Mice with bad ends: mouse models for the study of telomeres and telomerase in cancer and aging (original) (raw)
Abstract
Telomeres are capping structures at the ends of eukaryotic chromosomes, which consist of repetitive DNA bound to an array of specialized proteins. Telomeres are part of the constitutive heterochromatin and are subjected to epigenetic modifications. The function of telomeres is to prevent chromosome ends from being detected as damaged DNA. Both the length of telomere repeats and the integrity of the telomere-binding proteins are important for telomere protection. Telomere length is regulated by telomerase, by the telomere-binding proteins, as well as by activities that modify the state of the chromatin. Various mouse models with altered levels of telomerase activity, or mutant for different telomere-binding proteins, have been recently generated. Here, I will discuss how these different mouse models have contributed to our understanding on the role of telomeres and telomerase in cancer and aging.
Keywords: aging, cancer, mouse, telomerase, telomeres
The making of a telomerase scientist
The discovery of telomerase has fascinated me since my early days as a PhD student with Margarita Salas at the ‘Severo Ochoa' Molecular Biology Center in Madrid. It was exactly 20 years ago that telomerase activity was first discovered in the ciliate Tetrahymena by Greider and Blackburn (1985). They first named this activity ‘telomere terminal transferase' for its capacity to elongate telomeric primers in the absence of a DNA template. Soon after they discovered that telomerase was a ribonucleoprotein that used an essential RNA component as a template, and therefore had reverse transcriptase activity (Greider and Blackburn, 1987, 1989). The discovery of telomerase was not simply down to mere chance—its existence was predicted on the basis of DNA duplication, and its importance for cancer and aging soon became clear. Polymerases that replicate ends were not, however, entirely unfamiliar to me. During my PhD studies, I had already studied a DNA polymerase implicated in maintaining the ends of the linear genome of ø29 bacteriophage, through its ability to use a terminal protein as primer. As Margarita probably still recalls from my very first interview, I expressed an early interest in cancer and aging. How could I ever have predicted at that time though that chromosome ends and human diseases were indeed related?
Completion of my PhD studies thankfully coincided with Carol Greider setting up her own research group at the Cold Spring Harbor Laboratory in Long Island, NY (CSHL). The CSHL, as it turned out, had two special connections with telomere biology. Not only was it the home to the majority of McClintock's (1941) research work, during which time she had described the existence of a special structure at the ends of chromosomes that prevented them from being ‘sticky', but its then Director, Watson (1972), had predicted that material from the ends of chromosomes was lost every time that a cell divides due to the so-called end-replication problem. Consequently, when Carol accepted my application to work in her group, I was truly confident that I was making the right career choice. I can still recall that my project was ‘to identify the mouse telomerase RNA and to generate a knockout mouse'. Upon reflection, I am still astonished that we actually managed to achieve both objectives in less than 3 years. This of course was only possible thanks to the essential collaboration of many other scientists, especially those working at Carol's lab, at the Geron Corporation, as well as thanks to Han-Wong Lee who was involved in generating the mice. Without a doubt, the most exhilarating point of my scientific career to date is the discovery that the knockout mouse for the telomerase RNA did not show any detectable telomerase activity. Just as Titia de Lange had pointed out so poignantly, telomerase ‘was not essential for life, nor for sex', since the mice were viable and fertile as long as their telomeres were long enough. These mice have been the basis of a major part of my scientific contribution and, I hope, of interpreting the role of telomeres and telomerase in cancer and aging.
You can imagine my euphoria when last summer, Frank Gannon, Director of EMBO, called me up to announce that I had been awarded the 2004 EMBO Gold Medal. This award represents a two-fold triumph: not only was my work being consequently acknowledged by a large community of European scientists, but also telomerase was being recognized by EMBO as an interesting and important research subject. Back in 1993, however, when I was applying for fellowships to work with Carol, three main European agencies rejected my applications on the basis that the research subject was ‘still very new and uncertain', and ‘the mammalian genes were not even cloned'. It is to my great satisfaction to think that I, along with many other researchers, may have contributed to this complete turn-around in perception.
Telomeric chromatin
Vertebrate telomeres are composed of tandem repeats of the TTAGGG sequence, as well as of a number of associated proteins (Blackburn, 2001; Chan and Blackburn, 2002; de Lange, 2002). Telomeres are also characterized by having a 150–200 nucleotide-long 3′-overhang of the G-rich strand, the so-called G-strand overhang (de Lange, 2002). The length of the double-stranded TTAGGG track varies from ∼10 kb at human telomeres to >40 kb in mouse inbreed strains (Zijlmans et al, 1997). The current model is that telomeres can form a structure that physically hides the 3′-overhang from cellular activities that may be hazardous for its integrity, such as DNA repair activities and nucleases. The most accepted telomere structure model is based on electron microscopy studies, which suggest that the 3′-overhang can fold back and invade the double-stranded region of the telomere forming the so-called T-loop and generating a displacement loop, or D-loop (Griffith et al, 1999; Nikitina and Woodcock, 2004). T-loops have been recently proposed to represent a primordial mechanism for chromosome end protection (de Lange, 2004).
Proteins that bind to the double-stranded TTAGGG region, such as TRF1 and TRF2, or that bind to the single-stranded G-strand overhang, such as Pot 1, have been shown to influence both telomere capping and telomere length (Chong et al, 1995; Bilaud et al, 1997; Broccoli et al, 1997; van Steensel et al, 1998; Baumann and Cech, 2001; Loayza and De Lange, 2003). TRF1 and TRF2 have been also visualized at telomeric T-loops (Griffith et al, 1999), and demonstrated to be negative regulators of telomere length (Smogorzewska et al, 2000). TRF1 function is regulated by TIN2 (Kim et al, 1999), and by the poly(ADP-ribose) polymerases TANK1 (also known as tankyrase) and TANK2 (Smith et al, 1998; Kaminker et al, 2001). In particular, TIN2 is a TANK1 modulator and controls telomere length via the TRF1 protein complex; furthermore, TIN2 can also bind to the TRF2 complex (Kim et al, 2004; Ye and de Lange, 2004a; Ye et al, 2004b). In addition to its role at telomeres, TANK1 has been recently demonstrated to be essential for separation of sister chromatid telomeres during mitosis, suggesting the existence of a new telomere-specific cohesion which is regulated by poly(ADP-ribosylation) (Dynek and Smith, 2004). Finally, TRF1 interacts with Pot 1, and this interaction has been proposed to convey information from the double-stranded telomere region to the single-stranded 3′-overhang (Loayza and De Lange, 2003). Recently, a new Pot-1 interacting protein (PTOP/PIP1) has been identified and shown to be important for telomere length regulation by the TRF1 complex (Liu et al, 2004; Ye et al, 2004c). All these findings suggest that TRF1 forms a multi-protein complex, which is involved in telomere length control and that contains at least TRF1, TIN2, the TANK1 and TANK2 tankyrases, Pot-1 and PTOP/PIP1, and may also contain TRF2 through its interaction with TIN2 (Figure 1). Interestingly, the role of TRF1 in the context of the organism seems to go beyond telomeres, since mice with targeted deletion of TRF1 are embryonic lethal in the absence of loss of telomere capping or telomere shortening (Karlseder et al, 2003).
Figure 1.
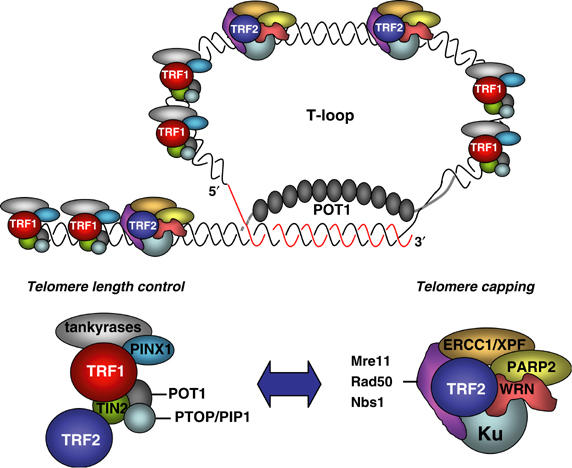
Telomere-binding proteins. Scheme showing the telomere in a T-loop conformation, as well as with different protein complexes found at mammalian telomeres. The TRF1 complex has been shown to influence telomere length, while the TRF2 complex has been shown to influence both telomere length and telomere capping.
TRF2 has been proposed to have a fundamental role in protecting the G-strand overhang from degradation, as well as in preventing telomeric fusions (van Steensel et al, 1998). TRF2 also recruits a number of proteins to the telomeres, many of which are involved in different DNA repair processes (Figure 1). In particular, TRF2 recruits the MRE11 complex to telomeres (Zhu et al, 2000). The MRE11 complex is composed of RAD50, MRE11 and NBS1 and is a key component of the homologous recombination (HR) and non-homologous end-joining pathways (NHEJ) involved in DNA double-strand break (DSB) repair. TRF2 also interacts with other DNA repair proteins such as PARP-2 (Dantzer et al, 2004), Ku proteins (Song et al, 2000), Werner (Opresko et al, 2004), and the nucleotide excision repair complex XPF/ERCC1 (Zhu et al, 2003) among others. Interestingly, XPF/ERCC1 has been identified as the exonuclease that resects the 3′-overhang in the absence of functional TRF2 (Zhu et al, 2003). In addition, TRF2 has been recently shown to specifically bind to ATM and to block the ATM-dependent DNA damage response, suggesting that TRF2 could be specifically inhibiting ATM activation at telomeres (Karlseder et al, 2004). Finally, TRF2 recruits hRAP1 to human telomeres. hRAP1 is the homologue of yeast RAP1 protein and its overexpression causes telomere elongation (Li et al, 2000; Li and de Lange, 2003). No mouse models for TRF2 are available to date.
Besides their known role in DNA repair, the different repair proteins present at telomeres also have a fundamental role in telomere metabolism. In particular, the study of Ku86- and DNA-PKcs-deficient mice has indicated that these proteins are also required for telomere protection (reviewed in Smith and Jackson, 1999; Goytisolo and Blasco, 2002). In particular, abrogation of either Ku86 or DNA-PKcs results in telomeric fusions characterized by showing TTAGGG repeats at the fusion point (Bailey et al, 1999, 2001; Hsu et al, 2000; Samper et al, 2000; Gilley et al, 2001; Goytisolo et al, 2001; Espejel et al, 2002a, 2002b). These end-to-end chromosome fusions are not the result of telomere shortening below a minimum length, but rather they are due to loss of telomere capping. In addition, these fusions have been shown to preferentially involve telomeres produced by leading strand synthesis, thus suggesting a role for these proteins in the post-replicative processing of the leading strand telomere, that is, to generate the 3′G-strand overhang (Bailey et al, 2001; Jaco et al, 2004).
Deficiency in either Ku86 or DNA-PKcs also influences telomere length, in accordance with a role for these proteins in generating or maintaining a proper telomere structure. In particular, both in plants and mice Ku86 acts as a negative regulator of telomerase (Espejel et al, 2002a; Riha et al, 2002). In contrast, human cells deficient for Ku86 show shorter telomeres and a dramatic loss of viability, suggesting important differences in the role of Ku86 at both human and mouse telomeres (Jaco et al, 2004; Myung et al, 2004). DNA-PKcs has been shown to cooperate with telomerase in telomere length maintenance, and mice doubly deficient for both activities show an accelerated rate of telomere loss (Espejel et al, 2002b). Also in agreement with this notion, single mutant DNA-PKcs mice show decreased telomere length with age, as well as with increasing mouse generations compared to the wild-type controls (Espejel et al, 2004). Besides their roles in telomere capping and telomere length regulation, Ku86 and DNA-PKcs have also been shown to be essential in signalling and processing critically short telomeres as damaged DNA (Espejel et al, 2002a, 2002b).
In addition to NHEJ, HR also plays a role in telomere biology. In particular, proteins involved in HR-mediated DNA repair, such as Rad54 and Rad51D, are important for telomere capping and telomere length regulation, suggesting that HR has an important role at mammalian telomeres (Jaco et al, 2003; Tarsounas et al, 2004). Since T-loop structures resemble in part an intermediate of HR, it has been proposed that HR activities may have an important role in the regulation of T-loops at telomeres (de Lange, 2004; Wang et al, 2004).
Epigenetic regulation of telomeric chromatin
Human and mouse telomeres show nucleosome arrays, suggesting that they may be subjected to histone modifications (Tommerup et al, 1994). Histone modifications include acetylation, methylation and phosphorylation, which in turn generate a repertoire of chromatin structures that can regulate various cellular responses (Jenuwein and Allis, 2001; Lachner et al, 2001). In particular, constitutive heterochromatin is found at transcriptionally inactive (‘silenced'), repetitive genomic regions, such those of pericentric chromatin, and it is characterized by hypermethylation of DNA, hypoacetylation of histones, and hypermethylation of histones H3 and H4. In particular, H3-K9 trimethylation by the Suv39h histone methyltransferases (HMTases) as well as H4-K20 trimethylation by the Suv4-20h HMTases are two main hallmarks of pericentric heterochromatin (Peters et al, 2001, 2003; Schotta et al, 2004). First, H3-K9 trimethylation creates a binding site for the heterochromatin protein 1 (HP1) family of proteins (Lachner et al, 2001), which mediate heterochromatin formation by recruiting the Suv4-20 HMTases (Schotta et al, 2004).
Telomeres have also been shown to be part of the constitutive heterochromatin in yeast and flies (Hecht et al, 1995; Savitsky et al, 2002; Cenci et al, 2003; Perrod and Gasser, 2003). Furthermore, yeast and flies defective for activities that modify the state of chromatin also have abnormal telomere function and telomere length regulation (Savitsky et al, 2002; Cenci et al, 2003; reviewed in Perrod and Gasser, 2003). In particular case of flies, HP1 mutations show defective telomere capping, as well as increased recombination at telomeres, suggesting that telomere function can be regulated by epigenetic modifications (Fanti et al, 1998; Cenci et al, 2003). In mice, it has been recently described that telomeres are enriched for trimethylated H3-K9 and for HP1, similar to pericentric chromatin (Garcia-Cao et al, 2004). Furthermore, it has been established that the activity of the Suv39h1 and Suv39h2 HMTases is required to maintain both H3-K9 trimethylation and HP1 binding at telomeres (Garcia-Cao et al, 2004). These findings suggest that telomeres have the hallmarks of constitutive heterochromatin, and predict that epigenetic errors at telomeres may also alter telomere function. In fact, mice doubly deficient for the Suv39h1 and Suv39h2 HMTases show abnormally elongated telomeres, suggesting that loss of heterochromatic features at telomeres results in a more ‘open' chromatin state, which in turn could facilitate the access of telomerase or other telomere-elongating activities to the chromosome end (Garcia-Cao et al, 2004) (Figure 2 for model of telomere heterochromatin assembly). Alternatively, loss of heterochromatic features at telomeres may alter the expression of telomere-length regulator genes, a phenomenon known as ‘telomere position effect' (TPE), which in turn is related to the property of telomeric chromatin to repress or silence neighboring genes. This phenomenon has been extensively studied in budding yeast (reviewed in Perrod and Gasser, 2003), but is less well understood in mammals (Baur et al, 2001; Koering et al, 2002). Similarly, epigenetic modifications could also regulate the binding of specific proteins, such as TRF1 and TRF2, to telomeres. Indeed, a reproducible increase in TRF1 binding per amount of TTAGGG repeats was detected in Suv39h double null telomeres, reflecting on a change in telomere architecture (Garcia-Cao et al, 2004).
Figure 2.
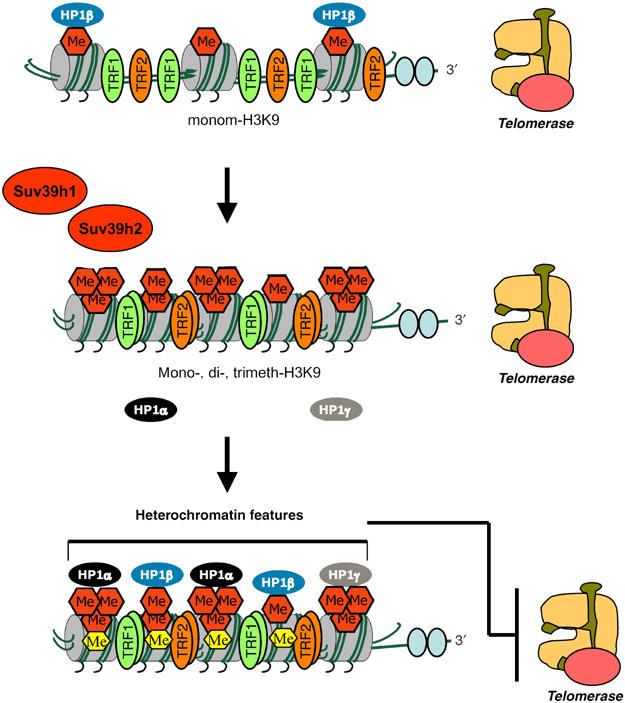
Assembly of telomeric heterochromatin. Mammalian telomeres contain features of the constitutive heterochromatin such as enrichment for H3-K9 di- and trimethylation, as well as binding of the HP1 family of proteins, similar to that previously described for pericentric hetochromatin. The Suv39h1 and Suv39h2 HMTases are required for the di-and trimethylation of H3-K9 at telomeres, which in turn recruits the HP1 proteins. Telomeric chromatin in SUV39DN cells also shows decreased binding of the HP1 proteins. These epigenetic modifications contribute to a ‘closed' chromatin state, which may regulate the access of telomerase to the telomeres.
Finally, these results predict that, besides the Suv39h HMTases, other activities that modify the state of the chromatin may also regulate telomere function (Jenuwein, 2001). In addition, the fact that epigenetic errors can alter telomere length in mammals may explain abnormal re-setting of telomere length in cloned animals (Shiels et al, 1999; Lanza et al, 2000).
The telomerase enzyme
Telomerase, the cellular reverse transcriptase that adds TTAGGG repeats onto pre-existent telomeres, is the main regulator of telomere length in mammalian cells (Collins and Mitchell, 2002). Telomerase consists of two essential components, a reverse transcriptase subunit known as Telomerase Reverse Transcriptase (Tert) and an RNA molecule or Telomerase RNA component (Terc), which contains the template for the synthesis of new telomeric repeats (Collins, 2000). Most human normal somatic cells do not have sufficient telomerase and undergo telomere attrition coupled to cell division (Harley et al, 1990). Telomere loss in the absence of telomerase activity in telomerase-deficient mice eventually results TTAGGG-exhausted chromosome ends, end-to-end chromosome fusions and loss of cell viability (Blasco et al, 1997; Lee et al, 1998; Herrera et al, 1999a). Re-introduction of telomerase prevents critical shortening of telomeres and allows viability both in cultured cells and in the context of the telomerase-deficient mouse (Bodnar et al, 1998; Hemann et al, 2001b; Samper et al, 2001), demonstrating that short telomeres trigger rapid loss of cell viability unless they are rescued by telomerase. In this regard, telomerase may also prevent critical telomere shortening in more than 90% of all human tumors, which reactivate telomerase at some point during their formation (Hiyama and Hiyama, 2002). It has been proposed that telomerase inhibition could be an effective way to abolish tumor growth by provoking telomere shortening to a critical length (Blasco, 2002). In addition, there is evidence that telomerase might enhance survival and promote proliferation independently of telomere length, favoring tumor growth even at stages when telomeres are sufficiently long (Mattson et al, 2001; Blasco, 2002) (see below).
Alternative mechanisms of telomere length maintenance
Some human cell lines and tumors that lack telomerase activity, however, are still able to maintain or elongate their telomeres by alternative mechanisms to telomerase, which have been termed alternative lengthening of telomeres (ALT) (Henson et al, 2002). ALT-positive cells are characterized by the simultaneous presence of long and short telomeres in the same nucleus, as well as by the co-localization of telomere-binding proteins and PML in the so-called ALT-associated PML bodies (APB) (Bryan et al, 1997; Dunham et al, 2000). Very little is known, however, on the mechanisms underlying ALT in mammalian cells. In yeast, HR and mismatch repair (MMR) pathways have been involved in telomerase-independent telomere elongation (Lundblad, 2002), suggesting that HR is one of the mechanisms for ALT-mediated rescue of short telomeres.
In the case of mammalian cells, there is increasing evidence that a number of factors can influence telomere length in the absence of significant changes in telomerase activity. Some of these factors are proteins with known roles in HR, such as Rad54, which is central to the HR DNA repair pathway. In particular, mice deficient for Rad54 show a significant loss of telomeric sequences in the absence of changes in telomerase activity (Jaco et al, 2003). These mice also show a higher frequency of end-to-end chromosome fusions, indicating a role for Rad54 in telomere capping (Jaco et al, 2003). More recently, Rad51D, a Rad51 paralog required for normal levels of genetic recombination, has been also shown to be required for telomere length maintenance and telomere capping (Tarsounas et al, 2004). The role of Rad54 and Rad51D in telomere length maintenance may also suggest that HR could be at the basis of the telomerase-independent telomere maintenance mechanisms in mammals.
In addition, activities that modify the state of the telomeric heterochromatin (i.e., Suv39h HMTases) are also likely to influence both telomerase action at telomeres as well as the ALT pathway (Garcia-Cao et al, 2004). Similarly, a connection between cell cycle regulators and telomere length has been recently established. In particular, abrogation of p107 and p130, two Rb-family members, results in a massive elongation of telomeres in the absence of changes in telomerase activity, suggesting a connection between cell cycle regulation and telomere length control (García-Cao et al, 2002).
The telomerase-deficient mouse model
A telomerase-deficient mouse model has been generated by the elimination of the gene encoding for the murine Terc gene, Terc−/− mice (Blasco et al, 1995, 1997). These mice are viable, but only a limited number of generations can be derived before loss of viability is observed due to telomere loss and increased end-to-end fusions (Blasco et al, 1997; Lee et al, 1998). The phenotypes associated to telomere dysfunction in these mice include (i) male and female infertility (Lee et al, 1998; Herrera et al, 1999a; Hemann et al, 2001a); embryonic mortality due to a defective closure of the neural tube (Herrera et al, 1999b); (ii) small size and severe intestinal atrophy (Herrera et al, 1999a; Rudolph et al, 1999); (iii) spleen atrophy and reduced proliferation of B and T lymphocytes (Lee et al, 1998; Herrera et al, 1999a); (iv) impaired germinal centre function (Herrera et al, 2000); (v) reduced angiogenic potential (Franco et al, 2002); (vi) reduced proliferative potential of the bone marrow stem cells (Samper et al, 2002); (vii) heart dysfunction (Leri et al, 2003); (viii) reduced proliferative capacity of adult neural stem cells (Ferron et al, 2004). These findings indicate that a minimal telomere length is necessary to maintain tissue homeostasis in the mouse, and predict that telomere shortening with age in humans may also lead to similar disease states, thus contributing to the pathobiology of aging. In this regard, a number of human premature aging syndromes, such as Werner's syndrome and Ataxia telangiectasia, have been modelled in mice only when in combination with telomerase deficiency and short telomeres in the context of the telomerase-deficient mouse model (Wong et al, 2003; Chang et al, 2004), suggesting that short telomeres are an important component in the pathobiology of these premature-aging diseases as well as possibly in diseases that are aging-related.
Importantly, it has been demonstrated that telomerase can re-elongate critically short telomeres in the context of the late-generation telomerase-deficient mice and prevent their premature aging phenotypes (Samper et al, 2001). In particular, telomerase is able to recognize short telomeres and to extend them, preventing the occurrence of end-to-end fusions and the appearance of phenotypes in these mice (Hemann et al, 2001b; Samper et al, 2001). These findings open the possibility of using telomerase re-introduction as a putative gene therapy for human premature aging syndromes that are characterized by a faster rate of telomere loss such as Dyskeratosis congenita (Collins and Mitchell, 2002), as well as for age-associated pathologies.
Finally, the telomerase-deficient mouse model has provided strong evidence that short telomeres suppress tumor progression, in agreement with the fact that telomerase activity is upregulated in most human tumors (González-Suárez et al, 2000). This tumor suppressor phenotype coincides with p53 upregulation in Terc−/− mice (González-Suárez et al, 2000). In fact, p53 has been proposed to be sensing short telomeres and contributing to cessation of growth (González-Suárez et al, 2000; Leri et al, 2003). Telomerase deficiency in combination with deficiencies in tumor suppressor genes other than p53 significantly reduce carcinogenesis (Chin et al, 1999; Greenberg et al, 1999; Artandi et al, 2000; Rudolph et al, 2001; Wong et al, 2003), suggesting that a telomerase inhibitor may be effective in cessation of tumor growth. Importantly, the antitumor effect of telomerase inhibitors may be enhanced in combination with genotoxic agents, as short telomeres also result in a higher sensitivity to these agents (Goytisolo et al, 2000; Wong et al, 2000). In particular, critically short telomeres and dysfunctional telomeres have been recently shown to interfere with the proper repair of DSB in the genome, thus increasing chromosomal instability and the sensitivity to genotoxic agents (Latre et al, 2003; Bailey et al, 2004).
A role for telomerase-promoting growth independent of telomere length
Telomerase activation during human tumor progression is though to be required to rescue critically short telomeres, thus allowing cell viability and tumor growth (Figure 3). Intriguingly, telomerase activity is also upregulated during mouse tumorigenesis, even though mice have much longer telomeres than humans (Blasco et al, 1996; Broccoli et al, 1996). This fact suggests that telomerase might have additional roles in promoting tumorigenesis, which are not solely mediated by telomere elongation. In support of this notion, first-generation (G1) telomerase-deficient mice, Terc−/− mice, which lack telomerase activity but still have long telomeres, were shown to have less skin tumors than wild-type mice following skin chemical carcinogenesis, indicating a negative impact of telomerase deficiency on tumor growth even in the presence of sufficiently long telomeres (González-Suárez et al, 2000).
Figure 3.

Role of telomerase in tumorigenesis. Telomerase is re-activated in more than 90% of all types of human tumors. Telomerase re-activation in tumors confers a proliferative advantage through two mechanisms: (i) rescue of critically short telomeres and prevention of cell death or cell arrest, (ii) telomere-length independent effects on survival and proliferation.
Additional evidence for a role of telomerase in promoting tumorigenesis independently of telomere length comes from the study of mice that overexpress the catalytic component of mouse telomerase (Martín-Rivera et al, 1998) in basal keratinocytes, the so-called K5-Tert mice (González-Suárez et al, 2001). K5-Tert mice show high levels of telomerase activity and long telomeres in the skin (González-Suárez et al, 2001). K5-Tert mice were found to be more susceptible to developing tumors than wild-type mice upon chemical carcinogenesis of the skin (González-Suárez et al, 2001). In addition, when these mice were left to age, they showed a decreased viability during the first year of life compared to the corresponding wild-type controls due to a significant increase in spontaneous tumors (González-Suárez et al, 2001, 2002). Mice with transgenic telomerase expression under a β-actin constitutive promoter, or under a thymus-specific promoter (Lck-Tert mice), also showed an increased incidence of spontaneous tumors (Artandi et al, 2002; Canela et al, 2004). Interestingly, K5-Tert mice that do not die from tumors during the first year of age show an increased survival at older ages, as well as a maximum lifespan extension compared to the wild-type littermates, which is coincidental with increased tissue fitness of the germ line and the kidney (González-Suárez et al, 2005). These findings suggest antagonistic roles of Tert in cancer and aging (González-Suárez et al, 2005). These findings are in line with data obtained from cultured cells, which also suggest a role for telomerase in enhancing survival and proliferation in the presence of very long telomeres. In particular, the epidermal growth factor receptor (EGFR) is upregulated in cells overexpressing Tert and this upregulation is required to mediate the telomere-length-independent effects of Tert overexpression on cell proliferation (Smith et al, 2003).
All together, these findings suggest that telomerase activation at early stages of tumor growth may actively promote tumor growth and survival even if telomeres are still sufficiently long, and that telomerase activation could favor tumorigenesis by at least two different mechanisms: by signaling proliferation and promoting growth independently of telomere length, and by rescuing tumor cells with critically short telomeres (Figure 3).
Figure 1.

María A Blasco
Acknowledgments
Research in the laboratory of MAB laboratory is funded by the MCYT (SAF2001-1869, GEN2001-4856-C13-08), by the Regional Government of Madrid, CAM (08.1/0054/01), by the European Union (TELOSENS FIGH-CT-2002-00217, INTACT LSHC-CT-2003-506803, ZINCAGE FOOD-CT-2003-506850, RISC-RAD F16R-CT-2003-508842), and the Josef Steiner Cancer Award 2003. MA Blasco's laboratory is funded by the MCyT (SAF2001-1869, GEN2001-4856-C13-08), by the Regional Government of Madrid (08.1/0054/01), European Union (TELOSENS FIGH-CT-2002-00217, INTACT LSHC-CT-2003-506803, ZINCAGE FOOD-CT-2003-506850, RISC-RAD FI6R-CT-2003-508842), and the Josef Steiner Award 2003.
References
- Artandi SE, Alson S, Tietze MK, Sharpless NE, Ye S, Greenberg RA, Castrillon DH, Horner JW, Weiler SR, Carrasco RD, DePinho RA (2002) Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc Natl Acad Sci USA 99: 8191–8196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406: 641–645 [DOI] [PubMed] [Google Scholar]
- Bailey SM, Conforth MN, Kurimasa A, Chen DJ, Goodwin EH (2001) Strand-specific postreplicative processing of mammalian telomeres. Science 293: 2462–2465 [DOI] [PubMed] [Google Scholar]
- Bailey SM, Cornforth MN, Ullrich RL, Goodwin EH (2004) Dysfunctional mammalian telomeres join with DNA double-strand breaks. DNA Repair 3: 349–357 [DOI] [PubMed] [Google Scholar]
- Bailey SM, Meyne J, Chen DJ, Kurimasa A, Li GC, Lehnert BE, Goodwin EH (1999) DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc Natl Acad Sci USA 96: 14899–14904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Cech TR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 [DOI] [PubMed] [Google Scholar]
- Baur JA, Zou Y, Shay JW, Wright WE (2001) Telomere position effect in human cells. Science 292: 2075–2077 [DOI] [PubMed] [Google Scholar]
- Bilaud T, Brun C, Ancelin K, Koering CE, Laroche T, Gilson E (1997) Telomeric localization of TRF2, a novel human telobox protein. Nat Genet 17: 236–239 [DOI] [PubMed] [Google Scholar]
- Blackburn EH (2001) Switching and signaling at the telomere. Cell 106: 661–673 [DOI] [PubMed] [Google Scholar]
- Blasco MA (2002) Telomerase beyond telomeres. Nat Rev Cancer 2: 627–632 [DOI] [PubMed] [Google Scholar]
- Blasco MA, Funk WD, Villeponteau B, Greider CW (1995) Functional characterization and developmental regulation of mouse telomerase RNA. Science 269: 1267–1270 [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34 [DOI] [PubMed] [Google Scholar]
- Blasco MA, Rizen M, Greider CW, Hanahan D (1996) Differential regulation of telomerase activity and its RNA component during multistage tumorigenesis. Nat Genet 12: 200–204 [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349–352 [DOI] [PubMed] [Google Scholar]
- Broccoli D, Godley LA, Donehower LA, Varmus HE, de Lange T (1996) Telomerase activation in mouse mammary tumors: lack of detectable telomere shortening and evidence for regulation of telomerase RNA with cell proliferation. Mol Cell Biol 16: 3765–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccoli D, Smogorzewska A, Chong L, de Lange T (1997) Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet 17: 231–235 [DOI] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR (1997) Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med 3: 1271–1274 [DOI] [PubMed] [Google Scholar]
- Canela A, Martín-Caballero J, Flores JM, Blasco MA (2004) Constitutive expression of Tert in thymocytes leads to increased incidence and dissemination of T-cell lymphoma in Lck-Tert mice. Mol Cell Biol 24: 4275–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci G, Siriaco G, Raffa GD, Kellum R, Gatti M (2003) The Drosophila HOAP protein is required for telomere capping. Nat Cell Biol 1: 82–84 [DOI] [PubMed] [Google Scholar]
- Chan SW-L, Blackburn EH (2002) New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 21: 553–563 [DOI] [PubMed] [Google Scholar]
- Chang S, Multan AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, DePinho RA (2004) Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet 36: 877–882 [DOI] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA (1999) p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97: 527–538 [DOI] [PubMed] [Google Scholar]
- Chong L, van Steensel B, Broccoli D, Erdjument-Bromage H, Hanish J, Tempst P, de Lange T (1995) A human telomeric protein. Science 270: 1663–1667 [DOI] [PubMed] [Google Scholar]
- Collins K (2000) Mammalian telomeres and telomerase. Curr Opin Cell Biol 12: 378–383 [DOI] [PubMed] [Google Scholar]
- Collins K, Mitchell JR (2002) Telomerase in the human organism. Oncogene 21: 564–579 [DOI] [PubMed] [Google Scholar]
- Dantzer F, Giraud-Panis MJ, Jaco I, Ame JC, Schultz I, Blasco MA, Koering CE, Gilson E, Menissier-de Murcia J, de Murcia G, Schreiber V (2004) Functional interaction between poly(ADP-ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol Cell Biol 24: 1595–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T (2002) Protection of mammalian telomeres. Oncogene 21: 532–540 [DOI] [PubMed] [Google Scholar]
- De Lange T (2004) T-loops and the origin of telomeres. Nat Rev Mol Cell Biol 5: 323–329 [DOI] [PubMed] [Google Scholar]
- Dunham MA, Neumann AA, Fasching CL, Reddel RR (2000) Telomere maintenance by recombination in human cells. Nat Genet 26: 447–450 [DOI] [PubMed] [Google Scholar]
- Dynek JN, Smith S (2004) Resolution of sister telomere association is required for progression through mitosis. Science 304: 97–100 [DOI] [PubMed] [Google Scholar]
- Espejel S, Franco S, Rodríguez-Perales S, Bouffler SD, Cigudosa JC, Blasco MA (2002a) Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J 21: 2207–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejel S, Franco S, Sgura A, Gae D, Bailey S, Taccioli G, Blasco MA (2002b) Functional interaction between DNA-PKcs and telomerase in telomere length maintenance. EMBO J 21: 6275–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejel S, Klatt P, Martín-Caballero JM, Flores J, Blasco MA (2004) Shorter telomeres, accelerated aging and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep 5: 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti L, Giovinazzo G, Berloco M, Pimpinelli S (1998) The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol Cell 2: 527–538 [DOI] [PubMed] [Google Scholar]
- Ferron S, Mira E, Franco S, Cano-Jaimez M, Bellmunt E, Ramirez C, Fariñas I, Blasco MA (2004) Telomere shortening and chromosomal instabilityabrogates proliferation of adult but not embryonic neural stem cells. Development 131: 4059–4070 [DOI] [PubMed] [Google Scholar]
- Franco S, Segura I, Riese H, Blasco MA (2002) Telomere length is a key molecular determinant of angiogenic potential in vivo: implications for cancer and aging. Cancer Res 62: 552–559 [PubMed] [Google Scholar]
- García-Cao M, Gonzalo S, Dean D, Blasco MA (2002) Role of the Rb family members in controlling telomere length. Nat Genet 32: 415–419 [DOI] [PubMed] [Google Scholar]
- Garcia-Cao M, O'Sullivan R, Peters AH, Jenuwein T, Blasco MA (2004) Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet 36: 94–99 [DOI] [PubMed] [Google Scholar]
- Gilley D, Tanaka H, Hande MP, Kurimasa A, Li GC, Oshimura M, Chen DJ (2001) DNA-PKcs is critical for telomere capping. Proc Natl Acad Sci USA 98: 15084–15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Suárez E, Flores JM, Blasco MA (2002) Coorperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol Cell Biol 22: 7291–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Suárez E, Geserick C, Flores JM, Blasco MA (2005) Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene [E-pub ahead of print: 31 January 2005] [DOI] [PubMed] [Google Scholar]
- González-Suárez E, Samper E, Flores JM, Blasco MA (2000) Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet 26: 114–117 [DOI] [PubMed] [Google Scholar]
- González-Suárez E, Samper E, Ramirez A, Flores JM, Martin-Caballero J, Jorcano JL, Blasco MA (2001) Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J 20: 2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goytisolo FA, Blasco MA (2002) Many ways to telomere dysfunction: in vivo studies using mouse models. Oncogene 21: 584–591 [DOI] [PubMed] [Google Scholar]
- Goytisolo FA, Samper E, Edmonson S, Taccioli GE, Blasco MA (2001) The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol Cell Biol 21: 3642–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goytisolo FA, Samper E, Martin-Caballero J, Finnon P, Herrera E, Flores JM, Bouffler SD, Blasco MA (2000) Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J Exp Med 192: 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RA, Chin L, Femino A, Lee KH, Gottlieb GJ, Singer RH, Greider CW, DePinho RA (1999) Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell 97: 515–525 [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43: 405–413 [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH (1987) The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51: 887–898 [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH (1989) A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337: 331–337 [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97: 503–514 [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460 [DOI] [PubMed] [Google Scholar]
- Hecht A, Laroche T, Strahl-Bolsinger S, Gasser SM, Grunstein M (1995) Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell 80: 583–592 [DOI] [PubMed] [Google Scholar]
- Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW (2001a) Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell 12: 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann MT, Strong MA, Hao LY, Greider CW (2001b) The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107: 67–77 [DOI] [PubMed] [Google Scholar]
- Henson JD, Neumann AA, Yeager TR, Reddel RR (2002) Alternative lengthening of telomeres in mammalian cells. Oncogene 21: 598–610 [DOI] [PubMed] [Google Scholar]
- Herrera E, Martinez AC, Blasco MA (2000) Impaired germinal center reaction in mice with short telomeres. EMBO J 19: 472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Samper E, Blasco MA (1999b) Telomere shortening in mTR−/− embryos is associated with failure to close the neural tube. EMBO J 18: 1172–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA (1999a) Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J 18: 2950–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K (2002) Clinical utility of telomerase in cancer. Oncogene 21: 643–649 [DOI] [PubMed] [Google Scholar]
- Hsu HL, Gilley D, Galande SA, Hande MP, Allen B, Kim SH, Li GC, Campisi J, Kohwi-Shigematsu T, Chen DJ (2000) Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev 14: 2807–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaco I, Muñoz P, Blasco MA (2004) Role of human Ku86 in telomere length maintenance and telomere capping. Cancer Res 64: 7271–7278 [DOI] [PubMed] [Google Scholar]
- Jaco I, Muñoz P, Goytisolo FA, Wesoly J, Bailey S, Taccioli G, Blasco MA (2003) Role of mammalian Rad54 in telomere length maintenance. Mol Cell Biol 23: 5572–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T (2001) Re-SET-ting heterochromatin by histone methyltransferases. Trends Cell Biol 11: 266–273 [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Kaminker PG, Kim SH, Taylor RD, Zebarjadian Y, Funk WD, Morin GB, Yaswen P, Campisi J (2001) TANK2, a new TRF1-associated PARP, causes rapid induction of cell death upon overexpression. J Biol Chem 276: 35891–35899 [DOI] [PubMed] [Google Scholar]
- Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JH, Lange T (2004) The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol 2, E240 (Epub 2004 August 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J, Kachatrian L, Takai H, Mercer K, Ignoran S, Jacks T, de Lange T (2003) Targeted deletion reveals an essential function for the telomere length regulator Trf1. Mol Cell Biol 23: 6533–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Beausejour C, Dávalos AR, Kaminker P, Heo SJ, Campisi J (2004) TIN2 mediates functions of TRF2 at human telomeres. J Biol Chem 279: 43799–43804 [DOI] [PubMed] [Google Scholar]
- Kim SH, Kaminker P, Campisi J (1999) TIN2, a new regulator of telomere length in human cells. Nat Genet 23: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koering CE, Pollice A, Zibella MP, Bauwens S, Puisieux A, Brunori M, Brun C, Martins L, Sabatier L, Pulitzer JF, Wilson E (2002) Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep 3: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner M, O'Carrol D, Rea S, Mechtler K, Jenuwein T (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116–120 [DOI] [PubMed] [Google Scholar]
- Lanza RP, Cibelli JB, Blackwell C, Cristofalo VJ, Francis MK, Baerlocher GM, Mak J, Schertzer M, Chavez EA, Sawyer N, Lansdorp PM, West MD (2000) Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 288: 665–669 [DOI] [PubMed] [Google Scholar]
- Latre L, Tusell L, Martín M, Miró R, Egozcue J, Blasco MA, Genescá A (2003) Shortened telomeres join DNA breaks interfering with their correct repair. Exp Cell Res 287: 282–288 [DOI] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, Greider CW, DePinho RA (1998) Essential role of mouse telomerase in highly proliferative organs. Nature 392: 569–574 [DOI] [PubMed] [Google Scholar]
- Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA (2003) Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure. EMBO J 22: 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, de Lange T (2003) Rap1 affects the length and heterogeneity of human telomeres. Mol Biol Cell 14: 5060–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T (2000) Identification of human Rap1: implications for telomere evolution. Cell 101: 471–483 [DOI] [PubMed] [Google Scholar]
- Liu D, Safari A, O'Connor MS, Chan DW, Laegeler A, Qin J, Songyang Z (2004) PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol 6: 673–680 [DOI] [PubMed] [Google Scholar]
- Loayza D, De Lange T (2003) POT1 as a terminal transducer of TRF1 telomere length control. Nature 423: 1013–1018 [DOI] [PubMed] [Google Scholar]
- Lundblad V (2002) Telomere maintenance without telomerase. Oncogene 21: 522–531 [DOI] [PubMed] [Google Scholar]
- Martín-Rivera L, Herrera E, Albar JP, Blasco MA (1998) Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc Natl Acad Sci USA 95: 10471–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Fu W, Zhang P (2001) Emerging roles for telomerase in regulating cell differentiation and survival: a neuroscientist's perspective. Mech Aging Dev 122: 659–671 [DOI] [PubMed] [Google Scholar]
- McClintock B (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26: 234–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K, Ghosh G, Fattah FJ, Li G, Kim H, Dutia A, Pak E, Smith S, Hendrickson EA (2004) Regulation of telomere length and suppression of genomic instability in human somatic cells by Ku86. Mol Cell Biol 24: 5050–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina T, Woodcock CL (2004) Chromatin loops at the ends of chromosomes. J Cell Biol 166: 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opresko PL, Otterlei M, Graakjaer J, Bruheim P, Dawut L, Kolvraa S, May A, Seidman MM, Bohr VA (2004) The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol Cell 14: 763–774 [DOI] [PubMed] [Google Scholar]
- Perrod S, Gasser SM (2003) Long-range silencing and position effects at telomeres and centromeres: parallels and differences. Cell Mol Life Sci 60: 2303–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, Kubicek S, Mechtler K, O'Sullivan RJ, Derijck AA, Perez-Burgos L, Kohlmaier A, Opravil S, Tachibana M, Shinkai Y, Martens JH, Jenuwein T (2003) Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell 12: 1577–1589 [DOI] [PubMed] [Google Scholar]
- Peters AH, O'Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T (2001) Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107: 323–337 [DOI] [PubMed] [Google Scholar]
- Riha K, Watson JM, Parkey J, Shippen DE (2002) Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO J 21: 2819–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA (1999) Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96: 701–712 [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Millard M, Bosenberg MW, DePinho RA (2001) Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 28: 155–159 [DOI] [PubMed] [Google Scholar]
- Samper E, Fernández P, Martín-Rivera L, Bernad A, Blasco MA, Aracil M (2002) Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood 99: 2767–2775 [DOI] [PubMed] [Google Scholar]
- Samper E, Flores JM, Blasco MA (2001) Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Rep 2: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samper E, Goytisolo FA, Slijepcevic P, van Buul PP, Blasco MA (2000) Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Rep 1: 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky M, Kravchuk O, Melnikova L, Georgiev P (2002) Heterochromatin protein 1 is involved in control of telomere elongation in Drosophila melanogaster. Mol Cell Biol 22: 3204–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T (2004) A silencing pathway to induce H3-K9 and H4-K20 tri-methylation at constitutive heterochromatin. Genes Dev 8: 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels PG, Kind AJ, Campbell KH, Waddington D, Wilmut I, Colman A, Schnieke AE (1999) Analysis of telomere lengths in cloned sheep. Nature 399: 316–317 [DOI] [PubMed] [Google Scholar]
- Smith GC, Jackson SP (1999) The DNA-dependent protein kinase. Genes Dev 13: 916–934 [DOI] [PubMed] [Google Scholar]
- Smith LL, Coller HA, Roberts JM (2003) Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol 5: 474–479 [DOI] [PubMed] [Google Scholar]
- Smith S, Giriat I, Schmitt A, de Lange T (1998) Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282: 1484–1487 [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T (2000) Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Jung D, Jung Y, Lee SG, Lee I (2000) Interaction of human Ku70 with TRF2. FEBS Lett 481: 81–85 [DOI] [PubMed] [Google Scholar]
- Tarsounas M, Muñoz P, Claas A, Smiraldo PH, Pittman DL, Blasco MA, West SC (2004) Telomere maintenance requires the RAD51D recombination/repair protein. Cell 117: 337–347 [DOI] [PubMed] [Google Scholar]
- Tommerup H, Dousmanis A, de Lange T (1994) Unusual chromatin in human telomeres. Mol Cell Biol 14: 5777–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92: 401–413 [DOI] [PubMed] [Google Scholar]
- Wang RC, Smogorzewska A, de Lange T (2004) Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119: 355–368 [DOI] [PubMed] [Google Scholar]
- Watson JD (1972) Origin of concatemeric T7DNA. Nat New Biol 239: 197–201 [DOI] [PubMed] [Google Scholar]
- Wong KK, Chang S, Weiler SR, Ganesan S, Chaudhuri J, Zhu C, Artandi SE, Rudolph KL, Gottlieb GJ, Chin L, Alt FW, DePinho RA (2000) Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat Genet 26: 85–88 [DOI] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA (2003) Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421: 643–648 [DOI] [PubMed] [Google Scholar]
- Ye JZ, de Lange T (2004a) TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat Genet 36: 618–623 [DOI] [PubMed] [Google Scholar]
- Ye JZ, Donigian JR, Van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, Chait BT, De Lange T (2004b) TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J Biol Chem 279: 47264–47271 [DOI] [PubMed] [Google Scholar]
- Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T (2004c) POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev 18: 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XD, Kuster B, Mann M, Petrini JH, Lange T (2000) Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet 25: 347–352 [DOI] [PubMed] [Google Scholar]
- Zhu XD, Niedernhofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T (2003) ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol Cell 12: 1489–1498 [DOI] [PubMed] [Google Scholar]
- Zijlmans JM, Martens UM, Poon SS, Raap AK, Tanke HJ, Ward RK, Lansdorp PM (1997) Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc Natl Acad Sci USA 94: 7423–7428 [DOI] [PMC free article] [PubMed] [Google Scholar]