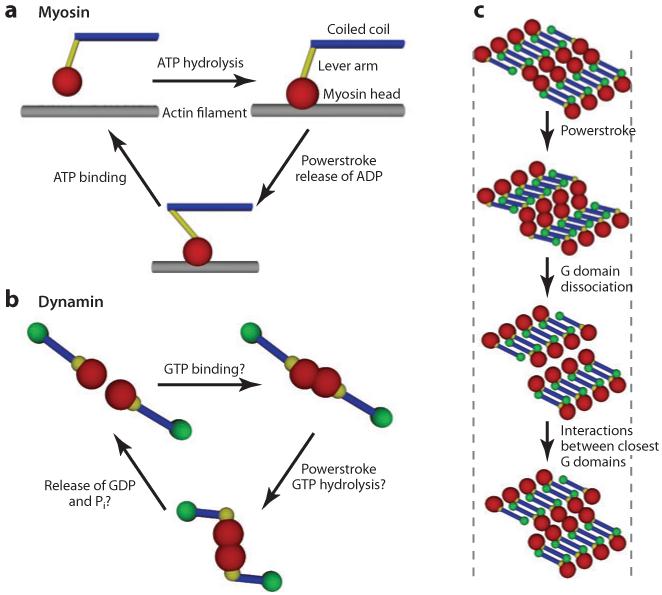
Figure 2.
Force-generating cycles for myosin and dynamin. (a) Actomyosin ATPase cycle. Upon ATP binding the myosin head (red ) detaches from the actin filament ( gray). After ATP hydrolysis myosin rebinds to actin. Release of ADP triggers the powerstroke: mechanical motion of the lever arm. (b) Putative dynamin GTPase cycle. In the case of dynamin, the powerstroke occurs during GTP hydrolysis. According to structural data, conformational changes are observed between the GMP-PCP-bound state and the GDP AIF4−-bound state. Thus, GDP release could be linked to dissociation of the G domain from its substrate, · i.e., the opposite G domain in the consecutive turn. GTP binding could then reactivate the G domain interactions. (c) GTPase cycles for two consecutive rows of four dimers. After each monomer completes GTP hydrolysis, the two adjacent turns have walked on each other, thus constricting the lipid tube. Note that GTPase cycles are not synchronized. Here cycles of the eight dimers are represented concomitantly for clarity.
Constriction of the Dynamin Helix
GTP hydrolysis leads to modifications in the dynamin helical structure. The earliest observation of a polymer conformational change was made on phosphatidylserine liposomes (75). When dynamin is added to these liposomes, it forms long membrane tubules decorated by the dynamin helix. When GTP is added, negative-stain electron microscopy shows that most of these tubules break into very small vesicles (20–30 nm diameter). However, some remain unbroken but clearly show a constricted appearance: The dynamin helix experiences a decrease in outer radius, from 25 to 20 nm, and in pitch, from 13 to 9 nm (75). Three-dimensional volume of the helical structure, reconstructed from cryo-EM images in the presence of GMP-PCP, shows a constricted state (87) compatible with the dimension measured from negative stain. The total number of dimers per helical turns decreases from 14.3 in the nonconstricted state to 13.3 in the constricted state (13), compatible with a torsion of the helix during GTP hydrolysis. Dynamin is a right-handed helix; thus, a right-handed twist of the helix reduces its pitch and diameter. This torsion has been experimentally observed (64) by attaching small beads to dynamin-coated tubules and following their rotation around the tube after the addition of GTP. Also, the supercoiling of the chloroplast division machinery upon GTP hydrolysis is probably dependent on torsion of dynamin polymer (86). A surprising result from structural studies is that constriction is obtained with nonhydrolyzable analogs, which do not promote fission. One reason could be that the use of GMP-PCP or GTPγS does not allow maximum constriction to be reached. Another plausible explanation is that constriction is mediated by torsion only partially, and the conformational change of dynamin thickens the coat, constricting the membrane (13, 49). But how is the GTP hydrolysis cycle linked to conformational change?
The first studies to address this issue were performed with dynamin helices associated with rigid nanorods made of galactoceramide, phosphatidylcholine, cholesterol, and PIP2 (73). The conformational change is observed when dynamin-coated nanorods are treated with GDP, whereas no change occurs when treated with GTPγS or when free of nucleotide. This finding is consistent with data on the structure of the GTPase domain, where the nucleotide-free structure is similar to the GTP-loaded state, as discussed above (60). Treatment with GTP shows intermediate behavior and the extension of some rings. In conclusion, the conformational change seems to be associated with the appearance of GDP, which has been confirmed by studies of several mutants of dynamin (48). However, there is a discrepancy between data on liposomes, where constriction is obtained with GTP and nonhydrolyzable analogs of GTP (87), and data on lipid nanorods, where deformation is obtained with GDP. This conformational change is visible at the whole polymer level, which uncommonly allows a direct visualization of the conformational change by standard EM techniques, but is driven by a molecular conformational change at the monomeric level. Only recently has the ΔPRD dynamin structure been obtained, visualizing the conformational change at the monomeric level (13).
A molecular view of the constriction process could be that dynamin GTPase domains of one helix turn walk onto the previous turn, forcing the turns to slide relative to one another. Recent structural data support this view and confirm the analogy we made with other molecular motors. At the molecular level, the crystal structure of dynamin without nucleotide or with GDP and aluminum fluoride [AlF4−, similar to the GDP + inorganic phosphate (Pi) conformation] identified an important conformational change: The angle between the GTPase domain and the stalk region (see Figure 2_b_) changes dramatically (25), which is compatible with the modification of the whole polymer structure observed in liposomes (see Figure 2). This modification is essentially due to the flexible element BSE, which induces a kink between the GTPase and the stalk in the constricted state (11). The BSE acts as a lever arm to transmit the movement to the rest of the structure. This mechanism to generate a powerstroke is very similar to that observed in myosins, as presented in Figure 2_a,b_ (74). In myosins, the ATPase domain is linked to the coiled-coil region by a flexible neck whose angle changes upon ATP hydrolysis. The sequence of conformational changes during the ATP hydrolytic cycle is now known in detail (74). Myosins walk on actin, and the angle between the myosin head (ATPase domain) and the actin filament changes upon release of ADP and Pi. In the case of dynamin, the difference between the structures of GMP-PCP and those of GDP · AlF4− suggests that the powerstroke is happening while GTP is hydrolyzed into GDP and Pi, consistent with data obtained on lipid nanorods (73). Another difference is that dynamin walks, not on a filament made of a different protein, but on the GTPase domains of the next turn, i.e., on its own filament. This peculiar helical structure constrains the conformational change because of the symmetry. As shown by Low & Löwe (45), the helical structure relies on direct GTPase domain interactions, and as a helical turn is intercalated into two other turns, bonds between several contiguous turns must be broken to allow sliding; otherwise, the whole helix cannot constrict. Bonds between GTPase domains are dependent on the nucleotide load of the dynamin GTPase domain (70). This property is directly reflected in the fact that GTP-bound dynamin polymerizes more easily than nucleotide-free dynamin because of tight bonds between GTPase domains of contiguous turns. We can then propose a putative molecular view of the GTP cycle of dynamin in which dynamin turn-to-turn bonds are transient (see Figure 2_b_), enabling relative sliding of turns (see Figure 2_c_). This would make the mechanism of dynamin even more similar to myosin, whose capability to contract is linked to its ability to bind actin, hydrolyze ATP and generate a powerstroke, and then detach to allow sliding of the filament while other myosin heads are pulling (see Figure 2_a_). In addition, this could explain why constriction is obtained with nonhydrolyzable analogs of GTP, which do not promote fission, and with GTP, which promotes fission. In the case of GTP, dynamin completes the full hydrolytic cycle, including detachment from the opposed dynamin turn, allowing the two juxtaposed turns to slide (Figure 2_c_). In the case of nonhydrolyzable analogs of GTP, constriction would thus be much less extended and stall at a larger radius, not promoting fission.
Dynamin, which was originally described as a mechanoenzyme, has a biochemistry and conformational change/GTP cycle similar to that of cytoskeletal molecular motors. Making an analogy to actin/myosin, one can see that dynamin is simultaneously the filament and the motors walking on the filaments combined into one helical structure. In addition to their amazing biochemistry and structural properties, cytoskeletal motors were one of the first systems for which single-molecule physics was essential to the understanding of their cycle and force-generating properties. Measuring forces, speeds, and displacements with nanophysics tools has led to a precise picture of the energy efficiency of these molecular motors. Recent membrane physics tools applied to dynamin have led investigators to decipher the kinetics and force generated by constriction.
Kinetics and Torque Generation by Dynamin Constriction
Dynamin constriction reduces the radius of the membrane tube enclosed in its helix. Membranes are viscoelastic sheets, and constriction is opposed by viscosity and elasticity of the membrane. Thus, dynamin must have enough strength to constrict the membrane tube. Dynamin generates rotational force, or torque, during its torsion/constriction conformational change. From the Canham-Helfrich theory, it is possible to evaluate the torque required for one turn of dynamin to constrict a membrane tube from a 10-nm radius (radius of nonconstricted dynamin) to a 5-nm radius (constricted radius in the presence of GMP-PCP), and values close to 500 pN · nm are found (51). In comparison, the rotational motor F1-ATPase generates maximal torques of 90 pN · nm (85). By evaluating the viscous drag on rotating beads attached to dynamin-coated tubules, and blocking them with magnetic tweezers, the maximum torque generated by dynamin was measured in the range of 1,000 pN · nm (51). The torque is large enough to constrict the membrane, making dynamin one of the most powerful nanomachines found in cells. Because 28 monomers of dynamin are needed to make one helical turn, many GTPs can be burned simultaneously. Dynamin torque increases linearly with the logarithm of the GTP concentration, as expected if the energy required for constriction comes primarily from the hydrolysis of GTP.
Another important parameter of constriction is how quickly dynamin constricts. As constriction induces torsional movements and reduces the radius of the membrane tube, it induces lipid flux within the membrane. One can thus expect constriction kinetics to be limited by membrane viscosity or membrane-helix friction. A theoretical study (42) has predicted that the dynamics of constriction could be damped by membrane-helix friction. Consistent with this prediction, the time required to complete constriction is quadratically dependent on the length of the dynamin coat. Long coats take more time to constrict than short coats (52). But the friction effect is observed only for tubes longer than 10 μm, far longer than dynamin helices found in vivo. In this study, the minimal time for constriction is a few hundred milliseconds, which has implications for discriminating between the several models proposed for the dynamin mechanism (see below).
In conclusion, structural data and biochemical data confirm that the dynamin polymer is able to constrict and that it generates enough strength to do so. GTP hydrolysis is coupled to a considerable conformational change at the monomeric level, driving the constriction of the whole polymer. Thus far, we have focused on the structure and the behavior of the dynamin helix. In the next section, we describe various models proposed in the literature and the facts supporting them. We discuss in light of all results which model is the most probable.
INDUCTION OF MEMBRANE FISSION
In a landmark paper (75) Sweitzer & Hinshaw showed that dynamin not only constricts but also chops membrane tubules into small vesicles, as seen by negative-stain electron microscopy. Later, Danino et al. (17) found that constriction, but not fission, was observed when tubules were treated in solution and observed by cryo-EM. According to the authors, a possible explanation for this difference was that the dynamin-coated tubules adsorbed to the microscopy grid prior to GTP treatment in the negative-stain protocol. This effect was later confirmed (64). Because constriction is not sufficient for fission, is the constriction model correct? Below, we detail different models that have emerged over the past 15 years and discuss their validity.
Molecular Switch GTPase
Because dynamin is a GTPase, its ability to function as a molecular switch, similar to the small G proteins, was called into question. In the GTP-bound form, dynamin would recruit other effectors to the neck of CCPs, which would promote fission. In support of this hypothesis, over-expression of Dyn1-K694A defective in self-assembly and of Dyn1-R725A defective in assembly-stimulated GTPase activity stimulates endocytosis (66). The authors proposed a model in which dynamin acts as a regulatory GTPase. Unassembled GTP-bound dynamin would be the active state that recruits fission machinery. Upon assembly, GTPase activity is stimulated and dynamin is inactivated. According to this model, assembly-defective mutants should stimulate endocytosis by impeding the hydrolysis of GTP, thus favoring the active GTP-bound state. However, this model was challenged by other experiments. Dyn1-I690K is defective in self-assembly (more strongly than Dyn1-K694A) and in assembly-stimulated GTPase activity. Overexpression of Dyn1-I690K in HeLa cells inhibits transferrin internalization (71). This dominant negative effect of a mutant defective in self-assembly shows that dynamin assembly is a required critical step in clathrin-mediated endocytosis. Also, as discussed above, the basic hydrolytic cycle of GTP by dynamin has properties similar to those of molecular motors, not of other molecular switch GTPases, which have very high affinity and low intrinsic GTPase activity. Moreover, a characteristic of switch GTPases is to have many partners that bind preferentially to the GTP-loaded conformation. Dynamin has many partners, but none of the described partners is binding specifically to the GTP state. Taken together, these results suggest that dynamin does not function as a molecular switch.
Spring Model
When dynamin is associated with lipid nanorods (73), the conformational change generates an extension of the pitch. The helical pitch increases from 11 to 20 nm when dynamin-coated nanorods are treated with GDP, whereas the pitch remains the same when the nanorods are treated with GTPγS or when free of nucleotide. The nanorods probably cannot be constricted because they are solid. In this case, as predicted by theory (42) and structural simulations (21), the conformational change observed in dynamin is an increased pitch rather than a constricted radius and a reduced pitch.
It was suggested that the membrane could be broken by rapid extension of the helix, tearing off the neck. Extended pitches of dynamin are seen in vivo (76), and theoretical modeling has been proposed to support this model (37). The validity of the spring model relies on the speed of the extension: If the dynamin helix extends faster than membrane can flow, then the membrane ruptures. If not, the membrane flows into the cylindrical volume of the helix and adjusts to the new conformation of the polymer without breaking. Thus, fission would occur if constriction were faster than the viscoelastic time of lipid membranes (the timescale below which membranes behave like an elastic solid rather than a viscous fluid), which is experimentally found to be less than 10 ms (7). As described above, the conformational change of dynamin occurs on the order of a few hundred milliseconds (52), more than 10 times slower than what is required. It seems unlikely that extension of the dynamin helix in a spring-like mode would generate enough drag in the lipid bilayer to tear the membrane.
Fission by Shearing
Another proposal that relies on the speed of constriction is the fission by shearing model, which proposes that torsion could lead to sufficient shearing, tearing off the membrane (41). As in the case of the spring model, how fast dynamin constricts is critical for this hypothetical model. Because it takes a few hundred milliseconds to constrict, it seems unlikely that dynamin constricts fast enough to shear off the membrane. A theoretical description of the conformational changes of dynamin-coated lipid tubes predicts three successive diffusive modes (42). The first mode occurs on a timescale of 100 μs and corresponds to very light (in the percent range) vertical expansion of the helix. During the second mode, which occurs within 100 ms, the tube radius decreases. In the last mode, which takes place within seconds, the helical pitch is reduced and the membrane is expelled from the helix. This theoretical model was supported by in vitro experiments (52), confirming that constriction occurs on timescales of 100 ms, which is longer than the viscoelastic time of membrane (less than 10 ms, see Reference 7). Therefore, membrane fission is not expected to occur by shearing.
Depolymerization
Warnock et al. (84) observed by sedimentation assay that dynamin structures are destabilized upon nucleotide treatment. The addition of 250 μM GTP releases 42% of dynamin structures preassembled at low ionic strength. Counterintuitively, GTPγS is more efficient in destabilizing dynamin structures: 58% of sedimentable dynamin is released with only 10 μM GTPγS. This would imply that GTP binding is sufficient to trigger dynamin disassembly. Hybrid polymer containing wild-type dynamin and the K44A mutant, which is defective in GTP binding and hydrolysis, is more stable upon GTPγS treatment, suggesting that a defect in GTP binding inhibits depolymerization. This finding led the authors to hypothesize that GTPase activity could induce cycles of dynamin disassembly and reassembly (84), given that in vivo, fast recycling of endocytic components is critical for synaptic vesicle production. Electron microscopy experiments also showed that dynamin helical structures are less organized after treatment with GTP (48, 73).
Two studies proposed a mechanism of membrane fission based on GTP-induced dynamin depolymerization. Bashkirov et al. (4) took advantage of electrophysiology techniques to follow in real time the variation in diameter of lipid nanotubes upon treatment with dynamin and GTP. By pulling a tube out of a black lipid membrane, with an electrophysiology pipette, and measuring the conductance as a function of the tube length, the authors calculated the radius of the nanotube (4). After adding GTP, the authors observed an increase in conductance, suggesting depolymerization. Then, the conductance instantaneously drops to zero, a signature of fission. This result is supported by live imaging of dynamin fission and biochemical assays on SUPER (supported bilayers with excess membrane reservoir) templates (58), showing that long coats are less efficient for fission than short coats are. The authors proposed that because long coats take more time to depolymerize, fission would be delayed. However, this result could be due to slower constriction for longer coats, as described above (52). Prior to fission, fluctuations of the dynamin coat punctae were observed, consistent with fluctuations in conductance for very short tubes in an electrophysiology assay (4). With this technique, very small radii of the membrane tube (ranging from 5 to 7 nm, including membrane thickness) inside the dynamin coat were measured prior to addition of GTP. The authors concluded that the dynamin polymer stabilizes the lipid nanotube in a state close to hemifission (a state in which the inner layer, but not the outer layer, of the membrane neck fuses onto itself). Then, GTP hydrolysis destabilizes the dynamin scaffold, releasing the membrane underneath, which can spontaneously hemifuse and break.
This proposition is counterintuitive, as membrane physics expects the constricted membrane to widen after release of the dynamin coat. Instabilities might appear if release was extremely fast, much faster than the viscoelastic time of membranes (less than 10 ms, close to 50 μs) (7), but as stated above, the conformational change of dynamin takes a few hundred milliseconds, and it is difficult to believe that dynamin would depolymerize much faster. Also, the timescales of dynamin fluctuations in both studies are in the 10-s range. Moreover, the generation of instabilities causing fission relies strictly on the fact that the radius of constricted non-GTP-treated dynamin tubes is extremely small, much smaller than the values found by other studies using various techniques (close to 10 nm) (17, 22, 63, 75, 78). Bashkirov et al. (4) neglected the Debye length (the screening length of electrostatic charges), which may account for smaller values (62). If the correct value is above 10 nm, it remains unlikely that the dynamin-coated tube can self-seal without further constriction (39). Finally, depolymerization of the dynamin coat following fission was not observed in other in vitro studies (51, 64, 75). The discrepancy may come from the fact that membrane-bound dynamin, but not polymerized dynamin, comes off the membrane upon GTP hydrolysis.
Depolymerization of dynamin is required in vivo, at least for the sake of endocytic component recycling; however, because the torque of dynamin receives most of its energy from GTP hydrolysis (51), it is unlikely that GTP energy is spent primarily on coat depolymerization. However, it is possible that depolymerization is a consequence of constriction, as stresses probably arise within the coat when it constricts.
Back to the Constriction Model
As stated above, the structure and biochemistry of dynamin support the hypothesis of a constricting helix. However, how constriction is involved in fission remained unclear until recently. The earliest model of dynamin proposed that constriction would reach the hemifission state, where the inner lipid layer of the tube would be removed, forming a cylindrical micelle structure locally. This hemifission state would resolve spontaneously to full fission. The largest radius of constriction needed to achieve spontaneous fission was theoretically calculated to be 3 nm (39). It remains unknown how much dynamin constricts upon GTP hydrolysis, even though cryo-EM three-dimensional reconstruction gives a constriction radius of 4.5 nm in the presence of GMP-PCP. This value is consistent with the fact that constriction is not sufficient for fission, as cryo-EM reconstructions show water-containing lumen and not a hemifissioned tube within the membrane tube.
Dynamin-mediated fission was shown to be nonleaky (4), confirming that it is mediated through a hemifission intermediate step. The hemifission intermediate step is also supported by the fact that lysolipids, which delay fusion by increasing the energy of the hemifusion intermediate (14), delay dynamin-mediated fission (51). This is consistent with the proposition that the hemifission and hemifusion intermediates are similar (38). How is this step reached? The explanation comesfrom a recent observation. Fission occurs at the edge between the dynamin-coated lipid and the pure lipid tube (51). Here, the membrane is highly stressed owing to mismatching radii: On one side of the tube, the radius is set by dynamin polymer, and on the other side, membrane tension and rigidity control the tube radius. The connective neck adopts a specific shape that locally increases the membrane elastic energy. This local increase facilitates fission at the edge of dynamin by reducing its energy barrier. Elastic parameters of the membrane, tension and rigidity, control the exact shape of the neck and its elastic energy. Theory predicts that the kinetics of the fission reaction depends exponentially on its energy barrier and thus on membrane tension and rigidity (Figure 3). By verifying this dependence experimentally, and calculating the local increase of membrane elastic energy, the energy barrier to fission is estimated to be 30–70 kBT. These values are similar to those of the energy barrier to fusion (43, 44).
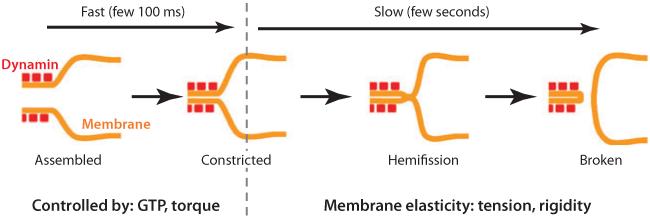
Figure 3.
Constriction model. Membrane fission occurs in two steps. In the first step, the dynamin helix constricts the lipid nanotube so that its radius decreases. This step is controlled by the concentration of GTP, as GTP is the energy source of dynamin, and the torque subsequently delivered. In the second step, the constricted tube spontaneously hemifuses and breaks. The kinetics of this step depends on membrane elasticity.
This picture of energetics explains several points. First, break is not random; it occurs where the membrane accumulates more stress (at the edge of the dynamin coat), not where it is the most constricted (below the dynamin coat). Second, tension and/or attachment to the substrate is needed for fission (17, 64), whereas constriction alone is not. Finally, fission kinetics is strongly dependent on the elastic parameters of the membrane: It takes approximately 1 min to break membranes at low tension (58) and tensed nanotubes break in a few seconds (4, 51, 52, 64). The dependence of fission time on tension also explains the previous observation that CCPs are blocked at the plasma membrane by hyperosmotic shock in live cells (30, 51), and that actin polymerization is required for dynamin-mediated fission in cultured cells (33).
CONCLUSION
After almost twenty years of researching how dynamin breaks membranes, several facts have been established. The dynamin helix constricts as shown by electron microscopy, biochemical, structural, and biophysical data. This constriction is necessary, but not sufficient, for fission, and appropriate control of the membrane elastic parameters is required. In cells, several machineries, such as actin, which has been involved in many fission reactions (33, 50), could help control membrane tension. Also, dynamin has many partners that have a role in membrane remodeling (69), and a future goal is to understand how the combined effects of dynamin and its partners change the dynamin fission reaction.
In conclusion, many hypotheses, all of which are supported by data, have been offered to explain how dynamin can break a lipid membrane. In our opinion, however, the earliest model of constriction, proposed on the basis of structural features of dynamin, is still supported by the clearest and most numerous data. Nonetheless, many aspects of the constriction model have to be confirmed, such as how much dynamin constricts upon GTP hydrolysis. All other models have less experimental support or can be reinterpreted as a refinement of the constriction model.
SUMMARY POINTS.
- The GTPase dynamin self-assembles into a helical polymer. At high concentration, its polymerization generates enough force to form membrane tubules.
- Upon hydrolysis of GTP, the helical polymer can constrict by torsion of the helix and a molecular conformational change, reducing the diameter of the membrane. This constriction is mediated by a conformational change that creates a powerstroke at the monomeric level.
- Constriction is required for fission, but it is not sufficient alone. Location and kinetics of membrane fission are controlled by local stress on the membrane, under the combined action of dynamin torque, membrane elasticity, and external pulling forces.
FUTURE ISSUES.
- Structural studies of dynamin with various GTP analogs, and kinetics studies of mutants and GTP fission reactions, should provide information about the limiting steps in the GTPase cycle of dynamin. The resulting findings should allow researchers to draw correlations between the nucleotide state and the conformational state.
- How do the interactions between dynamin activity and its partners modulate the kinetics of the fission reaction?
- Which aspects of the mechanism for dynamin-mediated membrane fission are general-izable to other dynamin-like proteins and to other fission machineries?
ACKNOWLEDGMENTS
The authors thank Martin Lenz for many helpful comments on the manuscript. We thank Stéphane Thore for his help in generating the molecular structure. Page constraints prevented us from citing all studies, especially work done on dynamin-like proteins, that have helped facilitate our understanding of dynamin. This work was supported by the Human Frontier Science Program Career Development Award (no. 0061/2008 to A.R.), a Swiss National Fund for Research grant (no. 31003A_130520/1 to A.R.), a European Research Council Starting Grant 2011 (no. 311536), the Société Académique de Genève, and the Swiss National Center for Competence in Research Program Chemical Biology.
Glossary
Membrane elasticity
physical property of the lipid bilayer that can be deformed (bent, stretched, or sheared) without breaking
Clathrin-coated pit (CCP)
membrane budding intermediate formed by polymerization of a clathrin cage on the plasma membrane
Phosphatidylinositol (4,5)-bisphosphate (PIP2)
a glycerophospholipid present in low amounts (below 1%) in plasma membranes; recruits proteins such as dynamin
Bundling signaling element (BSE)
structural domain of dynamin made of three α-helices; it acts as a lever arm between the GTPase domain and the stalk
ATP, GTP
nucleotides that fuel molecular motors upon being hydrolyzed into a bisphosphate (ADP or GDP) or an inorganic phosphate (Pi)
Powerstroke
corresponds to the transition between two molecular conformations giving rise to mechanical work
Torque
product of a force that rotates around an axis and the lever-arm distance
Hemifission
hypothetical intermediate state during fission reaction where the closest leaflets of the two bilayers have merged but the outer leaflets are still separate
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ahmadian MR, Hoffmann U, Goody RS, Wittinghofer A. Individual rate constants for the interaction of Ras proteins with GTPase-activating proteins determined by fluorescence spectroscopy. Biochemistry. 1997;36:4535–41. doi: 10.1021/bi962556y. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw CR. Muscle Contraction. Chapman & Hall; London/New York: 1993. p. 155. [Google Scholar]
- 3.Ban T, Heymann JAW, Song Z, Hinshaw JE, Chan DC. OPA1 disease alleles causing dominant optic atrophy have defects in cardiolipin-stimulated GTP hydrolysis and membrane tubulation. Hum. Mol. Genet. 2010;19:2113–22. doi: 10.1093/hmg/ddq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–86. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchiat C, Wang MD, Allemand J, Strick T, Block SM, Croquette V. Estimating the persistence length of a worm-like chain molecule from force-extension measurements. Biophys. J. 1999;76:409–13. doi: 10.1016/s0006-3495(99)77207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–32. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 7.Camley BA, Brown FL. Beyond the creeping viscous flow limit for lipid bilayer membranes: theory of single-particle microrheology, domain flicker spectroscopy, and long-time tails. Phys. Rev. E. 2011;84:021904. doi: 10.1103/PhysRevE.84.021904. [DOI] [PubMed] [Google Scholar]
- 8.Carr JF, Hinshaw JE. Dynamin assembles into spirals under physiological salt conditions upon the addition of GDP and γ-phosphate analogues. J. Biol. Chem. 1997;272:28030–35. doi: 10.1074/jbc.272.44.28030. [DOI] [PubMed] [Google Scholar]
- 9.Chappie JS, Acharya S, Leonard M, Schmid SL, Dyda F. G domain dimerization controls dynamin’s assembly-stimulated GTPase activity. Nature. 2010;465:435–40. doi: 10.1038/nature09032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappie JS, Acharya S, Liu YW, Leonard M, Pucadyil TJ, Schmid SL. An intramolecular signaling element that modulates dynamin function in vitro and in vivo. Mol. Biol. Cell. 2009;20:3561–71. doi: 10.1091/mbc.E09-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappie JS, Mears JA, Fang S, Leonard M, Schmid SL, et al. A pseudoatomic model of the dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell. 2011;147:209–22. doi: 10.1016/j.cell.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, et al. Multiple forms of dynamin are encoded by Shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–86. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Zhang P, Egelman E, Hinshaw JE. The stalk region of dynamin drives the constriction of dynamin tubes. Nat. Struct. Mol. Biol. 2004;v11:574–75. doi: 10.1038/nsmb762. [DOI] [PubMed] [Google Scholar]
- 14.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008;15:675–83. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark SG, Shurland DL, Meyerowitz EM, Bargmann CI, van der Bliek AM. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proc. Natl. Acad. Sci. USA. 1997;94:10438–43. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–34. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danino D, Moon KH, Hinshaw JE. Rapid constriction of lipid bilayers by the mechanochemical enzyme dynamin. J. Struct. Biol. 2004;147:259–67. doi: 10.1016/j.jsb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–27. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- 19.DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J. Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J. Cell Biol. 2009;186:793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyon JB, Zeitler B, Cheng J, Cheng AT, Cherone JM, et al. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat. Cell Biol. 2011;13:331–37. doi: 10.1038/ncb2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faelber K, Posor Y, Gao S, Held M, Roske Y, et al. Crystal structure of nucleotide-free dynamin. Nature. 2011;477:556–60. doi: 10.1038/nature10369. [DOI] [PubMed] [Google Scholar]
- 22.Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson SM, Brasnjo G, Hayashi M, Wölfel M, Collesi C, et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–74. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13(2):75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford MG, Jenni S, Nunnari J. The crystal structure of dynamin. Nature. 2011;477:561–66. doi: 10.1038/nature10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost A, Perera R, Roux A, Spasov K, Destaing O, et al. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–17. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao S, von der Malsburg A, Paeschke S, Behlke J, Haller O, et al. Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature. 2010;465:502–6. doi: 10.1038/nature08972. [DOI] [PubMed] [Google Scholar]
- 28.Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 1993;120:923–34. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, et al. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr. Biol. 2003;13:510–15. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 30.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinshaw JE, Schmid SL. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–92. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 32.Ingerman E, Nunnari J. A continuous, regenerative coupled GTPase assay for dynamin-related proteins. Methods Enzymol. 2005;404:611–19. doi: 10.1016/S0076-6879(05)04053-X. [DOI] [PubMed] [Google Scholar]
- 33.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J. Cell Biol. 2005;170:1021–27. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Iversen TG, Skretting G, van Deurs B, Sandvig K. Clathrin-coated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA. Proc. Natl. Acad. Sci. USA. 2003;100:5175–80. doi: 10.1073/pnas.0534231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 1989;9:3844–60. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozlov MM. Dynamin: possible mechanism of “Pinchase” action. Biophys. J. 1999;77:604–16. doi: 10.1016/S0006-3495(99)76917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozlov MM, Chernomordik LV. The protein coat in membrane fusion: lessons from fission. Traffic. 2002;3:256–67. doi: 10.1034/j.1600-0854.2002.030403.x. [DOI] [PubMed] [Google Scholar]
- 39.Kozlovsky Y, Kozlov MM. Membrane fission: model for intermediate structures. Biophys. J. 2003;85:85–96. doi: 10.1016/S0006-3495(03)74457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Goff L, Amblard F, Furst EM. Motor-driven dynamics in actin-myosin networks. Phys. Rev. Lett. 2002;88:018101. doi: 10.1103/PhysRevLett.88.018101. [DOI] [PubMed] [Google Scholar]
- 41.Lenz M, Morlot S, Roux A. Mechanical requirements for membrane fission: common facts from various examples. FEBS Lett. 2009;583:3839–46. doi: 10.1016/j.febslet.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Lenz M, Prost J, Joanny J. Mechanochemical action of the dynamin protein. Phys. Rev. E. 2008;78:11911. doi: 10.1103/PhysRevE.78.011911. [DOI] [PubMed] [Google Scholar]
- 43.Li F, Pincet F, Perez E, Eng WS, Melia TJ, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 2007;14:890–96. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- 44.Li F, Pincet F, Perez E, Giraudo CG, Tareste D, Rothman JE. Complexin activates and clamps SNAREpins by a common mechanism involving an intermediate energetic state. Nat. Struct. Mol. Biol. 2011;18:941–46. doi: 10.1038/nsmb.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Low HH, Löwe J. Dynamin architecture—from monomer to polymer. Curr. Opin. Struct. Biol. 2010;20:791–98. doi: 10.1016/j.sbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–89. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10:839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, et al. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–35. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 49.Mears JA, Ray P, Hinshaw JE. A corkscrew model for dynamin constriction. Structure. 2007;15:1190–202. doi: 10.1016/j.str.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell. Biol. 2010;12:645–54. doi: 10.1038/ncb2067. [DOI] [PubMed] [Google Scholar]
- 51.Morlot S, Galli V, Klein M, Chiaruttini N, Manzi J, et al. Membrane shape of the edge of the dynamin helix sets location and duration of the fission reaction. Cell. 2012;151:619–29. doi: 10.1016/j.cell.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morlot S, Lenz M, Prost J, Joanny JF, Roux A. Deformation of dynamin helices damped by membrane friction. Biophys. J. 2010;99:3580–88. doi: 10.1016/j.bpj.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muhlberg AB, Warnock DE, Schmid SL. Domain structure and intramolecular regulation of dynamin GTPase. EMBO J. 1997;16:6676–83. doi: 10.1093/emboj/16.22.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newton A, Kirchhausen T, Murthy V. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc. Natl. Acad. Sci. USA. 2006;103:17955–60. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obar RA, Collins CA, Hammarback JA, Shpetner HS, Vallee RB. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990;347:256–61. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- 56.Peter B. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–99. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 57.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 2004;5:133–47. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 58.Pucadyil TJ, Schmid SL. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–75. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, et al. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70:1100–14. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reubold TF, Eschenburg S, Becker A, Leonard M, Schmid S, et al. Crystal structure of the GTPase domain of rat dynamin 1. Proc. Natl. Acad. Sci. USA. 2005;102:13093–98. doi: 10.1073/pnas.0506491102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc. Natl. Acad. Sci. USA. 1997;94:8569–74. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roux A, Antonny B. The long and short of membrane fission. Cell. 2008;135:1163–65. doi: 10.1016/j.cell.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Roux A, Koster G, Lenz M, Sorre B, Manneville J-B, et al. Membrane curvature controls dynamin polymerization. Proc. Natl. Acad. Sci. USA. 2010;107:4141–46. doi: 10.1073/pnas.0913734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–31. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 65.Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, et al. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton’s tyrosine kinase. EMBO J. 1996;15:6241–50. [PMC free article] [PubMed] [Google Scholar]
- 66.Sever S, Muhlberg AB, Schmid SL. Impairment of dynamin’s GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–86. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]
- 67.Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–32. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 68.Shpetner HS, Vallee RB. Dynamin is a GTPase stimulated to high levels of activity by microtubules. Nature. 1992;355:733–35. doi: 10.1038/355733a0. [DOI] [PubMed] [Google Scholar]
- 69.Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 2000;1:161–72. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 70.Smirnova E, Shurland DL, Newman-Smith ED, Pishvaee B, van der Bliek AM. A model for dynamin self-assembly based on binding between three different protein domains. J. Biol. Chem. 1999;274:14942–47. doi: 10.1074/jbc.274.21.14942. [DOI] [PubMed] [Google Scholar]
- 71.Song BD, Yarar D, Schmid SL. An assembly-incompetent mutant establishes a requirement for dynamin self-assembly in clathrin-mediated endocytosis in vivo. Mol. Biol. Cell. 2004;15:2243–52. doi: 10.1091/mbc.E04-01-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorre B, Callan-Jones A, Manzi J, Goud B, Prost J, et al. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc. Natl. Acad. Sci. USA. 2012;109:173–78. doi: 10.1073/pnas.1103594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stowell MH, Marks B, Wigge P, McMahon HT. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nat. Cell. Biol. 1999;1:27–32. doi: 10.1038/8997. [DOI] [PubMed] [Google Scholar]
- 74.Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu. Rev. Biophys. 2010;39:539–57. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 75.Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–29. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 76.Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, et al. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–41. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 77.Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-γS in nerve terminals. Nature. 1995;374:186–90. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 78.Takei K, Slepnev V, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 79.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tuma PL, Collins CA. Activation of dynamin GTPase is a result of positive cooperativity. J. Biol. Chem. 1994;269:30842–47. [PubMed] [Google Scholar]
- 81.Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, et al. The role of dynamin 3 in the testis. J. Cell. Physiol. 2007;210:644–54. doi: 10.1002/jcp.20855. [DOI] [PubMed] [Google Scholar]
- 82.van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993;122:553–63. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Warnock DE, Baba T, Schmid SL. Ubiquitously expressed dynamin-II has a higher intrinsic GTPase activity and a greater propensity for self-assembly than neuronal dynamin-I. Mol. Biol. Cell. 1997;8:2553–62. doi: 10.1091/mbc.8.12.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warnock DE, Hinshaw JE, Schmid SL. Dynamin self-assembly stimulates its GTPase activity. J. Biol. Chem. 1996;271:22310–14. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- 85.Yasuda R, Noji H, Kinosita K, Jr, Yoshida M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell. 1998;93:1117–24. doi: 10.1016/s0092-8674(00)81456-7. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida Y, Kuroiwa H, Misumi O, Nishida K, Yagisawa F, et al. Isolated chloroplast division machinery can actively constrict after stretching. Science. 2006;313:1435–38. doi: 10.1126/science.1129689. [DOI] [PubMed] [Google Scholar]
- 87.Zhang P, Hinshaw JE. Three-dimensional reconstruction of dynamin in the constricted state. Nat. Cell Biol. 2001;3:922–26. doi: 10.1038/ncb1001-922. [DOI] [PubMed] [Google Scholar]