The multifaceted biology of plasmacytoid dendritic cells (original) (raw)
. Author manuscript; available in PMC: 2016 Mar 27.
Published in final edited form as: Nat Rev Immunol. 2015 Jul 10;15(8):471–485. doi: 10.1038/nri3865
Abstract
Plasmacytoid dendritic cells (pDCs) are a unique dendritic cell subset that specializes in the production of type I interferons (IFNs). pDCs promote antiviral immune responses and have been implicated in the pathogenesis of autoimmune diseases characterized by a type I IFN signature. However, pDCs can also induce tolerogenic immune responses. Here, we review recent progress from the field of pDC biology, focusing on: the molecular mechanisms that regulate pDC development and functions; the pathways involved in their sensing of pathogens and endogenous nucleic acids; the function of pDCs at mucosal sites; and their roles in infections, autoimmunity and cancer.
Keywords: plasmacytoid dendritic cell, interferon, transcription factor, virus, autoimmunity, cancer, mucosal immunity, human
Introduction
Plasmacytoid dendritic cells (pDCs) were originally described in human lymph nodes in the 1950s1. These cells were later found to secrete high amounts of type I interferon (IFN; i.e. IFN-α, IFN-β) in response to viruses2,3, thereby corresponding to the enigmatic natural IFN-producing cells identified in human peripheral blood4,5. pDCs specialize in producing type I IFNs following their recognition of viruses or self nucleic acids through Toll-like receptor (TLR) 7 and TLR96–8. However, pDCs can also secrete other pro-inflammatory cytokines and chemokines, including interleukin-6 (IL-6), IL-12, CXC-chemokine ligand 8 (CXCL8), CXCL10, CC-chemokine ligand 3 (CCL3) and CCL4. Moreover, expression of MHC class II and costimulatory molecules enables pDCs to present antigens to CD4+ T cells. Thus, the biology of pDCs is multifaceted (Figure 1).
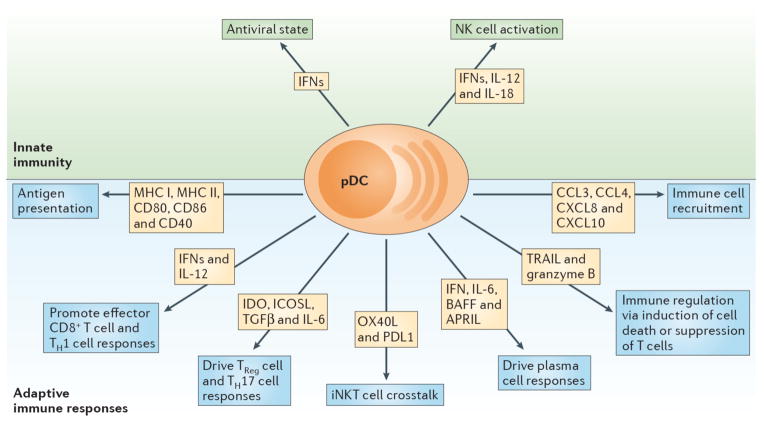
Figure 1. Diverse functions of pDCs.
pDCs are a prominent link between innate and adaptive immune responses. Their ability to rapidly produce type I IFNs (IFN-Is) during viral infections promotes an antiviral state by inducing expression of interferon stimulated genes and apoptosis of infected cells. Moreover, IFN-I, IL-12 and IL-18 enhance NK cell activation and effector functions such as IFN-γ secretion and lysis of target cells. Expression of MHC class I (MHCI) and MHC class II (MHCII) along with costimulatory markers including CD80, CD86 and CD40 enable pDCs to cross prime CD8+ T cells and present Ag to CD4+ T cells. Production of IFN-I and IL-12 by pDCs supports accumulation and effector functions of CD8+ T cells as well as the polarization of CD4+ T cells into T helper 1 cells (Th1). pDC expression of IDO and ICOSL or production of TGF-β and IL-6 promotes TReg or Th17 commitment, respectively. Crosstalk between pDCs and invariant NKT (iNKT) cells occurs via OX40/OX40L and PD-1/PD-L1 interactions and dampens antiviral adaptive immune responses. pDCs impact B cell activation, plasma cell generation and antibody secretion through production of IFN-I, IL-6, BAFF and APRIL. TRAIL and Granzyme B (GrB) serve as immunoregulatory factors that endow pDCs with the capacity to kill tumor cells, induce apoptosis of infected CD4+ T cells and suppress T cell proliferation. Finally, pDCs secrete chemokines such CXCL8, CXCL10, CCL3 and CCL4, which attract immune cells to sites of infection or inflammation.
The identification of pDCs in mice in 20019–11 has bolstered research in the pDC field, permitting exploration of the mechanisms involved in their development and their roles in innate and adaptive immunity. In addition to contributing to innate antiviral immunity, pDCs can participate in the priming of both immunogenic or tolerogenic adaptive immune responses. Human pDCs also continue to be extensively studied with a view to understanding their potential roles in the pathogenesis of autoimmune diseases, cancer and human immunodeficiency virus (HIV) infection. In this Review, we discuss the recent progress that has been made in these areas of mouse and human pDC biology. We begin by discussing the phenotype of pDCs and the mechanisms that control their development.
Phenotypes of human and mouse pDCs
Human pDCs lack expression of the lineage-associated markers CD3, CD19, CD14, CD16 and CD11c and selectively express the C-type lectin BDCA2 (also known as CLEC4C), and the Ig-superfamily receptor ILT712. Human pDCs also express CD4, CD68, ILT3 and the receptor for IL-3 (also known as CD123). Accordingly, IL-3 mediates human pDC survival in vitro3. CD2 is a cell adhesion molecule that is expressed by a subset of human pDCs13. CD2hi pDCs produce lysozyme, but whether this endows them with the capacity to lyse bacteria is unknown. The markers most commonly used to identify pDCs in mice are CD11c, B220, Ly6C, bone marrow stromal antigen 2 (BST2) and SIGLEC-H. In contrast to human pDCs, which are CD11c−, mouse pDCs express intermediate levels of CD11c. BST2 (also known as Tetherin14) is fairly specific to pDCs and plasma cells in steady-state conditions but is induced on many cell types following exposure to type I and type II IFNs15. In cell suspensions from primary and secondary lymphoid organs, SIGLEC-H expression is mainly confined to pDCs16,17 and is downregulated upon activation18. However, specialized macrophage subsets in the spleen, lymph nodes and brain also express SIGLEC-H17,19. In addition, studies in reporter mice have confirmed Siglech mRNA transcription in progenitor cells18,20,21. Mouse pDCs can also express CD8α and, like human pDCs, CD4. In peripheral tissues, the majority of mouse pDCs express CC chemokine receptor 9 (CCR9), Ly49Q and SCA1; however, in the bone marrow these markers distinguish pDC subsets that differ in developmental stage and/or activation state (Box 1).
Box 1. Heterogeneity of mouse pDCs in the bone marrow.
While CCR9, SCA1 and Ly49Q are expressed on the majority of mouse pDCs in the periphery, in the bone marrow these markers have unequal distribution, identifying pDC subsets that differ in their degree of maturation and their capacity to produce IFN-I or pro-inflammatory cytokines. CCR9− cells are pDC-like common DC precursors, whereas CCR9+ cells are fully differentiated pDCs. CCR9− pDC-like common DC precursors can respond to TLR stimulation and produce type I IFN and pro-inflammatory cytokines better than mature CCR9+ pDCs29. While CCR9− pDC-like common DC precursors are SCA1lo, CCR9+ pDCs in the bone marrow can be further divided into SCA1lo and SCA1hi subsets. SCA1lo pDCs are more efficient at producing IFN-α than SCA1hi pDCs and give rise to SCA1hi pDCs after activation or exposure to type I IFN217. Ly49Q− and Ly49Q+ pDCs secrete type I IFN in response to the synthetic TLR9 ligand CpG or herpes simplex virus (HSV), a DNA virus, but Ly49Q− pDCs respond poorly to stimulation with influenza virus, a RNA virus. Ly49Q− pDCs also appear to produce lower levels of pro-inflammatory cytokines after TLR stimulation compared to Ly49Q+ pDCs218. Two pDC subsets have been defined by CD9 expression219. The CD9+ subset has high type I IFN producing and T cell stimulatory capacities and may partially overlap with the nonplasmacytoid, high type I IFN producing DC subset described in the bone marrow220 and CCR9− pDC-like common DC precursors.
In general, studies on bone marrow pDC subsets concur that newly generated pDCs or their close precursors may be more efficient at producing type I IFN than mature pDCs in the bone marrow and in the periphery, at least in response to TLR agonists. However, it has also been reported that pDCs in the periphery and not in the bone marrow are the major source of type I IFN in mice infected with murine cytomegalovirus (MCMV)221. Most likely, the relative importance of bone marrow versus peripheral pDCs as sources of type I IFN depends not only on their intrinsic capacity but also on the degree of exposure to viruses or other stimuli that elicit a type I IFN response.
In conclusion, pDC subsets in bone marrow reflect different stages of development and/or activation and differ in their capacity to produce type I IFN versus pro-inflammatory cytokines as well as their impact on T cell activation and T cell effector or regulatory functions. Clonogenic assays and consistency among gating strategies and markers used to define pDCs will be essential to determine which populations contain mature pDCs versus those that are heterogeneous and can give rise to different subsets.
Development of pDCs
Progenitors and cytokines required for pDC development
A common DC progenitor (CDP) that generates both pDCs and classical DC (cDCs) but not other cell lineages has been identified in the bone marrow. The CDP is characterized by lack of lineage markers (LIN) and expression of Fms-like tyrosine kinase 3 (FLT3, also known as CD135), macrophage colony-stimulating factor receptor (M-CSFR, also known as CD115) and the receptor tyrosine kinase KIT (also known as CD117)22–26. Recently, a clonogenic progenitor downstream of CDP with prominent pDC potential has been reported27. This progenitor is LIN−KITint/loFLT3+IL-7Rα− and does not express M-CSFR. It expresses high levels of E2-2 (also known as TCF4), the transcription factor that defines the pDC lineage28, and can be derived from CDPs under conditions in which is E2-2 is upregulated, i.e. exposure to M-CSF or thrombopoietin. A subsequent step in pDC development is the generation of a CCR9− pDC-like common DC precursor that expresses some of the phenotypic markers of mature pDCs, such as CD11c, B220 and SiglecH, but has low or negligible levels of MHC class II and CCR9. This CCR9− pDC-like common DC precursor retains the potential to differentiate into either pDCs or cDCs, depending on the tissue environment29,30. Therefore, the conversion of progenitors into pDCs or cDCs may happen not only at the CDP stage of development, but also closer to terminal pDC differentiation.
Although many studies have focused on pDC differentiation within the myeloid lineage, there is also evidence that pDCs can originate from the lymphoid lineage. The lymphoid-primed multipotent progenitor, delineated as LIN−KIT+SCA1+CD34+FLT3+, can generate the M-CSFR− progenitor with prominent pDC potential described above27, which subsequently can differentiate into both Rag1-positive and Rag1-negative mature pDCs. pDCs of lymphoid origin may be distinct from pDCs of myeloid origin, but it has been reported that both myeloid- and lymphoid-derived pDCs can activate lymphoid-specific genetic programs31 such as nonproductive rearrangements of immunoglogulin or TCR genes and expression of pre-T cell receptor alpha32–34.
FLT3 and its ligand (FLT3L) are crucial for pDC development35. In the absence of FLT3 signaling, pDCs are reduced in number in the lymphoid organs and bone marrow of mice36,37. FLT3L promotes pDC development through activation of signal transducer and activator of transcription 3 (STAT3)- and phosphoinositide 3-kinase (PI3K)-dependent activation of mammalian target of rapamycin (mTOR)38,39. LAMTOR2 is a member of the Ragulator complex known to inhibit mTOR signaling. Conditional deletion of LAMTOR2 in CD11c+ cells (which include cDCs and pDCs) causes accumulation of FLT3 on the cell surface and massive expansion of pDC and cDC populations in older mice40. Recently, it was shown that FLT3L and type I IFN act synergistically to promote pDC development from common lymphoid progenitors by inducing upregulation of FLT341. In addition to FLT3 signaling, M-CSF (also known as CSF1) may support the generation of pDCs42. Indeed, pDCs can be derived from M-CSFR+ CDP23,27 and Csf1−/− mice have reduced numbers of both pDCs and cDCs43. By contrast, the growth factor GM-CSF inhibits FLT3L-driven pDC development44. GM-CSF-induced STAT5 signaling abolishes pDC-related gene expression in FLT3+ progenitors and inhibits the transcription factor interferon regulatory factor 8 (IRF8), which is crucial for pDC development, as discussed below45.
E2-2 expression guides pDC differentiation
E2-2 is the transcription factor essential for committing CDPs to the pDC lineage in both mice and humans28,46 (Figure 2). E2-2, along with the transcription factors E12, E47 and HEB (also known as TCF12), make up the E family of class I basic helix-loop-helix transcription factors47. E proteins form homodimers or heterodimers with each other and bind to conserved E box sequences, a process that can be perturbed by inhibitor of differentiation (ID) proteins. E2-2 binds to a large fraction of pDC-enriched genes and continuous expression of E2-2 in pDCs is required to maintain cell fate28,48. Deletion of E2-2 in mature pDCs causes the loss of pDC-associated markers, spontaneous differentiation into cDC-like cells, upregulation of MHC class II molecules and, ultimately, an increase in the ability to prime T cells48. These cDC-like cells are similar to the steady-state noncanonical CX3CR1+CD8α+ DC population that is related to pDCs49. CX3CR1+CD8α+ DCs require E2-2 for development, harbor Ig heavy-chain (IgH) D-J rearrangements and are enriched for pDC-specific genes; however, they do not produce type I IFN in response to influenza virus and lack surface expression of classical pDC markers like B220 and BST2. Thus, noncanonical CX3CR1+CD8α+ DCs may represent an alternative fate of pDC-committed cells that are unable to reach or maintain high enough levels of E2-2.
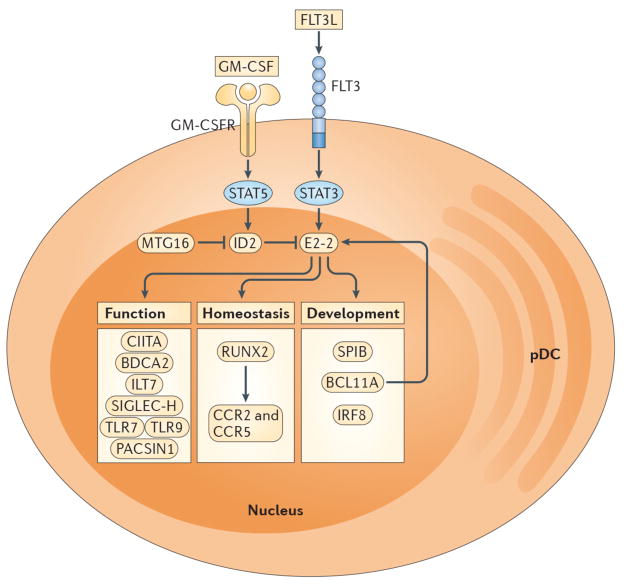
Figure 2. Regulation of pDC development and function.
The development of pDCs from CDPs is regulated by specific cytokines and transcription factors. FLT3L-STAT3 signaling promotes expression of E2-2, which is the master transcription factor required for pDC development. The absence of E2-2 or its deletion in mature pDCs results in the complete loss of pDCs or the differentiation of pDC-committed cells into cDC-like cells, respectively. In contrast, GM-CSF-STAT5 signaling blocks pDC differentiation during DC development by inducing expression of ID2, which is an antagonist of E2-2. The transcriptional cofactor MTG16 promotes pDC differentiation and restricts cDC development in part by repressing ID2. Transcriptional targets of E2-2 encode proteins associated with pDC development, homeostasis and function. SPIB, BCL11A and IRF8 are necessary for pDC differentiation and/or survival while RUNX2 controls pDC homeostasis through expression of the chemokine receptors CCR2 and CCR5, which permit egress from the bone marrow. CIITA promotes MHC class II expression. BDCA2, ILT7 and SIGLEC-H are markers that are selectively expressed by human or mouse pDCs and are involved in regulation of type I IFN production. TLR7, TLR9 and PACSIN1 enable pDC recognition of nucleic acids and pathogens (i.e. RNA and DNA viruses), resulting in type I IFN secretion and/or pro-inflammatory cytokine production.
ID2, a repressor of E2-2, is essentially absent in pDCs, but is prominently expressed in cDCs and supports CD8α+ DC development from CDPs28,50. pDC numbers are increased in _Id2_−/− mice50 whereas overexpression of ID2 or ID3 inhibits pDC development in vitro51. STAT3 and STAT5 help to regulate the balance between E and ID proteins during DC development by controlling the expression of E2-2 and ID2, respectively52. STAT3 stimulates FLT3L-dependent expression of E2-2, whereas STAT5 induces GM-CSF-dependent expression of ID2 (Figure 2). A recent study also identified a transcriptional cofactor of the ETO family, MTG16, as a key factor that represses ID2, thereby driving pDC differentiation and restricting cDC development (Figure 2)53.
Transcription factors targeted by E2-2
Transcriptional targets of E2-2 in pDCs encode proteins involved in the development, homeostasis and function of pDCs, including the transcription factors SPIB, BCL11A, IRF8, RUNX2 and CIITA; the cell surface markers BDCA2, ILT7, SIGLEC-H; and the nucleic acid sensors TLR7, TLR9 and PACSIN128,48,54 (Figure 2).
SPIB is a transcription factor critical for the differentiation of hematopoietic progenitors into pDCs and controls the survival of pDCs and their progenitors through induction of the anti-apoptotic gene BCL2-A155–58. pDC numbers are reduced in the bone marrow of _Spib_−/− mice but are increased in the periphery, suggesting impaired retention in the bone marrow59. Furthermore, _Spib_−/− pDCs exhibit attenuated expression of pDC-specific genes and display defects in type I IFN production following TLR7 or TLR9 stimulation. SPIB was also recently shown to be a novel immunohistochemical marker for the identification of human blastic pDC neoplasms56.
BCL11A is necessary for the expression of IL-7R and FLT3 in early hematopoietic progenitor cells and wildtype mice reconstituted with _Bcl11a_−/− fetal liver have severely reduced numbers of pDCs60. Moreover, genome-wide analyses of DNA binding has revealed that BCL11A regulates transcription of E2-2, Id2 and Mtg1661, thereby inducing a positive feedback loop for pDC development. IRF8 is essential for pDC and CD8α+ DC development: IRF8 deficiency results in the absence of pDCs and a reduction in the numbers of CD8α+ DCs62, as well as a decrease in all splenic DC subsets after competitive reconstitution of irradiated mice with wildtype and _Irf8_−/− bone marrow63. Loss of pDCs, cDCs and monocytes in an individual with an IRF8 mutation that impairs DNA binding and transactivation has also been reported64.
RUNX2 is a Runt family transcription factor that is required for expression of several pDC-enriched genes including the chemokine receptors CCR2 and CCR565. Mature pDCs in _Runx2_−/− mice accumulate in the bone marrow and fail to populate peripheral compartments due to reduced CCR5 expression. Additional transcription factors enriched in pDCs have been identified54,66, but their roles in pDC development, maintenance and activation have yet to be characterized. Other regulators of pDC development including microRNAs are discussed in Box 2.
Box 2. Additional regulators of pDC development.
Other transcriptional regulators are involved in pDC development, including IKAROS, PU.1, X box-binding protein 1 (XBP1), hypoxia-inducible factor 1α (HIF1α) growth factor independent 1 (GFI1) and nuclear polyadenylated RNA-binding protein 2 (NAB2). Mice that express low levels of IKAROS lack peripheral pDCs, but not other DC subsets, and are impaired in their ability to produce type I IFNs in response to TLR7 or TLR9 ligands222. PU.1 directly regulates FLT3 expression in a concentration-dependent manner and is necessary for both pDC and cDC development223. XBP1 is a target of the unfolded protein response sensor IRE-1α that maintains endoplasmic reticulum (ER) homeostasis and prevents activation of cell death pathways224. XBP1 deficiency results in impaired survival and reduced numbers of pDCs and cDCs225. Treatment of pDCs with the proteasome inhibitor Bortezomib reduces the active form of XBP1 and suppresses pDC survival and immunostimulatory functions by targeting ER homeostasis and the intracellular trafficking of TLRs226. Hypoxia-inducible factors influence hematopoiesis and maintain HSC function. Recently, it was shown that low oxygen content upregulates ID2 and suppresses FLT3L-induced pDC development, in a manner dependent on HIF1α227. GFI1 controls the development and functions of pDCs and cDCs in a STAT3-dependent manner228 and represses Rag transcription in pDCs229. NAB2 is a transcriptional corepressor that induces TRAIL expression in activated pDCs230. TRAIL-expressing pDCs are tumoricidal231 and induce apoptosis of CD4+ T cells in HIV viremic patients or after exposure to HTLV1232–234.
MicroRNAs represent another class of regulatory molecules that control different aspects of pDC development and function. miR-22 is induced in progenitor cultures by GM-CSF and it targets Irf8 mRNA for post-transcriptional repression235. Overexpression of miR-22 during DC development promotes the expansion of CD11b+ cDC populations at the expense of pDCs. Epigenetic or chromatin modifiers may also have a prominent role in DC lineage commitment and development. In support of this, histone deacetylation is required for PU.1 recruitment to target genes (including Flt3, Irf8 and PU.1 itself) and for pDC differentiation from progenitors236.
In conclusion, while E2-2 is the master transcription factor for pDC development, it is becoming clear that E2-2 is a component of a multi-protein complex that includes both positive and negative regulators. More studies are required to define the various components of this complex as well as the genomic regions that interact with this complex and drive the expression of molecules that define pDC phenotype and function.
Trafficking of pDCs
Initial studies showed that pDC migration is quite different from that of cDCs. Following their development in the bone marrow, pDCs circulate in the blood and reach T cell areas of lymph nodes mainly through high endothelial venules (HEVs) and not through afferent lymphatics67,68. In addition to secondary lymphoid organs, pDCs also migrate from blood into peripheral tissues. pDC migration involves CD62L, PSGL1, β1 and β2 integrins and multiple chemokine receptors, such as CXCR4, CCR7, CXCR3, CCR5, CCR2, CCR6, CCR10 and CCR9 (Figure 3)68,69.
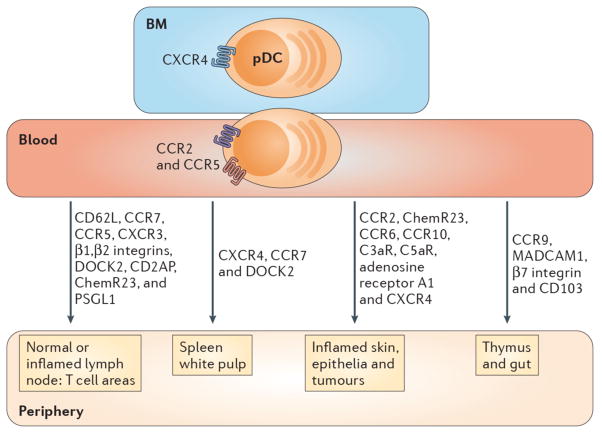
Figure 3. Factors influencing pDC migration.
pDCs express chemokine receptors and homing molecules that promote recruitment in the steady-state and during inflammation. Development of pDCs in bone marrow (BM) stromal cell niche requires CXCR4 expression, while pDC egress from the BM into the blood is dependent on CCR5 and CCR2. pDCs are attracted to tumors that produce CXCL12 (not shown) and the splenic white pulp via CXCR4. DOCK2, a hematopoietic cell-specific CDM family protein involved in CXCR4 signaling, is also necessary for pDC migration to spleen and lymph nodes (LNs). pDCs express CD62L, PSGL-1, β1/β2 integrins and the chemokine receptors CCR5, CXCR3 and CCR7 which mediate adhesion and chemotaxis to peripheral LNs and the splenic white pulp under normal and/or inflammatory conditions. Blood pDCs express the chemerin receptor, ChemR23, as well as A1-R, C3aR and C5aR, which may guide them to peripheral LNs and damaged tissues. CD2AP is an intracellular protein that regulates actin dynamics and promotes pDC migration to LNs under inflammatory settings. CCR2 drives the recruitment of pDCs to the skin following inflammation induced by the TLR7 agonist, Imiquimod. CCR6 and CCR10 are expressed by a subset of human tonsil pDCs and enable migration to inflamed epithelia producing CCL20 and CCL27 (not shown). CCR9 and its ligand CCL25 (not shown) promote trafficking of peripheral pDCs to the thymus and are required for pDC recruitment to the small intestine under both normal and inflammatory conditions. MAdCAM-1, β7 integrin and CD103 also influence pDC trafficking to the gut. Finally, pDCs express CX3CR1 (not shown), which may impact their homeostasis.
CXCR4 is required for the retention of pDCs in the bone marrow stromal niche and their development70. CXCR4 and CCR7 mediate the migration of pDCs into the splenic white pulp71. CXCR4 also promotes pDC recruitment to tumors that produce CXCL1272. During inflammation, CXCR3 and CCR5 drive pDC migration into inflamed tissues73,74. CCR2, which is expressed with a bimodal distribution on pDCs65, drives recruitment of pDCs to the skin following inflammation induced by topical application of imiquimod75. CCR6 and CCR10 are expressed by a subset of pDCs in human tonsil and facilitate migration to inflamed epithelia in response to CCL20 and CCL2776. pDC recruitment to the thymus and small intestine requires CCR977,78, whereas pDC migration into the colon is CCR9-independent79. MAdCAM-1 and β7 integrin were recently shown to promote pDC trafficking into the intestinal intraepithelial compartment80. pDCs can also migrate in response to engagement of receptors for chemerin (ChemR23), adenosine, as well as C3a and C5a, which are released at sites of tissue damage68. CX3CR1 is expressed by pDCs; however, its role in pDC migration and homeostasis is not well characterized.
Several studies have recently identified intracellular proteins that selectively impact pDC trafficking in the steady-state and in disease. Human and mouse pDCs express CD2-associated protein (CD2AP)81, an intracellular protein that regulates actin dynamics and promotes pDC migration to lymph nodes during inflammation82. DOCK2, a hematopoietic cell-specific CDM (Caenorhabditis elegans Ced-5, mammalian DOCK180 and Drosophila melanogaster myoblast city) protein, is necessary for migration of pDCs to lymph nodes and the periarteriolar lymphoid sheaths of the spleen83. Chemokine-induced Rac activation is severely impaired in _Dock2_−/− pDCs, resulting in the reduction of motility and the loss of polarity during chemotaxis83.
Innate sensing by pDCs
Sensing of pathogens and self nucleic acids by TLR7 and TLR9
The recognition of viruses or self nucleic acids by pDCs is mainly mediated by TLR7 and TLR9, which are located in endosomal compartments. Activation of these receptors in pDCs results in secretion of type I IFNs via the MyD88-IRF7 pathway as well as production of pro-inflammatory cytokines and chemokines via the MyD88-NF-κB pathway (reviewed in6–8). TLR7 senses RNA viruses, endogenous RNA and synthetic oligoribonucleotides, whereas TLR9 detects DNA viruses containing unmethylated CpG-rich DNA sequences, endogenous DNA and synthetic CpG oligodeoxyribonucleotides. Since pDCs constitutively express high levels of IRF7 and produce type I IFNs84, they are largely resistant to viral infection unless type I IFN signaling is compromised85. Therefore, uptake and recognition of viral nucleic acids by pDCs likely occurs via mechanisms that are independent of viral infection. Such mechanisms are not yet completely understood and are the subject of intensive investigation. In the case of hepatitis A virus, virions coated in host membrane enter pDCs through the phosphatidylserine receptor TIM186, while pDCs sense hepatitis C virus (HCV) via exosomes containing HCV RNA that are released from infected cells87. Direct contact of pDCs with vesicular stomatitis virus (VSV)-infected cells triggers type I IFN production in a manner that is TLR-dependent and more robust than free virus alone88. Viruses and endogenous nucleic acids can enter pDCs through Fc receptors when bound by antibodies during an immune response. Recently, it was documented that the inhibitory Fc receptor FcγRIIB promotes the uptake of Sendai virus immune complexes during a memory response and prevents type I IFN production by pDCs89. By contrast, endogenous nucleic acids are internalized as a complex with antinuclear antibodies via the activating Fc receptor FcγRIIA90–92.
Whether engagement of TLR7 or TLR9 results in the production of type I IFN or pro-inflammatory cytokines depends on the type of compartment in which these TLRs encounter their ligands (Figure 4)93,94. Multimeric CpG-A oligonucleotides aggregate in early endosomes where they activate the MyD88-IRF7 pathway that induces type I IFNs. In contrast, monomeric CpG-B is transferred to an endolysosomal compartment, where it activates the MyD88-NF-κB pathway that triggers expression of costimulatory molecules and pro-inflammatory cytokine secretion. IkappaB kinase-alpha (IKKα)95, osteopontin96 and mTOR97 are key components of the MyD88-IRF7 signaling pathway, while IRF5 is essential for MyD88-NF-κB signaling98,99. Trafficking of TLR9 to the subcellular compartment for type I IFN production is dependent on adapter protein 3 (AP3), as virus- or CpG-induced type I IFN expression is abolished in the absence of AP3100,101. Forward genetic approaches have further identified Slc15a4, which encodes the peptide/histidine transporter 1 (PHT1), and BLOC1 and BLOC2 Hermansky-Pudlak syndrome proteins as key components required for TLR9 signaling in pDCs but not in other cell types101. However, TLR9-mediated recognition of large DNA-containing immune complexes is independent of AP3102 and requires the convergence of phagocytic and autophagic pathways. This process is called microtubule-associated protein 1A/1B-light chain 3 (LC3)-associated phagocytosis (LAP) and involves autophagy-related proteins but not the conventional autophagic pre-initiation complex. Recognition of live, single stranded RNA (ssRNA) viruses by TLR7 in pDCs also occurs in a distinct subcellular compartment that requires transport of cytosolic viral replication intermediates into the lysosome by autophagy103.
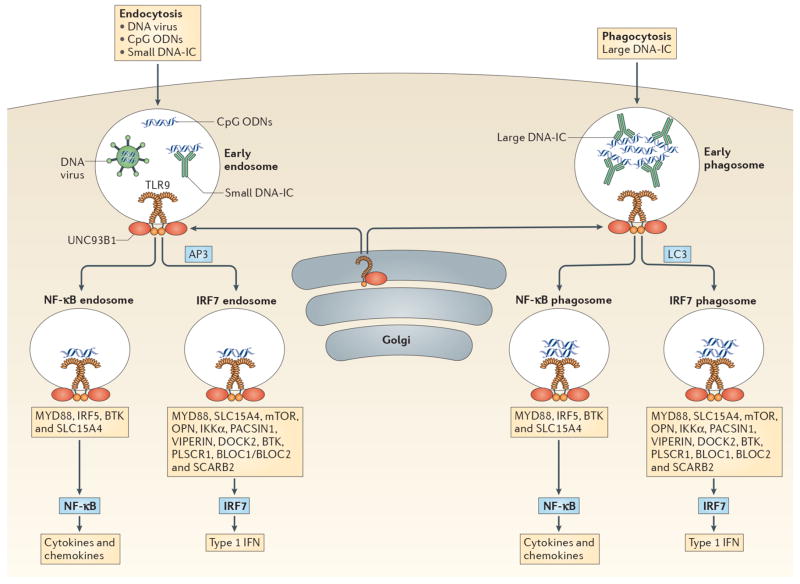
Figure 4. TLR9 signaling in pDCs.
pDCs sense DNA viruses, synthetic CpG oligodeoxyribonucleotides (ODN) and endogenous DNA through TLR9. All TLR9 signaling requires MyD88 but additional factors determine whether TLR9 engagement will result in type I IFN (IFN-I) or pro-inflammatory cytokine production. These factors include mode of ligand entry, and the intracellular compartment where TLR9 encounters its ligand. TLR9 is transported to appropriate intracellular compartments by UNC93B249,250 and requires cleavage in order to recruit MyD88251,252. DNA viruses, CpG ODN and small DNA immunecomplexes (DNA-ICs) enter pDCs through endocytosis and meet TLR9 in the early endosome. In contrast, large DNA-ICs are internalized by phagocytosis and encounter TLR9 and UNC93B in the early phagosome. If AP3 or LC3 are recruited then the IRF7 endosome or IRF7 phagosome is formed which leads to IFN-I production. Several molecules/pathways are involved in this process including IKKα, osteopontin (OPN), SLC15A4, BTK, BLOC1, BLOC2, DOCK2, PACSIN1, PLSCR1, VIPERIN, SCARB2 and the mTOR pathway. Alternatively, TLR9-containing compartments can form NF-κB endosomes or NF-κB phagosomes resulting in the production of pro-inflammatory cytokines and chemokines (i.e. IL-6, IL-12, TNF-α, etc). This process requires IRF5, BTK and SLC15A4. CpG ODN have different structures which results in trafficking to different compartments: CpG-A is transported to the IRF7 endosome and is a strong inducer of IFN-I, while CpG-B aggregates in the NF-κB endosome and is a potent stimulator of maturation and cytokine/chemokine production. CpG-C exhibits properties of both CpG-A and CpG-B in that it can induce both IFN-I and pro-inflammatory cytokines. TLR7 stimulation by viral and endogenous RNA may follow similar pathways.
Several additional molecules involved in TLR7/9 signaling have been identified in pDCs. The scavenger receptor SCARB2/LIMP-2 is highly expressed in pDCs and regulates TLR9-dependent type I IFN production by mediating TLR9 endosomal translocation and IRF7 nuclear translocation104. Phospholipid scramblase 1 (PLSCR1) is a TLR9-interacting protein that regulates TLR9 trafficking to endosomal compartments and type I IFN production in pDCs105. PACSIN1 is an endocytic adapter specifically expressed in mouse and human pDCs that is essential for TLR9-induced type I IFN secretion but not pro-inflammatory cytokine production54,106. VIPERIN is an IFN-inducible antiviral protein that promotes TLR7 and TLR9 signaling in pDCs by recruiting IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) to lipid bodies and inducing nuclear translocation of IRF7107. In addition to having a role in pDC migration, DOCK2 is essential for TLR7- and TLR9-mediated type I IFN production in pDCs, most likely because of its influence on actin cytoskeleton and vesicular trafficking108. In the absence of DOCK2, phosphorylation of IKKα and nuclear translocation of IRF7 are impaired in pDCs, but the capacity to produce pro-inflammatory cytokines is maintained. TLR9, but not TLR7, signaling is also dependent on Bruton’s tyrosine kinase (BTK)109. Blocking BTK inhibits all TLR9-induced responses in pDCs, including cytokine production and expression of costimulatory molecules. Additional studies are required to provide an integrated view of how all these molecules coordinate the trafficking and signaling properties of TLR7/9-containing vesicles in pDCs.
TLR2- and TLR12-mediated sensing in pDCs
Although TLR7 and TLR9 are important for pDC detection of viruses and self nucleic acids, recent evidence indicates that mouse pDCs also express TLR12 and TLR2, which permit detection of Toxoplasma gondii profilin110 and bacterial polysaccharide A (PSA)111, respectively. Although TLR11 and TLR12 can both recognize profilin, TLR12 is sufficient for profilin detection in pDCs and triggers the production of IL-12 and type I IFNs, which activate NK cells110. Furthermore, in contrast to Tlr11−/− mice, Tlr12−/− mice are highly susceptible to T. gondii infection, which suggests a critical role for pDCs in the induction of the innate immune response and host resistance. PSA is an immunomodulatory molecule expressed by the ubiquitous gut microorganism Bacteriodes fragilis112. pDCs exposed to PSA via TLR2 express MHC class II, inducible costimulatory ligand (ICOSL) as well as CD86, and stimulate IL-10 production by CD4+ T cells, all of which are required for protection against colitis111.
Other cytosolic sensors expressed by pDCs
Although TLRs have been established as the main innate receptors involved in pDC activation, the impact of cytosolic nucleic acid sensors on type I IFN or pro-inflammatory cytokine production by pDCs has just begun to be explored. CpG-A oligonucleotides selectively bind to the DExD/H-box helicase 36 (DHX36) and this is associated with the nuclear translocation of IRF7 and type I IFN production in pDCs113. By contrast, CpG-B oligonucleotides are bound by DHX9, and this results in NF-κB activation and the secretion of pro-inflammatory cytokines in pDCs. Additionally, in the absence of type I IFN signaling, viruses can replicate in pDCs, producing viral nucleic acid that can be detected in a RIG-I-like helicase-dependent manner85. Major cytosolic sensors of nucleic acids, such as cGAS and STING114, are likely to be investigated in pDCs in the near future as well.
Regulation of TLR7/TLR9 responses in pDCs
Regulatory receptors expressed by pDCs
Human and mouse pDCs express cell surface receptors that control the amplitude of type I IFN production and pDC activation state in response to TLR7 or TLR9 ligands115. Many of these receptors either contain intracellular tyrosine-based inhibitory motifs (ITIM) or they deliver intracellular signals through an associated adaptor, usually DNAX activation protein 12 (DAP12) or the γ chain of Fc receptors (FcRγ), which have an intracellular tyrosine-based activation motif (ITAM). Regulatory receptors associated with human pDCs include BDCA2116, ILT7117, NKp44118, CD300A/C119, DCIR (also known as CLEC4A)120, CD3290–92, BST2121 and leukocyte-associated immunoglobulin-like receptor 1 (LAIR1)122. Recently, it was shown that CD2-associated protein (CD2AP) positively regulates BDCA2 and/or FcRγ signaling by forming a complex with SHIP1 to inhibit the E3 ubiquitin ligase CBL123. Receptors that have been shown to regulate TLR7- and TLR9-driven responses in mouse pDCs include SIGLEC-H16, BST215, PDC-TREM124, Ly49Q125, PIR-B126 and EBI2127.
Regulation by microRNAs and hormones
MicroRNAs and hormones can also impact TLR7 and TLR9 signaling and pDC functions. Expression of miR-146a is induced upon engagement of TLR7 and TLR9, and this suppresses NF-κB activation and TLR-mediated signaling in pDCs128. miR-155 and its star-form partner miR-155* are expressed in pDCs after TLR7 signaling and have opposing effects on type I IFN production129. Shortly after TLR7 stimulation, miR-155* is induced and augments type I IFN expression by inhibiting IRAKM, whereas miR-155 induction occurs later and shuts down type I IFN production by targeting TAB2. miR-126 has a prominent role in promoting the survival and function of pDCs130. miR-126 targets the mTOR pathway and regulates the expression of genes including Tlr7, Tlr9, Nfkb1 and Kdr, which encodes the growth factor receptor VEGFR2. pDC homeostasis and the capacity to respond to TLR ligands are impaired in both _miR-126_−/− and _Kdr_−/− mice, indicating a role for VEGFR2 in supporting type I IFN production130.
Recently, it was shown that TLR7-mediated signaling in pDCs is enhanced by estrogen131. Notably, this would provide a mechanistic basis for the observation that pDCs from women produce more type I IFN in response to HIV than pDCs from men132.
pDCs as antigen-presenting cells
Because pDCs express MHC class II molecules as well as the costimulatory molecules CD40, CD80 and CD86, they can present antigens to CD4+ T cells, albeit not as efficiently as cDCs (reviewed in133,134). Antigen presentation by pDCs can lead to CD4+ T cell activation or tolerance induction, depending on the context (Figure 1). When pDCs receive activation signals through TLRs or other pattern recognition receptors, they act as immunogenic cells. When pDCs are either unstimulated or alternatively activated such that they express indoleamine 2,3-dioxygenase (IDO)135–138, ICOSL139, OX40L140, programmed death-ligand 1 (PD-L1)141 and/or granzyme B142, they promote tolerance to tumor cells, alloantigens and harmless antigens. Recent studies have proposed that pDCs that capture antigens in peripheral tissues utilize CCR9 to migrate to the thymus, where they induce deletion of antigen-specific thymocytes, thus contributing to immune tolerance77. It has also been reported that CCR9+ pDCs are potent inducers of regulatory T (TReg) cell function and can inhibit acute graft-versus-host disease induced by allogeneic CD4+ donor T cells in irradiated recipients143. This study defined pDCs as being either B220+CD11c+CCR9+ or B220+CD11c+CCR9−, the former being the tolerogenic subset; however, a later study argued that B220+CD11c+CCR9− cells are not a subset of pDCs, but rather precursors of lymphoid tissue-resident cDCs144. In a mouse model of lung inflammation, adoptive transfer of CD8α+ pDCs suppressed the development of airway hyper-reactivity by inducing TReg cells145; however, it has been reported that CD8 subunit expression by mouse pDCs is variable and does not define stable subsets146. Corroborating this, earlier studies found that CD8α is expressed by a fraction of pDCs in the steady-state but is induced on the majority of pDCs after activation9,147. Thus, the role of pDC subsets in tolerance remains to be further explored.
To selectively examine pDC antigen presentation capacity, antigens have been specifically targeted to pDCs by conjugating them to antibodies specific for pDC cell surface molecules. A recent study found that targeting antigens to BDCA2 promotes immunological tolerance by suppressing antigen-specific CD4+ T cell and antibody responses upon secondary exposure to antigen in the presence of adjuvant, a process that involves both preservation of TReg cells and a decrease in effector CD4+ T cells148. Antigens targeted to pDCs through DCIR are also presented to T cells120. In mice, it was shown that targeting a T cell epitope derived from myelin oligodendrocyte glycoprotein (MOG) to pDCs via SIGLEC-H delays onset and decreases disease severity in a model of MOG-induced experimental autoimmune encephalomyelitis (EAE) by reducing the expansion and polarization of Th1 and Th17 cell populations149. In contrast, antigen delivery to pDCs via BST2 in combination with TLR agonists generates robust cellular and humoral immunity and protects mice against subsequent viral infection or tumor growth150. Thus, targeting antigen to pDCs can either promote or inhibit antigen-specific immune responses, depending on the antigen formulation and mode of stimulation or delivery.
While in mouse models, pDCs have been mostly studied for their impact on antigen presentation to CD4+ T cells, human studies have focused on pDC antigen presentation to CD8+ T cells151,152. Human pDCs have recycling endosomes in which peptides can be continuously loaded on to MHC class I molecules, which enables efficient presentation of viral antigens to CD8+ T cells153. Through this mechanism, pDCs may contribute to adaptive responses during viral infections.
pDCs as a source of type I IFNs in viral infections
Acute viral infections
Type I IFN production by pDCs in response to acute viral infections is usually limited in time and amplitude (reviewed in154). pDC-secreted type I IFN is mostly evident at early timepoints in systemic infections such as MCMV, VSV, lymphocytic choriomeningitis virus (LCMV) and HSV-1, and mediates immediate containment of viral replication but becomes less important later on in the infection as other host cells become more dominant producers of type I IFNs. The impact of pDCs also depends on the route of infection. pDCs provide an important source of type I IFN in systemic infections, when the virus reaches the circulation. In contrast, the requirement for pDCs in type I IFN-mediated antiviral defense during local viral infections appears to be necessary only if other lines of defense are broken. For example, in pulmonary infection with Newcastle disease virus (NDV), alveolar macrophages are the primary source of type I IFN; if they are eliminated then pDC-derived type I IFN becomes important155. Only in the case of mouse hepatitis virus (MHV) and HSV-2 infections has pDC-derived type I IFN been shown to be critical for viral control in mice, reducing both morbidity and mortality156–158. A recent study found that individuals carrying inactivating mutations in the IRF7 gene have a severe defect in pDC function and are selectively susceptible to influenza virus infections159. It remains to be determined whether the pDC defect is sufficient to cause susceptibility to influenza or if IRF7 has a more general impact on the capacity of all cells to respond to the virus. Paradoxically, pDC responses to acute viral infections may not always be beneficial. Recent evidence indicates that excessive production of type I IFNs, probably by pDCs, during influenza virus infection in 129 mice can result in uncontrolled inflammation and TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis of bronchial epithelium160. Thus, the impact of pDCs on acute viral infections may vary considerably depending on the virus, route of infection and genetic background.
Chronic viral infections
The role of pDCs in chronic viral infections, such as HIV-1, is even more complex, involving pDC activation, trafficking, type I IFN production and interaction with CD4+ T cells (reviewed in161). HIV induces pDC activation through TLR7 but can also directly infect pDCs as they express CD4, CXCR4 and CCR5, which are co-receptors for viral entry. HIV promotes pDC trafficking to peripheral lymph nodes, and this is reflected by the reduced numbers of pDCs found in the blood of patients with HIV161. Moreover, new evidence indicates that pDCs upregulate the gut-homing markers α4β7 and CD103 and accumulate in the gut mucosa during SIV and HIV infections162,163. Interestingly, pDCs from elite controllers also maintain a gut-homing phenotype, suggesting that gut trafficking of pDCs is independent of viral load164.
pDCs may contribute to the chronicity of HIV infection through dysregulated activation and type I IFN production (reviewed in165). HIV-stimulated pDCs express low levels of maturation molecules, induce weak T cell responses and persistently secrete type I IFNs, due to HIV trafficking to early endosomes166. In addition, HIV-stimulated pDCs produce chemokines and express TRAIL, thereby promoting recruitment and apoptosis of T cells. HIV can also activate the noncanonical NF-κB pathway in pDCs, resulting in the expression of IDO and induction of TReg cells167. Noncanonical NF-κB signaling and induction of a tolerogenic pathway in pDCs stimulated with high doses of CpG has also been reported168. Studies in humanized mice have shown that depletion of pDCs prior to or during chronic HIV-1 infection severely reduces type I IFN production and increases viral replication169. However, HIV-1-induced CD4+ T cell death is curbed despite elevated viral loads, suggesting that pDCs suppress HIV-1 replication but also contribute to HIV-1 immunopathogenesis. Conversely, studies in simian immunodeficiency virus (SIV) infection have provided a different view. Blockade of TLR7- and TLR9-mediated IFN-α production by pDCs did not diminish immune activation during SIV infection170, suggesting that other sources of type I IFN may contribute to immunopathology.
The beneficial or detrimental effects of pDCs and type I IFNs in chronic viral infections may also depend on the timing of their action. During SIV infection, the timing of type I IFN production has a marked effect on the disease course171. Exogenous administration of IFN-α2a early on augments expression of antiviral genes and prevents systemic infection; however, sustained IFN-α2a treatment induces type I IFN desensitization, decreases antiviral gene expression, increases viral load and accelerates CD4+ T cell loss. In mouse, constitutive absence of pDCs or loss of their ability to signal through TLR7 and TLR9 in chronic LCMV infection appears to negatively impact T cell priming and viral clearance157,172. Moreover, early administration of exogenous type I IFN prevents chronic LCMV infection. However, late type I IFN administration has no beneficial effects173. In fact, blockade of type I IFN signaling during chronic LCMV infection improves T cell function and diminishes viral persistence174,175. Thus, there is a window of opportunity - i.e. early during infection - when type I IFNs and pDCs can be both beneficial and necessary to prevent or control chronic viral infection and preserve T cell numbers and functions.
Pathogenic functions of pDCs in autoimmunity
Role of pDCs in systemic lupus erythematosus
pDCs have been implicated in the pathogenesis of autoimmune diseases that are characterized by a type I IFN signature, such as systemic lupus erythematosus (SLE) (reviewed in176). In SLE, antinuclear antibodies form immune complexes with endogenous nucleic acids, which are delivered to pDC endosomal compartments via the Fc receptor CD32, where they activate TLR7 and TLR990–92. This process is enhanced by high mobility group box 1 (HMGB1)177. Neutrophils have been proposed to be a prominent source of endogenous nucleic acids for activation of pDCs in SLE. After exposure to anti-ribonucleoprotein antibodies, neutrophils release extracellular traps (NETs) containing chromatin DNA, the antimicrobial peptide LL-37 and HMGB1, which trigger the TLR9 pathway in pDCs, resulting in type I IFN secretion178,179. Counter to this hypothesis, lupus-prone MRL.Faslpr mice have markedly exacerbated lupus when crossed with mice lacking NADPH oxidase (NOX2), such that neutrophils are incapable of forming NETs180. Thus, endogenous nucleic acids that stimulate pDCs in SLE may be derived from other modes of cell death, such as necroptosis or pyroptosis, as well as from NADPH-independent release of mitochondrial DNA181.
Sustained activation of pDCs is also detrimental in SLE because it results in the resistance of pDCs to therapeutic glucocorticoids (GC), which are often administered to patients with SLE to control inflammation182, 183. Bioinformatics and functional analyses have suggested that TLR activation prevents GC-induced pDC apoptosis by inhibiting miR-29b and miR-29c, which promote pDC apoptosis by targeting MCL1 and BCL2184. Endogenous GC also have an impact on pDC homeostasis and survival185. Regulation of endogenous GC concentrations involves conversion of inactive substrates to active 11-hydroxyglucocorticoids by an enzyme complex containing 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and hexose-6-phosphate dehydrogenase (H6PDH). Under physiologic conditions, 11βHSD1-H6PDH increases the sensitivity of pDCs to GC-induced apoptosis; however, following activation with TLR ligands, the effects of enzyme activity are overridden.
Studies in various rodent models of SLE have confirmed that type I IFNs and pDCs contribute to disease pathogenesis. Male mice of the BXSB lupus-prone strain have a duplication of TLR7 and develop spontaneous autoimmunity. Blockade of type I IFN signaling early on in these mice reduces disease manifestations and extends survival186. Disruption of type I IFN signaling in MRL.Faslpr mice also provides some therapeutic benefit, however, it does not impact mortality. Additionally, autoimmune skin inflammation induced by tape stripping in lupus-prone (NZBxNZW)F(1) mice is ameliorated by antibody-mediated pDC depletion or by blocking TLR7 and TLR9 signaling with a bifunctional inhibitor187.
Genetic models that specifically address the contribution of pDCs in autoimmunity have been lacking until recently. Deletion of Irf8 in NZB mice and a mutation in Slc15a4 in C57BL/6.Faslpr mice abolish production of autoantibodies and disease manifestations188. While this study provides compelling evidence that pDCs and their TLR7/TLR9-mediated responses are damaging in SLE, IRF8 and SLC15A4 are not exclusively expressed by pDCs. In fact, B cell expression of SLC15A4 appears to be necessary for TLR7-induced expression of type I IFN and pathogenic antibody production in a mouse model of lupus189. Analysis of MRL.Faslpr mice lacking MyD88 showed that MyD88-dependent production of IFN-α by pDCs contributes to SLE pathogenesis, particularly B lymphopenia, but not to nephritis190. Two recent studies using lupus-prone mice that specifically lack pDCs have confirmed that pDCs are pathogenic in SLE. Global or CD11c-specific Tcf4 haplodeficiency in lupus-prone mice nearly abolishes autoantibody production and glomerulonephritis191, whereas transient depletion of pDCs prior to disease initiation in BXSB mice impairs expansion and activation of B and T cells and reduces antinuclear antibodies and expression of type I IFN-induced genes in tissues192. Taken together, these studies validate pDCs as a therapeutic target in SLE.
pDCs in other autoimmune diseases
Chronic activation of pDCs and dysregulated type I IFN production also appear to be contributing factors in initiating and/or promoting psoriasis and type I diabetes (T1D). In psoriasis, self nucleic acids form complexes with antimicrobial peptides which activate pDCs through TLR7 and TLR9193–195. Interestingly, the Vitamin D analogue calcipotriol, which is used to treat psoriatic skin lesions, triggers tolerogenic responses in cDCs and was recently shown to impair the capacity of both human and mouse pDCs to induce T cell proliferation and effector T cell differentiation196. In the nonobese diabetic (NOD) mouse model of T1D, activated B-1a cells produce DNA-reactive antibodies that stimulate release of cathelin-related antimicrobial peptide (CRAMP) from neutrophils; CRAMP subsequently binds to self DNA and activates pDCs through TLR9197.
The deleterious role(s) of pDCs in psoriasis and T1D have not been confirmed yet in genetic models, but blockade of type I IFN or antibody-mediated depletion of pDCs appears to confer protection in mouse models of these diseases197–199. Future studies will be necessary to address whether either blockade of type I IFN or depletion of pDCs are potential therapeutic avenues for intervention in psoriasis and T1D in human.
Gut-associated pDCs
As mucosal immunity and microbiome research is rapidly progressing, pDCs that reside within gut and gut-associated lymphoid tissues are also becoming the focus of increasing interest. In the TNBS-induced colitis model, PSA-induced protection is highly dependent on the ability of pDCs to induce the generation of IL-10-producing TReg cells111. pDCs from the liver and mesenteric lymph nodes are also important for suppression of T cell responses and induction of oral tolerance200. In contrast to splenic pDCs, pDCs from Peyer’s patches are unable to produce type I IFN after TLR7 or TLR9 stimulation201. pDCs that have been developed in the presence of type I IFN resemble Peyer’s patch pDCs, produce inflammatory cytokines, stimulate Th17 cell generation and fail to secrete IFNα after TLR engagement202. Therefore, factors expressed at mucosal sites inhibit type I IFN secretion by pDCs, but do not block the ability of pDCs to prime naïve T cells and trigger TReg and Th17 differentiation203,204.
Gut-associated pDCs are also efficient at generating mucosal B cell responses under normal conditions and during infection with a gut-tropic virus. In the steady-state, pDCs from Peyer’s patches and mesenteric lymph nodes induce T cell-independent IgA production205. Specifically, the microbiota activates stromal cells to produce type I IFN, which drives pDC expression of BAFF and APRIL that facilitate IgA responses in the gut. Viral clearance and protection against reinfection with rotavirus, an intestinal pathogen, is highly dependent on B cells206. Type I IFN production by human and mouse pDCs exposed to rotavirus appears to be necessary for B cell activation, virus-specific antibody secretion and viral clearance during infection206. How the microbiota influences pDC functions is largely unknown, but an earlier study found that colonization of the intestine with a restricted microflora resulted in lysis of pDCs by activated cytotoxic T lymphocytes207. Therefore, specific microbial communities are likely to have a profound impact on pDC homeostasis and function through both direct and indirect mechanisms.
Concluding remarks
Although many immune functions of pDCs have now been described, many questions still remain concerning their complex biology. Tuberculosis in humans is associated with a type I IFN signature208 and pDC accumulation in lymph nodes209. So what is the role of pDCs in tuberculosis and in bacterial infections in general? Can bacterial products activate pDCs and what are the sensors for these products? Although pDCs accumulate in the microenvironment of some tumors, they are reduced in other cancers such as chronic lymphocytic leukemia (CLL)210. What is the impact of pDCs in anti-tumor immune responses and can we exploit them to generate effective T cell responses (Box 3)?
Box 3. pDCs and cancer.
The recruitment of pDCs to tumors is often associated with poor prognosis as tumor-infiltrating pDCs tend to be tolerogenic rather than immunogenic. Unstimulated or alternatively activated pDCs can induce TReg cells through expression of IDO135–138 or ICOSL139. The accumulation of IDO-expressing cells in tumor draining lymph nodes has been associated with worse clinical outcomes in patients with malignant tumors including breast carcinoma137. The infiltration of pDCs in primary tumors of patients with invasive nonmetastatic breast cancer correlates with shorter survival times, suggesting that pDCs contribute to the progression of breast cancer237. Breast tumor-associated pDCs are poor producers of type I IFN and favor the expansion of TReg cells238. The mechanisms suppressing type I IFN production and immunogenic capacity of breast tumor infiltrating pDCs include tumor cell-derived TGF-β and TNF-α239, both TGF-β and TNF-α inhibit type I IFN secretion by pDCs201,240. ICOSL expression on pDCs also appears to correlate with breast cancer progression by supporting amplification of IL-10 producing TReg cells241.
Recruitment of pDCs to ovarian tumors is driven by CXCL12 and is associated with impaired T cell responses and poor prognosis72,242. Compared to pDCs in blood or ascites, ovarian tumor-associated pDCs produce less type I IFN and pro-inflammatory cytokines/chemokines in response to TLR stimulation and induce IL-10 production from allogeneic naive CD4+ T cells, suggesting that they play a critical role in the induction of immune tolerance and progression of ovarian cancer243.
Human pDCs may also contribute to cancer progression via the production and release of the pro-apoptotic molecule granzyme B12, which suppresses T cell proliferation. Activation of pDCs through TLR agonists and CD40 ligand negatively regulates granzyme B expression and the ability of pDCs to suppress T cells142. In contrast to NK cells, pDCs do not release the pore-forming protein perforin, and therefore are unable to kill tumor target cells by release of lytic granules.
On the other hand, pDCs can promote immunogenic anti-tumor responses if appropriately stimulated. Injection of activated pDCs loaded with tumor-associated peptides into metastatic melanoma patients leads to favorable CD4 and CD8 T cell responses, indicating that vaccination strategies employing activated pDCs might be an attractive therapeutic strategy to overcome immune tolerance in certain types of cancer244. In an orthotopic murine mammary tumor model, intratumoral administration of a TLR7 ligand results in tumor-associated pDC activation and has a potent anti-tumor effect245. In the B16 melanoma mouse model, TLR-stimulated pDCs mediate tumor killing through expression of TRAIL and granzyme B75 as well as through the activation of NK cells246. The ability of pDCs to produce cytolytic mediators and the functional relevance of this capacity in the elimination of target cells and immune regulation has been extensively debated; however, it has been shown that the FSME-IMMUN vaccine against tick-borne encephalitis stimulates pDCs to upregulate neural cell adhesion molecule 1 (NCAM1, also known as CD56) and TRAIL expression, thereby generating a subset of pDCs that combines the abilities of interferon production and antigen presentation with that of lysing target cells247. CD56 expression is also typical of several neoplastic human pDC tumors248.
Although SLE is generally associated with a type I IFN signature, it has been recently shown that pDC production of type I IFN is impaired in patients carrying a variant of the PTPN22 gene predisposing to SLE211. This observation raises the question of whether pDC and type I IFN depletion is a valid strategy for all SLE patients or should be adopted after stratification of the patients for pDC activity. Recent studies suggest that pDC dysfunction in autoimmune and inflammatory diseases may extend to scleroderma212, Sjögren syndrome213, Wiskott-Aldrich syndrome214 and atherosclerosis215. Is there a role for pDCs in other inflammatory diseases such as IBD? Is manipulation or elimination of pDCs a feasible therapeutic strategy for some of these diseases? Whether pDCs are valid targets during viral infections remains a matter of debate. While pDC activation may be beneficial at early stages of infection, depletion of pDCs may be considered during chronic viral infections, such as HIV, when type I IFN may exert negative effects.
Addressing these and other questions in mouse models will require advanced tools to deplete pDCs in vivo. The development of CLEC4C-DTR transgenic mice has provided a method for specific but transient pDC depletion216. Conditional targeting of E2-2 in CD11c+ cells has enabled constitutive depletion of pDCs157, although CX3CR1+CD8α+ DCs49, which are closely related to pDCs, and possibly B cell subsets expressing CD11c may also be affected. It will be important to continue to identify pDC-specific genes that can be used for development of pDC lineage-specific deficient mice and pDC-specific Cre deleters. With the recognition of pDC involvement in human pathologies, it is predicted that these cells will be more extensively evaluated for diagnostic and prognostic purposes. Indeed, it will be interesting to explore the therapeutic potential of monoclonal antibodies that selectively target pDCs in many of the disease scenarios discussed above.
Key Points.
- This Review provides an introduction to human and mouse plasmacytoid dendritic cells (pDCs) and their importance in the immune system.
- We discuss the phenotypes of human and mouse pDCs and the mechanisms that control their development.
- We explain how pDCs sense nucleic acids and microbial products as well as their potential role in viral infections and diseases characterized by a type I interferon (IFN) signature such as SLE, psoriasis and type I diabetes.
- We discuss the emerging field of microbiome research and the impact of pDCs on mucosal immune responses.
- We describe how pDCs can be tolerogenic or immunogenic in the context of cancer.
- Finally, we end by raising several questions regarding the validity of targeting pDCs or their type I IFN production as a therapeutic strategy.
Acknowledgments
The authors greatly appreciate the feedback and helpful suggestions from Drs. M. Cella, S. Gilfillan, B. Reizis, M. Sanjuan and A. Krug. M.S. was supported by K01DK095972 from NIDDK.
Glossary terms
star-form partner
The less abundant of the two strands of microRNA (miRNA) that are generated during miRNA biogenesis. Star-form miRNAs may have important functions in miRNA regulatory networks
noncanonical NF-κB pathway
Nuclear factor-κB (NF-κB) signaling that is induced by certain members of the tumour necrosis factor receptor superfamily and predominantly activates p52–RELB NF-κB complexes. By contrast, canonical (or classical) NF-κB signaling typically involves the activation of p50–RELA dimers and is induced by a wider range of pro-inflammatory stimuli
B-1a cells
A subset of innate-like B cells
Biographies
Marco Colonna obtained his MD degree from Parma University, Italy, and completed post-doctoral training at Roswell Park Memorial Institute and Harvard University. He established an independent laboratory at the Basel Institute for Immunology in 1994 and became Professor of Pathology and Immunology at the Washington University in St. Louis in 2001. He focuses on innate immunoreceptors, a field in which his accomplishments encompass identification of the KIR receptors and HLA-C polymorphisms as their inhibitory ligands, as well as the LILR and TREM receptors. Through analysis of the cellular distribution of these receptors, he identified pDCs and Innate Lymphoid Cells, which are a major focus of his laboratory.
Melissa Swiecki received her PhD in Microbiology from the University of Alabama at Birmingham in 2007. She joined the Colonna laboratory in 2007 as a postdoctoral trainee and then as Research Instructor. She studied the impact of pDCs on antiviral responses as well as surface receptors that influence pDC homeostasis and function. She is currently a Scientist in Discovery Immunology at Janssen Research & Development, LLC.
Footnotes
Competing interests statement
M. Swiecki is currently a Scientist at Janssen Research & Development, LLC.
References
- 1.Lennert K, Remmele W. Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes. Acta Haematol. 1958;19:99–113. doi: 10.1159/000205419. [DOI] [PubMed] [Google Scholar]
- 2.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 3.Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 4.Howell DM, Feldman M, Siegal FP, Pettera L, Fitzgerald-Bocarsly P. Peripheral blood of AIDS patients contains cells capable of providing accessory function for the natural killer cell-mediated, lysis of herpes simplex virus-infected targets despite low interferon-alpha production. J Acquir Immune Defic Syndr. 1993;6:15–23. [PubMed] [Google Scholar]
- 5.Perussia B, Fanning V, Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4:120–137. [PubMed] [Google Scholar]
- 6.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 7.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Asselin-Paturel C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 10.Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 11.Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facchetti F, Vermi W, Mason D, Colonna M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003;443:703–717. doi: 10.1007/s00428-003-0918-8. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 15.Blasius AL, et al. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 16.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 18.Puttur F, et al. Absence of Siglec-H in MCMV Infection Elevates Interferon Alpha Production but Does Not Enhance Viral Clearance. PLoS Pathog. 2013;9:e1003648. doi: 10.1371/journal.ppat.1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopatz J, et al. Siglec-h on activated microglia for recognition and engulfment of glioma cells. Glia. 2013;61:1122–1133. doi: 10.1002/glia.22501. [DOI] [PubMed] [Google Scholar]
- 20.Satpathy AT, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiecki M, et al. Cell depletion in mice that express diphtheria toxin receptor under the control of SiglecH encompasses more than plasmacytoid dendritic cells. J Immunol. 2014;192:4409–4416. doi: 10.4049/jimmunol.1303135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naik SH, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 23.Onai N, et al. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 24.Reizis B. Regulation of plasmacytoid dendritic cell development. Curr Opin Immunol. 2010;22:206–211. doi: 10.1016/j.coi.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12:101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 27.Onai N, et al. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity. 2013;38:943–957. doi: 10.1016/j.immuni.2013.04.006. This study identified a M-CSFR− CDP that can be derived from the previously described M-CSFR+ CDP (reference 23) or from lymphoid-primed multipotent progenitors. The authors show that M-CSFR− CDPs preferentially give rise to CCR9− pDC-like CDPs (described in references 29 and 30) and mature CCR9+ pDCs. [DOI] [PubMed] [Google Scholar]
- 28.Cisse B, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlitzer A, et al. Identification of CCR9− murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood. 2011;117:6562–6570. doi: 10.1182/blood-2010-12-326678. [DOI] [PubMed] [Google Scholar]
- 30.Schlitzer A, et al. Tissue-specific differentiation of a circulating CCR9− pDC-like common dendritic cell precursor. Blood. 2012;119:6063–6071. doi: 10.1182/blood-2012-03-418400. [DOI] [PubMed] [Google Scholar]
- 31.Shigematsu H, et al. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Res PC, Couwenberg F, Vyth-Dreese FA, Spits H. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 1999;94:2647–2657. [PubMed] [Google Scholar]
- 33.Bendriss-Vermare N, et al. Human thymus contains IFN-alpha-producing CD11c(−), myeloid CD11c(+), and mature interdigitating dendritic cells. J Clin Invest. 2001;107:835–844. doi: 10.1172/JCI11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelayo R, et al. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 36.Waskow C, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eidenschenk C, et al. Flt3 permits survival during infection by rendering dendritic cells competent to activate NK cells. Proc Natl Acad Sci U S A. 2010;107:9759–9764. doi: 10.1073/pnas.1005186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- 39.Sathaliyawala T, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheffler JM, et al. LAMTOR2 regulates dendritic cell homeostasis through FLT3-dependent mTOR signalling. Nat Commun. 2014;5:5138. doi: 10.1038/ncomms6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen YL, et al. A type I IFN-Flt3 ligand axis augments plasmacytoid dendritic cell development from common lymphoid progenitors. J Exp Med. 2013;210:2515–2522. doi: 10.1084/jem.20130536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fancke B, Suter M, Hochrein H, O’Keeffe M. M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood. 2008;111:150–159. doi: 10.1182/blood-2007-05-089292. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald KP, et al. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol. 2005;175:1399–1405. doi: 10.4049/jimmunol.175.3.1399. [DOI] [PubMed] [Google Scholar]
- 44.Gilliet M, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esashi E, et al. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity. 2008;28:509–520. doi: 10.1016/j.immuni.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagasawa M, Schmidlin H, Hazekamp MG, Schotte R, Blom B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur J Immunol. 2008;38:2389–2400. doi: 10.1002/eji.200838470. [DOI] [PubMed] [Google Scholar]
- 47.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–916. doi: 10.1016/j.immuni.2010.11.023. References 28 and 48 were major discoveries in the pDC field because they identified the transcription factor E2-2 as the master regulator of pDC development/function and found that its continuous expression is critical for maintaining the fate of mature pDCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bar-On L, et al. CX3CR1+ CD8alpha+ dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:14745–14750. doi: 10.1073/pnas.1001562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hacker C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 51.Spits H, Couwenberg F, Bakker AQ, Weijer K, Uittenbogaart CH. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J Exp Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li HS, et al. The signal transducers STAT5 and STAT3 control expression of Id2 and E2-2 during dendritic cell development. Blood. 2012;120:4363–4373. doi: 10.1182/blood-2012-07-441311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh HS, et al. ETO family protein Mtg16 regulates the balance of dendritic cell subsets by repressing Id2. J Exp Med. 2014;211:1623–1635. doi: 10.1084/jem.20132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robbins SH, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rissoan MC, et al. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100:3295–3303. doi: 10.1182/blood-2002-02-0638. [DOI] [PubMed] [Google Scholar]
- 56.Montes-Moreno S, et al. SPIB, a novel immunohistochemical marker for human blastic plasmacytoid dendritic cell neoplasms: characterization of its expression in major hematolymphoid neoplasms. Blood. 2013;121:643–647. doi: 10.1182/blood-2012-08-447599. [DOI] [PubMed] [Google Scholar]
- 57.Schotte R, Nagasawa M, Weijer K, Spits H, Blom B. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J Exp Med. 2004;200:1503–1509. doi: 10.1084/jem.20041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karrich JJ, et al. The transcription factor Spi-B regulates human plasmacytoid dendritic cell survival through direct induction of the antiapoptotic gene BCL2-A1. Blood. 2012;119:5191–5200. doi: 10.1182/blood-2011-07-370239. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki I, et al. Spi-B is critical for plasmacytoid dendritic cell function and development. Blood. 2012;120:4733–4743. doi: 10.1182/blood-2012-06-436527. [DOI] [PubMed] [Google Scholar]
- 60.Wu X, et al. Bcl11a controls Flt3 expression in early hematopoietic progenitors and is required for pDC development in vivo. PLoS One. 2013;8:e64800. doi: 10.1371/journal.pone.0064800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ippolito GC, et al. Dendritic cell fate is determined by BCL11A. Proc Natl Acad Sci U S A. 2014;111:E998–1006. doi: 10.1073/pnas.1319228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiavoni G, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becker AM, et al. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hambleton S, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawai CM, et al. Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J Exp Med. 2013;210:2151–2159. doi: 10.1084/jem.20130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller JC, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. References 54 and 66 are valuable resources for future investigation because they provide a comprehensive comparative analysis of DC subsets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 68.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Seth S, et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol. 2011;186:3364–3372. doi: 10.4049/jimmunol.1002598. [DOI] [PubMed] [Google Scholar]
- 70.Kohara H, et al. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12-CXCR4 chemokine signaling. Blood. 2007;110:4153–4160. doi: 10.1182/blood-2007-04-084210. [DOI] [PubMed] [Google Scholar]
- 71.Umemoto E, et al. Constitutive plasmacytoid dendritic cell migration to the splenic white pulp is cooperatively regulated by CCR7- and CXCR4-mediated signaling. J Immunol. 2012;189:191–199. doi: 10.4049/jimmunol.1200802. [DOI] [PubMed] [Google Scholar]
- 72.Zou W, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 73.Krug A, et al. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J Immunol. 2002;169:6079–6083. doi: 10.4049/jimmunol.169.11.6079. [DOI] [PubMed] [Google Scholar]
- 74.Diacovo TG, Blasius AL, Mak TW, Cella M, Colonna M. Adhesive mechanisms governing interferon-producing cell recruitment into lymph nodes. J Exp Med. 2005;202:687–696. doi: 10.1084/jem.20051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drobits B, et al. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sisirak V, et al. CCR6/CCR10-mediated plasmacytoid dendritic cell recruitment to inflamed epithelia after instruction in lymphoid tissues. Blood. 2011;118:5130–5140. doi: 10.1182/blood-2010-07-295626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hadeiba H, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. This study demonstrates that peripheral pDCs are able to capture antigen and migrate to the thymus where they delete antigen-reactive thymocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wendland M, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wurbel MA, McIntire MG, Dwyer P, Fiebiger E. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS One. 2011;6:e16442. doi: 10.1371/journal.pone.0016442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clahsen T, Pabst O, Tenbrock K, Schippers A, Wagner N. Localisation of dendritic cells in the gut epithelium requires MAdCAM-1. Clin Immunol. 2014;156:74–84. doi: 10.1016/j.clim.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Marafioti T, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111:3778–3792. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- 82.Srivatsan S, Swiecki M, Otero K, Cella M, Shaw AS. CD2-associated protein regulates plasmacytoid dendritic cell migration, but is dispensable for their development and cytokine production. J Immunol. 2013;191:5933–5940. doi: 10.4049/jimmunol.1300454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gotoh K, et al. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood. 2008;111:2973–2976. doi: 10.1182/blood-2007-09-112169. [DOI] [PubMed] [Google Scholar]
- 84.Kim S, et al. Self-priming determines high type I IFN production by plasmacytoid dendritic cells. Eur J Immunol. 2014;44:807–818. doi: 10.1002/eji.201343806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumagai Y, et al. Cutting Edge: TLR-Dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-{alpha} production in plasmacytoid dendritic cells. J Immunol. 2009;182:3960–3964. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 86.Feng Z, et al. Human pDCs preferentially sense enveloped hepatitis A virions. J Clin Invest. 2014;125:169–176. doi: 10.1172/JCI77527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dreux M, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. References 86 and 87 define mechanisms involved in the uptake and sensing of hepatitis A virus and hepatitis C virus by pDCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frenz T, et al. Independent of plasmacytoid dendritic cell (pDC) infection, pDC triggered by virus-infected cells mount enhanced type I IFN responses of different composition as opposed to pDC stimulated with free virus. J Immunol. 2014;193:2496–2503. doi: 10.4049/jimmunol.1400215. [DOI] [PubMed] [Google Scholar]
- 89.Flores M, et al. FcgammaRIIB Prevents Inflammatory Type I IFN Production from Plasmacytoid Dendritic Cells during a Viral Memory Response. J Immunol. 2015;194:4240–4250. doi: 10.4049/jimmunol.1401296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bave U, et al. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 91.Barrat FJ, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Means TK, et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 94.Guiducci C, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoshino K, et al. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 96.Shinohara ML, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takaoka A, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 99.Purtha WE, Swiecki M, Colonna M, Diamond MS, Bhattacharya D. Spontaneous mutation of the Dock2 gene in Irf5−/− mice complicates interpretation of type I interferon production and antibody responses. Proc Natl Acad Sci U S A. 2012;109:E898–904. doi: 10.1073/pnas.1118155109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blasius AL, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. References 100 and 101 describe the proteins involved in the bifurcation of TLR9 signals and selective receptor trafficking within the endosomal system in pDCs that lead to type I IFN production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henault J, et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. These findings highlight a major role for nonconventional autophagy in inflammation and provide a mechanism for how DNA-immune complexes are sensed by TLR9 in pDCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 104.Guo H, et al. SCARB2/LIMP-2 Regulates IFN Production of Plasmacytoid Dendritic Cells by Mediating Endosomal Translocation of TLR9 and Nuclear Translocation of IFN Regulatory Factor 7. J Immunol. 2015 doi: 10.4049/jimmunol.1402312. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Talukder AH, et al. Phospholipid scramblase 1 regulates Toll-like receptor 9-mediated type I interferon production in plasmacytoid dendritic cells. Cell Res. 2012;22:1129–1139. doi: 10.1038/cr.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Esashi E, Bao M, Wang YH, Cao W, Liu YJ. PACSIN1 regulates the TLR7/9-mediated type I interferon response in plasmacytoid dendritic cells. Eur J Immunol. 2012;42:573–579. doi: 10.1002/eji.201142045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saitoh T, et al. Antiviral protein Viperin promotes Toll-like receptor 7- and Toll-like receptor 9-mediated type I interferon production in plasmacytoid dendritic cells. Immunity. 2011;34:352–363. doi: 10.1016/j.immuni.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 108.Gotoh K, et al. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J Exp Med. 2010;207:721–730. doi: 10.1084/jem.20091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Lau KY, Jung J, Ravindran P, Barrat FJ. Bruton’s tyrosine kinase regulates TLR9 but not TLR7 signaling in human plasmacytoid dendritic cells. Eur J Immunol. 2014;44:1130–1136. doi: 10.1002/eji.201344030. [DOI] [PubMed] [Google Scholar]
- 110.Koblansky AA, et al. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38:119–130. doi: 10.1016/j.immuni.2012.09.016. This reference is the first to show that pDCs utilize the previously uncharacterized TLR12 to sense T. gondii profilin and contribute to host resistance to T. gondii infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. 2014;15:413–423. doi: 10.1016/j.chom.2014.03.006. Here it is reported that pDCs sense the immunomodulatory molecule polysaccharide A via TLR2 and mediate protection against colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 113.Kim T, et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 115.Bao M, Liu YJ. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell. 2013;4:40–52. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dzionek A, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cao W, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fuchs A, Cella M, Kondo T, Colonna M. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood. 2005;106:2076–2082. doi: 10.1182/blood-2004-12-4802. [DOI] [PubMed] [Google Scholar]
- 119.Ju X, Zenke M, Hart DN, Clark GJ. CD300a/c regulate type I interferon and TNF-alpha secretion by human plasmacytoid dendritic cells stimulated with TLR7 and TLR9 ligands. Blood. 2008;112:1184–1194. doi: 10.1182/blood-2007-12-127951. [DOI] [PubMed] [Google Scholar]
- 120.Meyer-Wentrup F, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 121.Cao W, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonaccorsi I, et al. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNalpha production. PLoS One. 2010;5:e15080. doi: 10.1371/journal.pone.0015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bao M, et al. CD2AP/SHIP1 complex positively regulates plasmacytoid dendritic cell receptor signaling by inhibiting the E3 ubiquitin ligase Cbl. J Immunol. 2012;189:786–792. doi: 10.4049/jimmunol.1200887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Watarai H, et al. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci U S A. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tai LH, et al. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205:3187–3199. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitsuhashi Y, et al. Regulation of plasmacytoid dendritic cell responses by PIR-B. Blood. 2012;120:3256–3259. doi: 10.1182/blood-2012-03-419093. [DOI] [PubMed] [Google Scholar]
- 127.Chiang EY, Johnston RJ, Grogan JL. EBI2 is a negative regulator of type I interferons in plasmacytoid and myeloid dendritic cells. PLoS One. 2013;8:e83457. doi: 10.1371/journal.pone.0083457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karrich JJ, et al. MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood. 2013;122:3001–3009. doi: 10.1182/blood-2012-12-475087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou H, et al. miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood. 2010;116:5885–5894. doi: 10.1182/blood-2010-04-280156. [DOI] [PubMed] [Google Scholar]
- 130.Agudo J, et al. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seillet C, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 132.Meier A, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 134.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid Dendritic Cells: Recent Progress and Open Questions. Annu Rev Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pallotta MT, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 136.Fallarino F, et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–3754. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- 137.Munn DH, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boasso A, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ito T, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diana J, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 141.Diana J, et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J Exp Med. 2011;208:729–745. doi: 10.1084/jem.20101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jahrsdorfer B, et al. Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion. Blood. 2010;115:1156–1165. doi: 10.1182/blood-2009-07-235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hadeiba H, et al. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Segura E, Wong J, Villadangos JA. Cutting edge: B220+CCR9− dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J Immunol. 2009;183:1514–1517. doi: 10.4049/jimmunol.0901524. [DOI] [PubMed] [Google Scholar]
- 145.Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8alpha(+)beta(−) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 2012;5:432–443. doi: 10.1038/mi.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brown AS, Bourges D, Ang DK, Hartland EL, van Driel IR. CD8 subunit expression by plasmacytoid dendritic cells is variable, and does not define stable subsets. Mucosal Immunol. 2014;7:200–201. doi: 10.1038/mi.2013.91. [DOI] [PubMed] [Google Scholar]
- 147.Dalod M, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chappell CP, et al. Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance. J Immunol. 2014;192:5789–5801. doi: 10.4049/jimmunol.1303259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Loschko J, et al. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J Immunol. 2011;187:6346–6356. doi: 10.4049/jimmunol.1102307. [DOI] [PubMed] [Google Scholar]
- 150.Loschko J, et al. Antigen delivery to plasmacytoid dendritic cells via BST2 induces protective T cell-mediated immunity. J Immunol. 2011;186:6718–6725. doi: 10.4049/jimmunol.1004029. [DOI] [PubMed] [Google Scholar]
- 151.Hoeffel G, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 152.Mouries J, et al. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Di Pucchio T, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Y, Swiecki M, McCartney SA, Colonna M. dsRNA sensors and plasmacytoid dendritic cells in host defense and autoimmunity. Immunol Rev. 2011;243:74–90. doi: 10.1111/j.1600-065X.2011.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumagai Y, et al. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 156.Cervantes-Barragan L, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cervantes-Barragan L, et al. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Swiecki M, Wang Y, Gilfillan S, Colonna M. Plasmacytoid Dendritic Cells Contribute to Systemic but Not Local Antiviral Responses to HSV Infections. PLoS Pathog. 2013;9:e1003728. doi: 10.1371/journal.ppat.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ciancanelli MJ, et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat Commun. 2014;5:3864. doi: 10.1038/ncomms4864. References 159 and 160 provide evidence that pDC-derived type I IFN may be protective or pathogenic during influenza infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Manches O, Frleta D, Bhardwaj N. Dendritic cells in progression and pathology of HIV infection. Trends Immunol. 2014;35:114–122. doi: 10.1016/j.it.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reeves RK, et al. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J Infect Dis. 2012;206:1462–1468. doi: 10.1093/infdis/jis408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lehmann C, et al. Longitudinal analysis of distribution and function of plasmacytoid dendritic cells in peripheral blood and gut mucosa of HIV infected patients. J Infect Dis. 2014;209:940–949. doi: 10.1093/infdis/jit612. [DOI] [PubMed] [Google Scholar]
- 164.Li H, Goepfert P, Reeves RK. Short Communication: Plasmacytoid Dendritic Cells from HIV-1 Elite Controllers Maintain a Gut-Homing Phenotype Associated with Immune Activation. AIDS Res Hum Retroviruses. 2014;30:1213–1215. doi: 10.1089/aid.2014.0174. For many years it has been observed that pDCs are reduced in the blood of HIV-infected individuals and references 162, 163 and 164 show that this is because pDCs exhibit a gut-homing phenotype and accumulate in the gut mucosa during SIV and HIV infections. [DOI] [PubMed] [Google Scholar]
- 165.O’Brien M, Manches O, Bhardwaj N. Plasmacytoid dendritic cells in HIV infection. Adv Exp Med Biol. 2013;762:71–107. doi: 10.1007/978-1-4614-4433-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.O’Brien M, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121:1088–1101. doi: 10.1172/JCI44960. This report demostrates that HIV activates pDCs through TLR7 in the early endosome, which skews pDCs toward a partially matured, persistently type I IFN-producing phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Manches O, Fernandez MV, Plumas J, Chaperot L, Bhardwaj N. Activation of the noncanonical NF-kappaB pathway by HIV controls a dendritic cell immunoregulatory phenotype. Proc Natl Acad Sci U S A. 2012;109:14122–14127. doi: 10.1073/pnas.1204032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Volpi C, et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun. 2013;4:1852. doi: 10.1038/ncomms2874. [DOI] [PubMed] [Google Scholar]
- 169.Li G, et al. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog. 2014;10:e1004291. doi: 10.1371/journal.ppat.1004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kader M, et al. Blocking TLR7- and TLR9-mediated IFN-alpha production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog. 2013;9:e1003530. doi: 10.1371/journal.ppat.1003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sandler NG, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. This reference shows that the timing of type I IFN-induced innate responses in acute SIV infection impacts overall disease course and outweighs the detrimental consequences of increased immune activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Blasius AL, Krebs P, Sullivan BM, Oldstone MB, Popkin DL. Slc15a4, a gene required for pDC sensing of TLR ligands, is required to control persistent viral infection. PLoS Pathog. 2012;8:e1002915. doi: 10.1371/journal.ppat.1002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang Y, et al. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11:631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilson EB, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. References 174 and 175 demonstrate that blockade of chronic type I IFN signaling during persistent viral infection restores T cell responses and promotes viral clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 178.Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. References 178 and 179 reveal a role for NETs in SLE pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Morshed M, et al. NADPH oxidase-independent formation of extracellular DNA traps by basophils. J Immunol. 2014;192:5314–5323. doi: 10.4049/jimmunol.1303418. [DOI] [PubMed] [Google Scholar]
- 182.Guiducci C, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. This study found that stimulation of pDCs through TLR7 and TLR9 accounts for the reduced activity of glucocorticoids to inhibit the type I IFN pathway in SLE patients and in lupus-prone mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lepelletier Y, et al. Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid predendritic cells (pDCs) Blood. 2010;116:3389–3397. doi: 10.1182/blood-2010-05-282913. [DOI] [PubMed] [Google Scholar]
- 184.Hong Y, et al. miR-29b and miR-29c are involved in Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid dendritic cells. PLoS One. 2013;8:e69926. doi: 10.1371/journal.pone.0069926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Soulier A, et al. Cell-intrinsic regulation of murine dendritic cell function and survival by prereceptor amplification of glucocorticoid. Blood. 2013;122:3288–3297. doi: 10.1182/blood-2013-03-489138. [DOI] [PubMed] [Google Scholar]
- 186.Baccala R, et al. Anti-IFN-alpha/beta receptor antibody treatment ameliorates disease in lupus-predisposed mice. J Immunol. 2012;189:5976–5984. doi: 10.4049/jimmunol.1201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guiducci C, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baccala R, et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc Natl Acad Sci U S A. 2013;110:2940–2945. doi: 10.1073/pnas.1222798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kobayashi T, et al. The histidine transporter SLC15A4 coordinates mTOR-dependent inflammatory responses and pathogenic antibody production. Immunity. 2014;41:375–388. doi: 10.1016/j.immuni.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 190.Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sisirak V, et al. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med. 2014;211:1969–1976. doi: 10.1084/jem.20132522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rowland SL, et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med. 2014;211:1977–1991. doi: 10.1084/jem.20132620. References 188, 191 and 192 provide strong evidence that depletion of pDCs or blocking their ability to produce type I IFN is an attractive therapeutic strategy for the treatment of SLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 194.Ganguly D, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lande R, et al. Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self-DNA. Eur J Immunol. 2014;45:203–213. doi: 10.1002/eji.201344277. [DOI] [PubMed] [Google Scholar]
- 196.Karthaus N, et al. Vitamin D controls murine and human plasmacytoid dendritic cell function. J Invest Dermatol. 2014;134:1255–1264. doi: 10.1038/jid.2013.501. [DOI] [PubMed] [Google Scholar]
- 197.Diana J, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 198.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li Q, et al. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Goubier A, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. Cutting edge: Peyer’s patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol. 2007;179:2690–2694. doi: 10.4049/jimmunol.179.5.2690. [DOI] [PubMed] [Google Scholar]
- 202.Li HS, et al. Cell-intrinsic role for IFN-alpha-STAT1 signals in regulating murine Peyer patch plasmacytoid dendritic cells and conditioning an inflammatory response. Blood. 2011;118:3879–3889. doi: 10.1182/blood-2011-04-349761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bonnefoy F, et al. TGF-beta-exposed plasmacytoid dendritic cells participate in Th17 commitment. J Immunol. 2011;186:6157–6164. doi: 10.4049/jimmunol.1002497. [DOI] [PubMed] [Google Scholar]
- 204.Michea P, et al. Epithelial control of the human pDC response to extracellular bacteria. Eur J Immunol. 2013;43:1264–1273. doi: 10.1002/eji.201242990. [DOI] [PubMed] [Google Scholar]
- 205.Tezuka H, et al. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 206.Deal EM, Lahl K, Narvaez CF, Butcher EC, Greenberg HB. Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses. J Clin Invest. 2013;123:2464–2474. doi: 10.1172/JCI60945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fujiwara D, et al. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and commensal microbiota. J Immunol. 2008;180:5843–5852. doi: 10.4049/jimmunol.180.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Berry MP, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Facchetti F, et al. Plasmacytoid monocytes (so-called plasmacytoid T cells) in granulomatous lymphadenitis. Hum Pathol. 1989;20:588–593. doi: 10.1016/0046-8177(89)90248-7. [DOI] [PubMed] [Google Scholar]
- 210.Saulep-Easton D, et al. Cytokine-driven loss of plasmacytoid dendritic cell function in chronic lymphocytic leukemia. Leukemia. 2014;28:2005–2015. doi: 10.1038/leu.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang Y, et al. PTPN22 variant R620W associates with reduced TLR7-induced type 1 Interferon in SLE. Arthritis Rheum. 2015 doi: 10.1002/art.39211. In press. [DOI] [PubMed] [Google Scholar]
- 212.van Bon L, et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med. 2014;370:433–443. doi: 10.1056/NEJMoa1114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ronnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Curr Opin Rheumatol. 2013;25:248–253. doi: 10.1097/BOR.0b013e32835c7e32. [DOI] [PubMed] [Google Scholar]
- 214.Prete F, et al. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J Exp Med. 2013;210:355–374. doi: 10.1084/jem.20120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sage AP, et al. MHC Class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation. 2014;130:1363–1373. doi: 10.1161/CIRCULATIONAHA.114.011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Niederquell M, et al. Sca-1 expression defines developmental stages of mouse pDCs that show functional heterogeneity in the endosomal but not lysosomal TLR9 response. Eur J Immunol. 2013;43:2993–3005. doi: 10.1002/eji.201343498. [DOI] [PubMed] [Google Scholar]
- 218.Kamogawa-Schifter Y, et al. Ly49Q defines 2 pDC subsets in mice. Blood. 2005;105:2787–2792. doi: 10.1182/blood-2004-09-3388. [DOI] [PubMed] [Google Scholar]
- 219.Bjorck P, Leong HX, Engleman EG. Plasmacytoid dendritic cell dichotomy: identification of IFN-alpha producing cells as a phenotypically and functionally distinct subset. J Immunol. 2011;186:1477–1485. doi: 10.4049/jimmunol.1000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.O’Keeffe M, et al. Nonplasmacytoid, high IFN-alpha-producing, bone marrow dendritic cells. J Immunol. 2012;188:3774–3783. doi: 10.4049/jimmunol.1101365. [DOI] [PubMed] [Google Scholar]
- 221.Zucchini N, et al. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int Immunol. 2008;20:45–56. doi: 10.1093/intimm/dxm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Allman D, et al. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108:4025–4034. doi: 10.1182/blood-2006-03-007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Carotta S, et al. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32:628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 224.Janssens S, Pulendran B, Lambrecht BN. Emerging functions of the unfolded protein response in immunity. Nat Immunol. 2014;15:910–919. doi: 10.1038/ni.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hirai M, et al. Bortezomib suppresses function and survival of plasmacytoid dendritic cells by targeting intracellular trafficking of Toll-like receptors and endoplasmic reticulum homeostasis. Blood. 2011;117:500–509. doi: 10.1182/blood-2010-05-284737. [DOI] [PubMed] [Google Scholar]
- 227.Weigert A, et al. HIF-1alpha is a negative regulator of plasmacytoid DC development in vitro and in vivo. Blood. 2012;120:3001–3006. doi: 10.1182/blood-2012-03-417022. [DOI] [PubMed] [Google Scholar]
- 228.Rathinam C, et al. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity. 2005;22:717–728. doi: 10.1016/j.immuni.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 229.Chow KT, Schulz D, McWhirter SM, Schlissel MS. Gfi1 and gfi1b repress rag transcription in plasmacytoid dendritic cells in vitro. PLoS One. 2013;8:e75891. doi: 10.1371/journal.pone.0075891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Balzarolo M, et al. The transcriptional regulator NAB2 reveals a two-step induction of TRAIL in activated plasmacytoid DCs. Eur J Immunol. 2012;42:3019–3027. doi: 10.1002/eji.201242385. [DOI] [PubMed] [Google Scholar]
- 231.Tel J, et al. Tumoricidal activity of human dendritic cells. Trends Immunol. 2014;35:38–46. doi: 10.1016/j.it.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci U S A. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stary G, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 234.Colisson R, et al. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood. 2010;115:2177–2185. doi: 10.1182/blood-2009-06-224741. [DOI] [PubMed] [Google Scholar]
- 235.Li HS, Greeley N, Sugimoto N, Liu YJ, Watowich SS. miR-22 controls Irf8 mRNA abundance and murine dendritic cell development. PLoS One. 2012;7:e52341. doi: 10.1371/journal.pone.0052341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chauvistre H, et al. Dendritic cell development requires histone deacetylase activity. Eur J Immunol. 2014;44:2478–2488. doi: 10.1002/eji.201344150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Treilleux I, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 238.Sisirak V, et al. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–5197. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 239.Sisirak V, et al. Breast cancer-derived transforming growth factor-beta and tumor necrosis factor-alpha compromise interferon-alpha production by tumor-associated plasmacytoid dendritic cells. Int J Cancer. 2013;133:771–778. doi: 10.1002/ijc.28072. [DOI] [PubMed] [Google Scholar]
- 240.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Faget J, et al. ICOS is associated with poor prognosis in breast cancer as it promotes the amplification of immunosuppressive CD4 T cells by plasmacytoid dendritic cells. Oncoimmunology. 2013;2:e23185. doi: 10.4161/onci.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Labidi-Galy SI, et al. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology. 2012;1:380–382. doi: 10.4161/onci.18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Labidi-Galy SI, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–5434. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 244.Tel J, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. References 75 and 244 show that activated pDCs can directly eliminate tumor cells in a mouse model of melanoma and promote anti-vaccine T cell responses in patients with metastatic melanoma. [DOI] [PubMed] [Google Scholar]
- 245.Le Mercier I, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73:4629–4640. doi: 10.1158/0008-5472.CAN-12-3058. [DOI] [PubMed] [Google Scholar]
- 246.Liu C, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tel J, et al. Human plasmacytoid dendritic cells are equipped with antigen-presenting and tumoricidal capacities. Blood. 2012;120:3936–3944. doi: 10.1182/blood-2012-06-435941. [DOI] [PubMed] [Google Scholar]
- 248.Petrella T, et al. ‘Agranular CD4+ CD56+ hematodermic neoplasm’ (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002;26:852–862. doi: 10.1097/00000478-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 249.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 250.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 251.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Park B, et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]