Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel (original) (raw)
Abstract
Introduction
Atherosclerotic cardiovascular disease (ASCVD) starts early, even in childhood.1,2 Non-invasive imaging in the PESA (Progression of Early Subclinical Atherosclerosis) study revealed that 71% and 43% of middle-aged men and women, respectively, have evidence of subclinical atherosclerosis.3 Extensive evidence from epidemiologic, genetic, and clinical intervention studies has indisputably shown that low-density lipoprotein (LDL) is causal in this process, as summarized in the first Consensus Statement on this topic.4 What are the key biological mechanisms, however, that underlie the central role of LDL in the complex pathophysiology of ASCVD, a chronic and multifaceted lifelong disease process, ultimately culminating in an atherothrombotic event?
This second Consensus Statement on LDL causality discusses the established and newly emerging biology of ASCVD at the molecular, cellular, and tissue levels, with emphasis on integration of the central pathophysiological mechanisms. Key components of this integrative approach include consideration of factors that modulate the atherogenicity of LDL at the arterial wall and downstream effects exerted by LDL particles on the atherogenic process within arterial tissue.
Although LDL is unequivocally recognized as the principal driving force in the development of ASCVD and its major clinical sequelae,4,5 evidence for the causal role of other apolipoprotein B (apoB)-containing lipoproteins in ASCVD is emerging. Detailed consideration of the diverse mechanisms by which these lipoproteins, including triglyceride (TG)-rich lipoproteins (TGRL) and their remnants [often referred to as intermediate-density lipoproteins (IDL)], and lipoprotein(a) [Lp(a)], contribute not only to the underlying pathophysiology of ASCVD but also potentially to atherothrombotic events, is however beyond the focus of this appraisal.6–14
The pathophysiological and genetic components of ASCVD are not fully understood. We have incomplete understanding, for example, of factors controlling the intimal penetration and retention of LDL, and the subsequent immuno-inflammatory responses of the arterial wall to the deposition and modification of LDL. Disease progression is also affected by genetic and epigenetic factors influencing the susceptibility of the arterial wall to plaque formation and progression. Recent data indicate that these diverse pathophysiological aspects are key to facilitating superior risk stratification of patients and optimizing intervention to prevent atherosclerosis progression. Moreover, beyond atherosclerosis progression are questions relating to mechanisms of plaque regression and stabilization induced following marked LDL-cholesterol (LDL-C) reduction by lipid-lowering agents.15–19 Finally, the potential implication of high-density lipoprotein (HDL) and its principal protein, apoAI, as a potential modulator of LDL atherogenicity remains unresolved.20 It was, therefore, incumbent on this Consensus Panel to identify and highlight the missing pieces of this complex puzzle as target areas for future clinical and basic research, and potentially for the development of innovative therapeutics to decrease the burgeoning clinical burden of ASCVD.
Trancytosis of low-density lipoprotein across the endothelium
Apolipoprotein B-containing lipoproteins of up to ∼70 nm in diameter [i.e. chylomicron remnants, very low-density lipoproteins (VLDL) and VLDL remnants, IDL, LDL, and Lp(a)] can cross the endothelium (Figure 1).21–29 Low-density lipoprotein, as the most abundant atherogenic lipoprotein in plasma, is the key deliverer of cholesterol to the artery wall. Many risk factors modulate the propensity of LDL and other atherogenic lipoproteins to traverse the endothelium and enter the arterial intima.30 Despite the relevance of LDL endothelial transport during atherogenesis, however, the molecular mechanisms controlling this process are still not fully understood.31
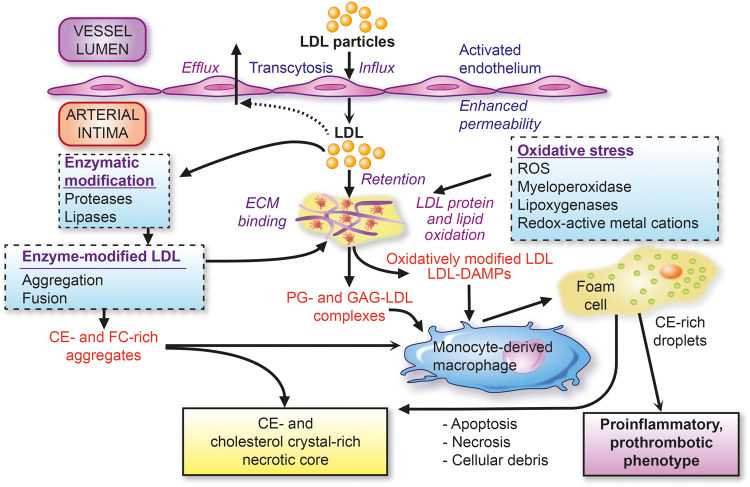
Figure 1.
Low-density lipoprotein (LDL) as the primary driver of atherogenesis. Key features of the influx and retention of LDL in the arterial intima, with ensuing pathways of modification leading to (i) extracellular cholesterol accumulation and (ii) formation of cholesteryl ester droplet-engorged macrophage foam cells with transformation to an inflammatory and prothrombotic phenotype. Both of these major pathways favour formation of the plaque necrotic core containing cellular and extracellular debris and LDL-cholesterol-derived cholesterol crystals. CE, cholesteryl ester; DAMPs, damage-associated molecular patterns; ECM, extracellular matrix; FC, free cholesterol; GAG, glycosaminoglycans; PG, proteoglycans; ROS, reactive oxygen species.
A considerable body of evidence in recent years32 has challenged the concept that movement of LDL occurs by passive filtration (i.e. as a function of particle size and concentration) across a compromised endothelium of high permeability.33 Studies have demonstrated that LDL transcytosis occurs through a vesicular pathway, involving caveolae,34–36 scavenger receptor B1 (SR-B1),37 activin receptor-like kinase 1 (ALK1),38 and the LDL receptor.32 However, although the LDL receptor appears to mediate LDL transcytosis across the blood–brain barrier,39 proprotein convertase subtilisin/kexin type 9 (PCSK9)-directed degradation of the LDL receptor has no effect on LDL transcytosis40; thus, LDL transport across the endothelium in the systemic circulation seems to be LDL receptor-independent.32 Indeed, new evidence shows that LDL transcytosis across endothelial cell monolayers requires interaction of SR-B1 with a cytoplasmic protein.40 More specifically, LDL induces a marked increase in the coupling of SR-B1 (through an eight-amino-acid cytoplasmic tail domain) to the guanine nucleotide exchange factor dedicator of cytokinesis 4 (DOCK4); both SR-B1 and DOCK4 are required for LDL transport.41 Interestingly, expression of SR-B1 and DOCK4 is higher in human atherosclerotic arteries than in normal arteries.41
Oestrogens significantly inhibit LDL transcytosis by down-regulating endothelial SR-BI.42 This down-regulation is dependent on the G-protein-coupled oestrogen receptor and explains why physiological levels of oestrogen reduce LDL transcytosis in arterial endothelial cells of male but not female origin. These findings offer one explanation for why women have a lower risk than men of ASCVD before but not after the menopause.43,44 Transcytosis of LDL across endothelial cells can also be increased, for example, by activation of the NOD-like receptor containing domain pyrin 3 (NLRP3) inflammasome,45 the multiprotein cytosolic complex that activates expression of the interleukin-1 (IL-1) family of cytokines, or by hyperglycaemia.46 In contrast, rapid correction of hypercholesterolaemia in mice improved the endothelial barrier to LDL.47 The mechanisms that underlie increased rates of LDL transcytosis during hypercholesterolaemia remain unclear; improved understanding offers potential for therapies targeting early events in atherosclerosis.48
Factors affecting retention of low-density lipoprotein in the artery wall
Subendothelial accumulation of LDL at lesion-susceptible arterial sites is mainly due to selective retention of LDL in the intima, and is mediated by interaction of specific positively charged amino acyl residues (arginine and lysine) in apoB100 with negatively charged sulfate and carboxylic acid groups of arterial wall proteoglycans.49 Genetic alteration of either the proteoglycan-binding domain of apoB100 or the apoB100-binding domain of arterial wall proteoglycans greatly reduces atherogenesis.49,50 Thus, the atherogenicity of LDL is linked to the ability of its apoB100 moiety to interact with arterial wall proteoglycans,50,51 a process influenced by compositional changes in both the core and surface of the LDL particle. For example, enrichment of human LDL with cholesteryl oleate enhances proteoglycan-binding and atherogenesis.52 In addition, apoE, apoC-III, and serum amyloid A increase the affinity of LDL for arterial wall proteoglycans.49,53–55
Autopsy studies in young individuals demonstrated that atherosclerosis-prone arteries develop intimal hyperplasia, a thickening of the intimal layer due to accumulation of smooth muscle cells (SMCs) and proteoglycans.56,57 In contrast, atherosclerosis-resistant arteries form minimal to no intimal hyperplasia.57–59 Surgical induction of disturbed laminar flow in the atherosclerosis-resistant common carotid artery of mice has been shown to cause matrix proliferation and lipoprotein retention,60 indicating that hyperplasia is critical to the sequence of events leading to plaque formation.
Although the propensity to develop atherosclerosis varies markedly across different sites in the human vasculature, it is notable at branches and bifurcations where the endothelium is exposed to disturbed laminar blood flow and low or fluctuating shear stress.61 These mechanical forces may modulate gene and protein expression and induce endothelial dysfunction and intimal hyperplasia. Formation of atherosclerotic lesions in vessels exhibiting intimal hyperplasia also occurs following surgical intervention, as exemplified by vascular changes following coronary artery bypass surgery.62 A number of the genetic variants strongly associated with ASCVD in genome-wide association studies (GWAS) occur in genes that encode arterial wall proteins, which either regulate susceptibility to LDL retention or the arterial response to LDL accumulation.63 This topic is discussed in more detail below.
Low-density lipoprotein particle heterogeneity
Low-density lipoprotein particles are pseudomicellar, quasi-spherical, and plurimolecular complexes. The lipidome accounts for ∼80% by weight and involves >300 distinct molecular species of lipids (Meikle and Chapman, unpublished observations), whereas the proteome is dominated by apoB100 (one molecule per LDL particle).64–66 ApoB100, one of the largest mammalian proteins (∼550 kDa), maintains the structural integrity of particles in the VLDL-LDL spectrum and, in contrast to smaller apolipoproteins, remains with the lipoprotein particle throughout its life cycle.
At circulating particle concentrations of ∼1 mmol/L, LDL is the principal carrier of cholesterol (2000–2700 molecules per particle, of which ∼1700 are in esterified form) in human plasma. Low-density lipoprotein is also the major carrier of vitamin E, carotenoids, and ubiquinol, but a minor carrier of small, non-coding RNAs compared with HDL, although the proatherogenic microRNA miR-155 is abundant in LDL.66–68
Low-density lipoprotein comprises a spectrum of multiple discrete particle subclasses with different physicochemical, metabolic, and functional characteristics (Box 1).64,66,67,69–84,90–98 In people with normal lipid levels, three major subclasses are typically recognized: large, buoyant LDL-I (density 1.019–1.023 g/mL), LDL-II of intermediate size and density (density 1.023–1.034 g/mL), small dense LDL-III (density 1.034–1.044 g/mL); and a fourth subfraction of very small dense LDL-IV (density 1.044–1.063 g/mL) is present in individuals with elevated TG levels 64,75,81,90,99 Low-density lipoprotein-cholesterol measured routinely in the clinical chemistry laboratory is the sum of cholesterol in these subclasses and in IDL and Lp(a).100,101
Box 1.
Differences in physicochemical, metabolic, and functional characteristics between the markedly heterogenous low-density lipoprotein subclasses
- Particle diameter, molecular weight, hydrated density, net surface charge, % weight lipid and protein composition (CE, FC, TG, PL, and PRN), and N-linked glycosylation of apoB100.
- Particle origin (liver and intravascular remodelling from precursor particles).
- Residence time in plasma (turnover half-life).
- Relative binding affinity for the cellular LDL receptor.
- Conformational differences in apoB100.
- Relative susceptibility to oxidative modification under oxidative stress (e.g. conjugated diene and LOOH formation).
- Relative binding affinity for arterial wall matrix proteoglycans and thus potential for arterial retention.
- Relative content of minor apolipoproteins, including apoC-III and apoE.
- Relative content of lipoprotein-associated phospholipase A2.
- Relative acceptor activities for neutral lipid transfer/exchange (CE and TG) mediated by CETP.
apo, apolipoprotein; CE, cholesteryl ester; CETP, cholesteryl ester transfer protein; FC, free cholesterol; LOOH, lipid hydroperoxide; PL, phospholipid; PRN, protein; TG, triglyceride.
Factors affecting the low-density lipoprotein subfraction profile
Very low-density lipoprotein-TG levels are a major determinant of the LDL subfraction profile. As plasma TG levels rise, the profile shifts from a predominance of large particles to small dense LDL.64,66,74,77–79,90,99 Sex is also a key factor; men are more likely to produce small dense LDL than women at a given TG level, with the underlying mechanism attributed to higher hepatic lipase activity.74,79,90 In metabolic models explaining the generation of small LDL species (LDL-III and LDL-IV), cholesteryl ester transfer protein (CETP)-mediated transfer of TG molecules from VLDL (and potentially chylomicrons) to the core of LDL particles in exchange for cholesteryl esters is a critical step.102 The LDL particle may be subsequently lipolyzed by hepatic lipase to remove both TG from the core and phospholipid from the surface, thereby producing a new, stable but smaller and denser particle.64,74,75,79
Plasma TG levels in the fasting state are regulated by VLDL production in the liver, residual intestinal production of apoB48-containing VLDL-sized particles,103 the activities of lipoprotein and hepatic lipases, and the rate of particle clearance by receptor-mediated uptake. The liver can produce a range of particles varying in size from large VLDL1, medium-sized VLDL2, to LDL, depending on hepatic TG availability.92 The rate of VLDL production is also influenced by metabolic factors, such as insulin resistance, and lipolysis and clearance of VLDL are markedly affected by apoC-III and angiopoietin-like 3 (ANGPTL3) content and lipase activities.91,94 The LDL subclass profile is principally determined by the nature of the secreted VLDL particles, their circulating concentrations, the activities of lipases and neutral lipid transfer proteins including CETP, tissue LDL receptor activity, and the affinity of LDL particles to bind to the receptor, which is, in turn, a function of the conformation of apoB100 within the particle.69,104,105 These factors are critical determinants of the amount and overall distribution of LDL particle subclasses, as well as their lipidomic profile and lipid load.64,69,70,74,75
Individuals with plasma TG in the range 0.85–1.7 mmol/L (75–150 mg/dL) release VLDL1 and VLDL2 from the liver,91,93 which are delipidated rapidly to IDL and then principally to LDL of medium size;64,66,99 thus, the LDL profile is dominated by LDL-II (Figure 2A). In contrast, people with low plasma TG levels (<0.85 mmoL/L or 75 mg/dL) have highly active lipolysis and generally low hepatic TG content. Consequently, hepatic VLDL tend to be smaller and indeed some IDL/LDL-sized particles are directly released from the liver.74–76 The LDL profile displays a higher proportion of larger LDL-I (_Figure 2B_) and is associated with a healthy state (as in young women). However, this pattern is also seen with familial hypercholesterolaemia (FH), in which LDL levels are high77,99 because of overproduction of small VLDL and reduced LDL clearance due to low receptor numbers.76 Finally, formation of small dense LDL is favoured when plasma TG levels exceed 1.7 mmol/L (150 mg/dL),79,80 and especially at levels >2.23 mmol/L (200 mg/dL) due to VLDL overproduction (as in insulin-resistant states, such as Type 2 diabetes and metabolic syndrome), and potentially when lipolysis is defective due to high apoC-III content [which inhibits lipoprotein lipase (LPL) action and possibly VLDL particle clearance].78,95 An LDL subfraction profile in which small particles predominate (Figure 2C) is part of an atherogenic dyslipidaemia in which remnant lipoproteins are also abundant. As particle size decreases and the conformation of apoB100 is altered, LDL receptor binding affinity is attenuated, resulting in a prolonged residence time in plasma (Box 2).64,78–80
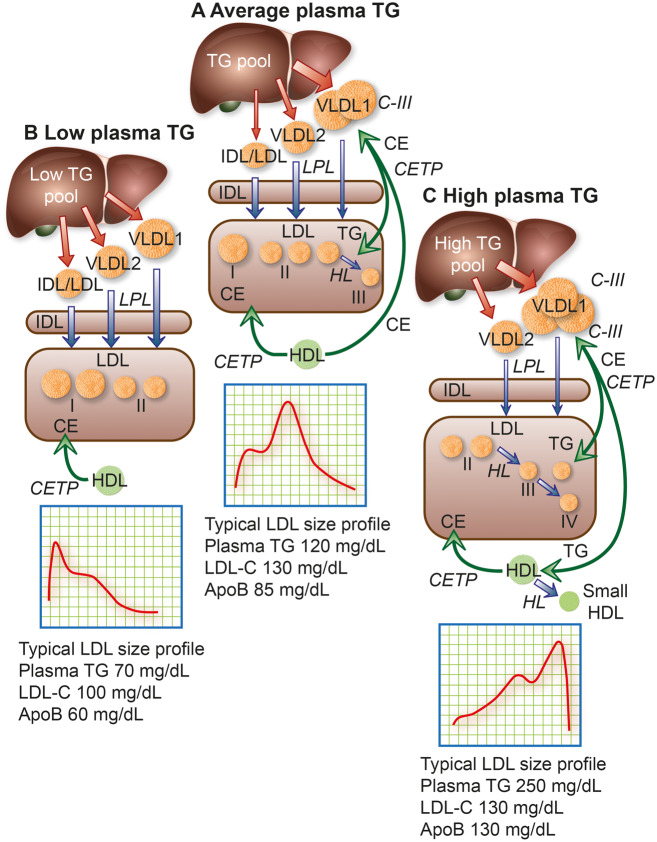
Figure 2.
Model of the metabolic interrelationships between low-density lipoprotein (LDL) subfractions and their hepatic precursors. The liver produces apolipoprotein (apo)B100-containing particles ranging in size from large triglyceride (TG)-rich very low-density lipoprotein (VLDL) 1, through small VLDL2 and intermediate-density lipoprotein (IDL) to LDL.74 The hepatic TG content (TG pool) affects the profile of the secreted particles.99 Secreted VLDL undergoes lipolysis and remodelling to form remnants/IDL; LDL is then formed via the actions of lipoprotein lipase (LPL), hepatic lipase (HL), and cholesteryl ester transfer protein (CETP). (A) In people with population average TG levels, about half the lipolytic remnants (which correspond to IDL based on density and size) in this pathway are cleared relatively efficiently and the remainder are converted mainly to LDL-II, which has higher LDL receptor affinity and shorter residence time than the LDL arising from VLDL1.74,79,82,83 The composition of IDL-derived LDL is modulated both by CETP-mediated transfer of cholesteryl esters (CE) from high-density lipoprotein (HDL) and by CETP-mediated transfer of TG from VLDL and their remnants.102,106 (B) In individuals with low plasma TG, LDL-I and -II predominate. Clearance of these lipoproteins is rapid and LDL-cholesterol (LDL-C) and apoB concentrations are low. (C) Individuals with elevated plasma TG levels overproduce VLDL1 and have reduced lipolysis rates due in part to inhibition of LPL activity by their abundant content of apoC-III, an LPL inhibitor. Very low-density lipoprotein 1 remodelling gives rise to remnants within the VLDL size range that are enriched in apoE; such circulating remnants can be removed by several mechanisms, primarily in the liver, including the LDL receptor-related protein, heparan sulfate proteoglycans, and LDL receptor.107–109 Hepatic clearance of VLDL1-derived remnant particles may, however, be slowed by enrichment with apoC-III.78 Very low-density lipoprotein 1 and VLDL2 are targeted by CETP, which exchanges core CE in LDL for TG in both VLDL1 and VLDL2. Hydrolysis of TG by HL action then shrinks LDL particles to preferentially form small, dense LDL-III in moderate hypertriglyceridaemia, or even smaller LDL-IV in severe hypertriglyceridaemia; such small dense LDL exhibit attenuated binding affinity for the LDL receptor, resulting in prolonged plasma residence (Box 2). Together, this constellation of lipoprotein changes, originating in increased levels of large VLDL1 and small dense LDL, represents a lipid phenotype designated atherogenic dyslipidaemia,6–8,74,75,79–81,110 a key feature of metabolic syndrome and Type 2 diabetes.6–8,78–80 Typical LDL subfraction patterns are indicated together with relevant plasma lipid and apoB levels. Note that when small dense LDL is abundant, apoB is elevated more than LDL-C. The width of the red arrows reflects the quantity of apoB/particle production and release from the liver, while the width of the blue arrows depicts relative lipolytic efficiency.
Box 2.
The distinct biological features of small dense low-density lipoprotein
- Prolonged plasma residence time reflecting low LDL receptor binding affinity.
- Increased affinity for LDL receptor-independent cell surface binding sites.
- Small particle size favouring enhanced arterial wall penetration.
- Elevated binding affinity for arterial wall proteoglycans favouring enhanced arterial retention.
- Elevated susceptibility of PL and CE components to oxidative modification, with formation of lipid hydroperoxides.
- Elevated susceptibility to glycation.
- Enrichment in electronegative LDL.
- Preferential enrichment in lipoprotein-associated phospholipase A2.
- Preferential enrichment in apoC-III.
References: 54,55,64,66,69–75,78,79,81–85,105,111–113
apo, apolipoprotein; CE, cholesteryl ester; PL, phospholipid.
Low-density lipoprotein as the primary driver of atherogenesis
All LDL particles exert atherogenicity to variable degrees, which can be influenced by the proteome, lipidome, proteoglycan binding, aggregability, and oxidative susceptibility.64,96,97 The atherogenic actions of LDL in arterial tissue have multiple origins. Broadly, these encompass:
- Formation of macrophage-derived foam cells upon phagocytic uptake of aggregated LDL particles, or LDL in which lipid and/or protein components have undergone covalent modification, triggering uptake by scavenger receptors. Aggregation may occur by non-enzymatic or enzymatically induced mechanisms. Oxidation of lipids (phospholipids, cholesteryl esters, and cholesterol) or apoB100 can occur enzymatically (e.g. by myeloperoxidase) or non-enzymatically (e.g. by reactive oxygen species liberated by activated endothelial cells or macrophages).
- Release of bioactive proinflammatory lipids (e.g. oxidized phospholipids) or their fragments (e.g. short-chain aldehydes) subsequent to oxidation, which may exert both local and systemic actions.
- Formation of extracellular lipid deposits, notably cholesterol crystals, upon particle denaturation.
- Induction of an innate immune response, involving damage-associated molecular patterns (DAMPs, notably oxidation-specific epitopes and cholesterol crystals). Damage-associated molecular patterns promote recruitment of immuno-inflammatory cells (monocyte-macrophages, neutrophils, lymphocytes, and dendritic cells) leading to local and potentially chronic inflammation that can induce cell death by apoptosis or necrosis, thereby contributing to necrotic core formation.
- Induction of an adaptive immune response subsequent to covalent modification of apoB100 by aldehydes or apoB100 degradation with the activation of antigen-specific T-cell responses and anti-bodies.114–118
Beyond LDL, additional apoB-containing lipoproteins (<70 nm diameter) can exacerbate the atherogenic process; these include Lp(a) (which is composed of apo(a) covalently linked to the apoB of LDL and is a major carrier of proinflammatory oxidized phospholipids) and cholesterol-enriched remnant particles metabolically derived from TGRL.6,7,11,13,26,119 Whereas the classic TG-poor LDL requires modification for efficient uptake by arterial macrophages, remnant particles are taken up by members of the LDL receptor family in their native state.107,120 There is also evidence that LPL-mediated hydrolysis of TG from incoming remnant particles enhances the inflammatory response of arterial macrophages, 121,122 and that the internalization of remnants induces lysosomal engorgement and altered trafficking of lipoprotein cholesterol within the cell, 123 thus inducing endoplasmic reticulum stress and activation of apoptosis disproportionate to the cholesterol cargo delivered.
Low-density lipoprotein subfraction profile affects atherogenicity
Under defined cardiometabolic conditions, a specific LDL subclass may become more prominent as the driver of atherogenesis. Several biological properties of small dense LDL could confer heightened coronary heart disease (CHD) risk (Box 2). Certainly, small dense LDL appears to enter the arterial intima faster than larger LDL.111 However, the significant metabolic inter-relationships of small dense LDL with abnormalities of other atherogenic apoB-containing lipoproteins, particularly increased concentrations of VLDL and remnant lipoproteins, have created challenges in assessing the independent contributions of small dense LDL to CHD.81 Nevertheless, in several recent large prospective cohort studies,98,124,125 and the placebo group of a large statin trial,126 concentrations of small dense LDL but not large LDL predicted incident CHD independent of LDL-C. The heterogenous proteomic and lipidomic profiles of LDL particles may also affect their pathophysiologic activity. For example, small dense LDL is preferentially enriched in apoC-III and glycated apoB relative to larger LDL.85,112 Additionally, the small dense LDL subclass includes an electronegative LDL species associated with endothelial dysfunction.113 Moreover, the unsaturated cholesteryl esters in the lipidome of small dense LDL are markedly susceptible to hydroperoxide formation under oxidative stress.73
Low-density lipoprotein particle profiles may also reflect specific genetic influences on LDL metabolism that concomitantly influence CHD risk.98 A notable example is a common non-coding DNA variant at a locus on chromosome 1p13 that regulates hepatic expression of sortilin, as well as other proteins, 127 and is strongly associated with both LDL-C levels and incident myocardial infarction.128 The major risk allele at this locus is preferentially associated with increased levels of small dense LDL, 127 but the mechanistic basis for this association is unknown.
The residence time of LDL in the circulation may be the critical factor in the relationship between plasma LDL subclass level and atherosclerosis risk, as it determines both exposure of arterial tissue to LDL particles and the potential of LDL to undergo proatherogenic intravascular modifications, such as oxidation. Increased plasma residence time can result from deficiency or dysfunction of LDL receptors, as in FH, or from structural or compositional features of LDL particles that impair their binding affinity for LDL receptors, as for small dense LDL.82,83 Indeed, there is evidence of a lower fractional catabolic rate and longer plasma residence time for small dense LDL than for larger LDL in combined hyperlipidaemia.84
Responses elicited by low-density lipoprotein retained in the artery wall
Retention and subsequent accumulation of LDL in the artery wall triggers a number of events that initiate and propagate lesion development.21,50 Due to the local microenvironment of the subendothelial matrix, LDL particles are susceptible to oxidation by both enzymatic and non-enzymatic mechanisms, which leads to the generation of oxidized LDL (oxLDL) containing several bioactive molecules including oxidized phospholipids.129,130 Oxidized LDL, in turn, initiates a sterile inflammatory response by activating endothelial cells to up-regulate adhesion molecules and chemokines that trigger the recruitment of monocytes—typically inflammatory Ly6Chi monocytes—into the artery wall.131 The importance of oxidized phospholipids in the inflammatory response of the vascular wall has been demonstrated through the transgenic expression of an oxidized phospholipid-neutralizing single-chain antibody, which protected atherosclerosis-prone mice against lesion formation.132 Newly recruited monocytes differentiate into macrophages that can further promote the oxidation of LDL particles, which are then recognized and internalized by specific scavenger receptors giving rise to cholesterol-laden foam cells.133 Several other modifications of retained LDL, including enzymatic degradation or aggregation, have also been shown to promote its uptake by macrophages. Macropinocytosis of native LDL may also contribute to this process.134,135
Macrophages exhibiting different phenotypes, ranging from classical inflammatory subtypes to alternatively activated anti-inflammatory macrophages, have been identified in atherosclerotic lesions.136,137 Macrophage polarization appears to depend on the microenvironment, where different pro- and anti-inflammatory inducers are present together with complex lipids, senescent cells, and hypoxia.137 Thus, macrophage behaviour is a dynamic process adapting to the microenvironment, thereby allowing macrophage subsets to participate in almost every stage of atherosclerosis.138
Several DAMPs, generated by modification of retained LDL, induce the expression of pro-inflammatory and pro-thrombotic genes in macrophages by engaging pattern recognition receptors, such as toll-like receptors (TLRs). In particular, recognition of oxLDL by a combination of TLR4-TLR6 and the scavenger receptor CD36 triggers NFκB-dependent expression of chemokines, such as CXCL1, resulting in further recruitment of monocytes.139 Such leucocyte recruitment is tightly controlled in a stage-specific manner by a diverse range of chemokines and their receptors.140 At later stages of plaque development, the pool of intimal macrophages is largely maintained by self-renewal, which increases the burden of foam cells in the plaque. Moreover, SMCs may take up cholesterol-rich lipoproteins to become macrophage-like cells that contribute to the number of foam cells in advanced lesions.141
An important consequence of lipid loading of macrophages is the formation of cholesterol crystals, which activate an intracellular complex, the NLRP3 inflammasome, to promote local production of IL-1β and IL-18.142–144 The persistent presence of lipid-derived DAMPs in the artery wall, together with continuous expression of inflammatory cytokines and recruitment of phagocytes (whose role is to remove the triggers of inflammation), sustains this inflammatory response. It also facilitates an active cross-talk with several other arterial cells, including mast cells, which in turn become activated and contribute to plaque progression by releasing specific mediators.145
The recruitment of myeloid cells is also accompanied by the infiltration of both CD4+ and CD8+ T cells that display signs of activation and may interact with other vascular cells presenting molecules for antigen presentation, such as major histocompatibility complex II.146 Analyses of the T-cell receptor repertoire of plaque-infiltrating T cells demonstrated an oligoclonal origin of these T cells and suggest expansion of antigen-specific clones. Indeed, T cells with specificity for apoB-derived epitopes have been identified, linking adaptive immune responses to the vascular retention of LDL (Figure 3).147
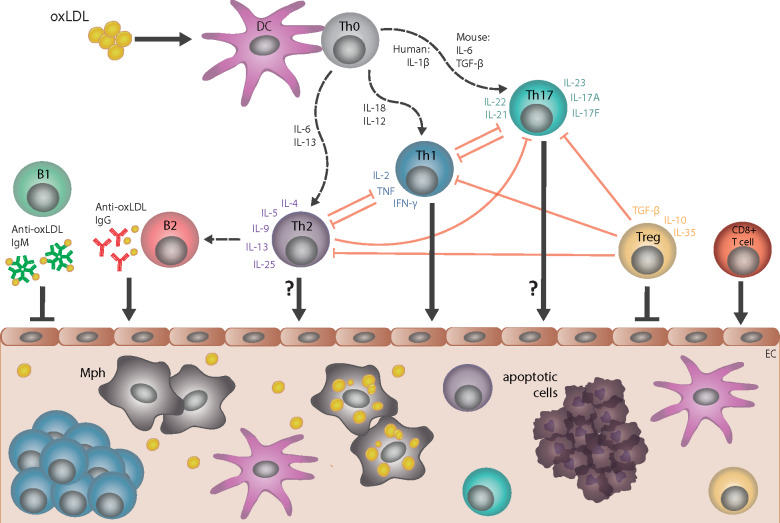
Figure 3.
Cellular and humoral immune responses in atherosclerosis. Dendritic cells (DC) take up several forms of modified low-density lipoprotein (LDL), including oxidized LDL (oxLDL), and present specific epitopes (e.g. apolipoprotein B peptides) to naive T cells (Th0), which induces differentiation into CD4+ T helper 1 (Th1), T helper 2 (Th2), T helper 17 (Th17), or T regulatory (T reg) cell subtypes; multiple cytokines control such differentiation. CD4+ T-cell subtypes, together with specific cytokines that they secrete, provide help to B cells and regulate the activity of other T-cell subtypes. The pro-atherogenic role of interferon gamma (IFN-γ)-secreting Th1 cells and the anti-atherogenic effect of interleukin-10/transforming growth factor beta (IL-10/TGF-β)-secreting T regulatory cells are well established. However, the role of Th2 and Th17 in atherogenesis is less clear, as opposing effects of cytokines associated with these respective subtypes have been described. Cytotoxic CD8+ T cells can promote atherogenesis. Anti-oxLDL immunoglobulin (Ig)M antibodies produced by B1 cells are atheroprotective, whereas anti-oxLDL IgG antibodies produced by B2-cell subsets are likely pro-atherogenic. All of these cell types may infiltrate the arterial wall at sites of ongoing plaque development, with the possible exception of Th2 and Th17 cell types. EC, endothelial cell; Mph, monocyte-derived macrophage.
Interferon-gamma (IFNγ)-secreting CD4+ Th1 cells promote atherogenesis, but this response is dampened by T regulatory cells expressing transforming growth factor beta (TGF-β) and IL-10.148 The role of CD4+ Th2 and Th17 cells is less clear, but CD8+ cytotoxic T cells also seem to promote atherogenesis.149 Distinct roles for different B-cell subsets have been reported, and although only small numbers of B cells are found in atherosclerotic lesions, both immunoglobulin (Ig)G and IgM antibodies derived from such cells accumulate.150,151 Many of these antibodies have specificity for oxLDL and, in an isotype-dependent manner, trigger activation of complement, further modulating the inflammatory response.152
Thus, retention and subsequent modification of LDL elicits both innate and adaptive cellular and humoral immune responses that drive inflammation in the artery wall. Disrupting this vicious cycle by targeting inducers and mediators may provide alternative approaches to halting atherogenesis at specific stages (Box 3). Proof of concept for this therapeutic strategy has been provided in a secondary prevention trial in which patients were treated with a statin in combination with the anti-IL-1β antibody canakinumab.154
Box 3.
Cell-specific responses to retained and modified low-density lipoprotein
- Oxidized LDL initiates a sterile inflammatory response by activating endothelial cells to up-regulate adhesion molecules and chemokines, triggering the recruitment of monocytes that differentiate into macrophages.
- Modifications of retained LDL promote its uptake by macrophages leading to cholesterol-laden foam cells.
- Smooth muscle cells also take up cholesterol-rich lipoproteins and significantly contribute to the number of foam cells in advanced lesions.
- Lesional macrophages contain subsets with different phenotypes, ranging from classical inflammatory subtypes to alternatively activated anti-inflammatory macrophages.
- DAMPs, formed when retained LDL become modified, induce the expression of pro-inflammatory and pro-thrombotic genes in macrophages by engaging PRRs, such as TLRs.
- Lipid loading of macrophages may lead to formation of cholesterol crystals, which activate the NLRP3 inflammasome, leading to production of IL-1β and IL-18.
- T cells and B cells are found in atherosclerotic lesions. The B cells have specificity for oxidized LDL, which also triggers the activation of complement, further modulating the inflammatory response.
References: 129,130,132,133,136–143,145–148,150–153
DAMPs, damage-associated molecular patterns; IL, interleukin; PRRs, pattern recognition receptors; TLRs, toll-like receptors.
Defective cellular efferocytosis and impaired resolution of inflammation
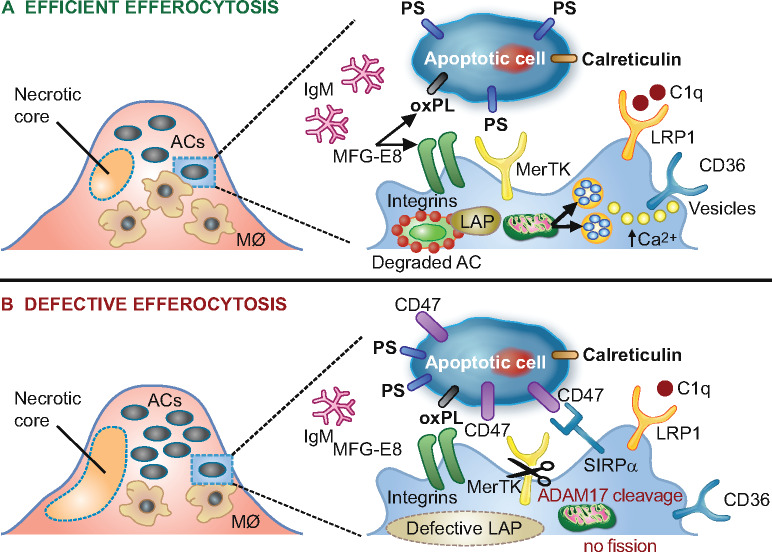
The efficient clearance of dying cells by phagocytes, termed efferocytosis, is an important homeostatic process that ensures resolution of inflammatory responses (Figure 4).155,156 This involves recognition of several ‘eat-me’ signals, such as phosphatidylserine exposure on apoptotic cells, by their respective receptors on macrophages, as well as bridging molecules that mediate binding. Moreover, ‘don’t-eat-me’ signals, such as CD47, also play a critical role and influence atherogenesis.157 Uptake of apoptotic cells is associated with increased expression of the anti-inflammatory cytokines TGF-β and IL-10 and decreased expression of pro-inflammatory IL-8 and IL-1β by macrophages.158 Efficient efferocytosis thereby protects against atherogenesis by removing cellular debris and creating an anti-inflammatory milieu. Uptake of cellular debris also favours the production of various specialized pro-resolving lipid mediators, such as lipoxins, resolvins, and maresins that are actively involved in resolving inflammation.159
Figure 4.
Schematic representation of processes involved in lesional efferocytosis. (A) Externalized ‘eat me’ signals including phosphatidylserine (PS), calreticulin, and oxidized phospholipids (oxPL) are recognized by their respective receptors, Mer tyrosine kinase (MerTK), low-density lipoprotein-receptor-related protein 1 (LRP1), as well as integrin αvβ3 and CD36 on macrophages; such recognition is facilitated either directly or mediated by bridging molecules such as growth arrest-specific 6 for PS, complement protein C1q for calreticulin and milk fat globule-epidermal growth factor 8 (MFG-E8) for oxPL. Calcium-dependent vesicular trafficking events driven by mitochondrial fission and LC3-associated phagocytosis (LAP) promote phagolysosomal fusion and the hydrolytic degradation of apoptotic cells. Simultaneously, natural immunoglobulin (Ig)M antibodies with reactivity towards oxidation-specific epitopes further enhance the efficient clearance of dying cells via complement receptors. (B) In advanced atherosclerosis, one or more of these mechanisms are dysfunctional and can lead to defective efferocytosis, propagating non-resolving inflammation and plaque necrosis. Additional processes contributing to impaired efferocytosis include ADAM-17-mediated cleavage of MerTK as well as the inappropriate expression of the ‘don’t eat me’ signal CD47 on apoptotic cell surfaces. ACs, apoptotic cells.
In chronic inflammation, the general pro-inflammatory environment alters the expression of molecules that regulate efferocytosis, so that oxLDL particles in atherosclerotic lesions compete for uptake by macrophages.129,160 As a result, efferocytosis becomes defective and resolution of inflammation, which is mainly driven by modified LDL, is impaired. Under such conditions, apoptotic cells accumulate and undergo secondary necrosis, promoting the release of several DAMPs that further propagate inflammation. Impaired clearance of apoptotic cells results in the formation of necrotic cores that contribute to unstable plaques and plaque rupture (Box 4). Thus, defective efferocytosis may be a potential therapeutic target to promote resolution of inflammation in atherosclerosis.
Box 4.
Efficient vs. impaired efferocytosis
- Efficient efferocytosis removes cellular debris and modified forms of low-density lipoprotein, and creates an anti-inflammatory milieu.
- Impaired efferocytosis in atherosclerosis results in non-resolving inflammation.
- Impaired clearance of apoptotic cells contributes to formation of necrotic core in atherosclerotic lesions
- Genetically modified mice with enhanced/restored efferocytosis protects from atherosclerosis, indicating novel therapeutic strategies.
How does plaque composition and architecture relate to plaque stability?
Our knowledge of the intricate relationships between plaque stability and the cellular and non-cellular components of plaque tissue, together with their spatial organization, is incomplete. Local SMCs respond to insults exerted by progressive oxLDL accumulation170 by proliferating and ultimately changing their phenotype to fibroblast- and ostechondrogenic-like cells;171 the latter produce extracellular matrix, regulate calcification and contribute (through SMC death) to necrotic core formation. This ‘healing’ response is the major source of key components of advanced plaques but is highly heterogenous. Furthermore, the determinants of this response are diverse, and its interaction with LDL-driven inflammation is poorly understood. Depending on the pathways that predominate in development of a lesion, segments of an atherosclerotic artery may remain quiescent, exhibit chronic stenosis, or precipitate an acute, life-threatening thrombus.
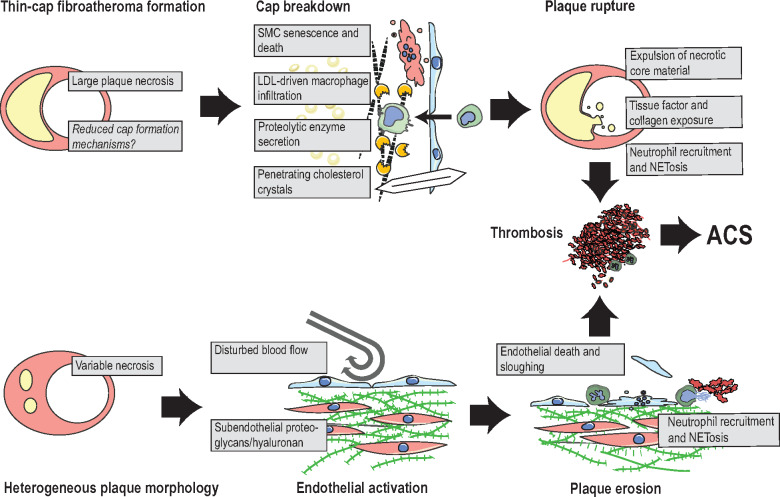
Lesions that develop substantial lipid cores, which almost reach the luminal surface, are at risk of rupturing with subsequent thrombus formation (Figure 5). In this event, the thin cap of fibrous tissue between the lipid core and blood is torn, allowing blood to enter and often core material to leak out. Cholesterol crystals, which can be seen protruding through the plaque surface around sites of rupture, may contribute to final disintegration of the residual cap tissue.172 Ruptured lesions are also typically large with intraplaque angiogenesis and often have little previous stenosis due to extensive expansive remodelling (Box 5).
Figure 5.
Proposed mechanisms of plaque rupture and plaque erosion. Rupture: lesions that develop extensive necrosis and only sparse fibrous cap tissue are at risk of plaque rupture. Suggested final processes that precipitate rupture include senescence and death of residual cap smooth muscle cells (SMC), degradation of the fibrous matrix by macrophage-secreted proteolytic enzymes, and cholesterol crystals, which may penetrate cap tissue. These processes expose the prothrombotic plaque interior and result in neutrophil-accelerated thrombosis. Erosion: lesions that are complicated by erosion typically display variable amounts of plaque necrosis, but are frequently characterized by subendothelial accumulation of proteoglycans and hyaluronan. Current hypotheses suggest that the combination of disturbed blood flow and endothelial activation by immune activators, e.g. hyaluronan fragments, leads to neutrophil recruitment with neutrophil extracellular trapsosis, endothelial cell apoptosis/sloughing, and thrombus formation. ACS, acute coronary syndrome; NETosis, cell death by neutrophil extracellular traps.
Box 5.
Plaque rupture and erosion
- Plaques developing substantial necrosis that reach the luminal surface can rupture and precipitate thrombus.
- Ruptured plaques are often large, non-stenotic, and vascularized lesions with protruding cholesterol crystals, but the causal role of these features is unresolved.
- Thrombus can form on other types of plaques by plaque erosion. The process is less well-understood but may involve combinations of flow disturbance, vasospasm, and neutrophil-generated endothelial shedding.
- Plaque progression and rupture are influenced by both biological and mechanical factors, highlighting plaque composition as a major factor in resistance to mechanical stress.
- Lowering of low-density lipoprotein levels appears more effective in reducing the risk for plaque rupture than for plaque erosion.
Plaque rupture accounts for the majority of coronary thrombi at autopsy (73%), 173 and in survivors of ST-elevation myocardial infarction (STEMI) examined by optical coherence tomography (∼70%), 174,175 but is less common (∼43–56%) in culprit lesions of non-ST segment elevation myocardial infarction (NSTEMI).175,176 Lesions without lipid cores or with thick fibrous caps are not at risk of rupture but may produce a thrombus in response to plaque erosion. In these cases, the plaque is intact but lacks endothelial cells, and neutrophils predominate at the plaque-thrombus interface. The underlying lesion is frequently, but not always, rich in the glycosaminoglycan hyaluronan and SMCs.173 The mechanism leading to intravascular thrombosis is not yet clear, but experiments with mouse arteries have shown that subendothelial hyaluronan and disturbed blood flow render the endothelium vulnerable to neutrophil-mediated denudation and thrombosis.177 Vasospasm has also been proposed as the initiating event in plaque erosion.178
Rupture requires a specific plaque morphology (thin-cap fibro-atheroma) and is a strong prothrombotic stimulus, whereas erosion complicates earlier lesion types and provides a subtler thrombogenic stimulus. Plaque progression and potentially plaque rupture are influenced by the complex interaction between biological and mechanical factors, indicating that plaque composition is a major factor in its resistance to mechanical stress.179 Erosion favours a higher fraction of thrombi in younger, especially female, patients and in patients with less severe atherosclerosis with few thin-cap fibroatheromas,173,174 and more frequently affects lesions exposed to local (disturbed blood flow near bifurcations) or systemic (smoking) prothrombotic factors.56
Low-density lipoprotein-lowering therapies mitigate key mechanisms of plaque rupture, i.e. lipid core formation and LDL-driven inflammation and degeneration of caps. Statin therapy lowers the rate of events but also shifts the presentation of acute coronary syndromes from STEMI towards NSTEMI, indicating that LDL lowering is less efficient in counteracting erosion mechanisms.176,180 Successful implementation of LDL lowering in patients with established plaques may, therefore, leave a residual burden of thrombosis caused by plaque erosion, thus emphasizing the need for alternative types of prevention and therapy.
Fibrous cap matrix components: guardians of cardiovascular peace?
Lesions that rupture form predominantly in arterial regions with thick pre-existing arterial intima. When the lipid core develops in the deep part of the intima at these sites, it is initially separated from the lumen by normal intima but is gradually replaced by a more compact layer of SMCs and collagen-rich matrix that spreads underneath the endothelium.181 This structure, called the fibrous cap in areas where it overlies lipid core, prevents rupture as long as it is not excessively thin: 95% of ruptured plaques have cap thickness <65 μm (by definition thin-cap fibroatheroma).182 It is uncertain to what extent such thin caps result from degradation of an initially thick cap or from failure to form thick-cap tissue in the first place. From a therapeutic viewpoint, the relationship of LDL-C levels to fibrous cap thickness is of relevance.183 Thus, frequency-driven optical coherence tomography imaging of coronary arteries selected for percutaneous intervention in statin-treated patients with CHD revealed that those with LDL-C levels <1.3 mmol/L (50 mg/dL) were more likely to have fibrous plaque and thick fibrous caps (51.7% and 139.9 μm, respectively).183
Lineage tracking of SMCs showed that fibrous caps in mice form by massive clonal expansion of a few pre-existing SMCs.184,185 These findings are consistent with earlier studies of X chromosome inactivation patterns in human lesions, which indicated the existence of similar large clonal populations in SMC-rich lesion areas.186 If substantial SMC clonal expansion does indeed occur during human cap formation, this may contribute to the replicative senescence and limited repair potential that characterize cap SMCs.187
Several processes leading to cap degradation have been described. Cap collagen and elastin fibres are long-lived with little spontaneous turnover, but invading macrophages, recruited as a result of LDL-driven plaque inflammation, secrete matrix metalloproteinases and cathepsins that break down the matrix.188 Together with SMC and macrophage death, such proteolysis progressively converts cap tissue into lipid core and predisposes it to rupture (Box 6).
Box 6.
Fibrous cap
- The fibrous cap, between the necrotic core and the lumen, protects against rupture.
- Processes integral to both tissue degeneration and reduced cap formation may be involved in the genesis of thin-cap fibroatheromas.
- Caps form by oligoclonal expansion of smooth muscle cells in experimental models, and there is suggestive evidence for the same process in humans.
- Degradation of cap tissue involves inflammatory cell invasion with secretion of proteolytic enzymes. Mechanical effects of local cholesterol crystals may also contribute.
References: 181–191
How does calcification impact plaque architecture and stability?
Arterial calcification is an established marker of atherosclerotic disease,192,193 and the severity of coronary artery calcification is a strong predictor of cardiovascular morbidity and mortality.194,195 Yet whether coronary artery calcium (CAC) is simply a marker of advanced disease, or whether it increases risk of plaque rupture, is unclear.
Clinical, animal, and in vitro studies implicate hyperlipidaemia-induced inflammation in the genesis and progression of arterial calcification.196–201 Although statins were expected to prevent and/or reverse vascular calcification, clinical studies showed that, despite benefit on mortality,202 treatment increased progression of coronary artery calcification.203–206 Moreover, elite male endurance athletes have higher CAC scores than less physically active individuals, but experience fewer cardiovascular events.207–209
This paradox raises the question of whether calcified plaque architecture influences rupture vulnerability, either positively or negatively. Understanding in this area, however, remains limited. By using finite element analysis, rigid deposits (calcification) embedded in a distensible material (vessel wall) under tension are shown to create focal stress that is concentrated at areas of compliance mismatch at the surfaces of the deposits, 210 rendering them prone to debonding or rupture. The mineral surfaces found in carotid arteries and those in skeletal bone are remarkably similar and characterized by abundant proteoglycans.211 The chemical nature and architecture of that surface bonding may be critical in determining whether calcium deposits promote plaque rupture or stability.
Clinical studies provide varying results with respect to the association of calcification with plaque rupture. Histological analysis showed that patients who died of acute myocardial infarction had more CAC than controls, but the CAC did not colocalize closely with the unstable plaque.212 Computed tomographic (CT) analyses of patients with acute coronary syndrome, however, showed that the culprit lesions tended to have dispersed or ‘spotty’ calcification (∼0.2–3 mm), whereas stable lesions tended to have contiguous calcium deposits (≥3 mm).213 Based on this and other findings, 214,215 the presence of a spotty pattern of calcium deposits is now considered a feature of a ‘high-risk’ plaque.
A new imaging modality using positron emission tomography (PET)216 detects smaller calcium deposits that are below the resolution of CT (∼200–500 μm)217 and intravascular ultrasound (∼200 μm lateral resolution). In human and animal studies, 18F-NaF PET-CT imaging, which has higher sensitivity for calcium mineral, 218 identified high-risk, vulnerable lesions.218–221
Taken together, these findings suggest that calcification is not a clear marker; mineral features may vary in quality and microarchitecture, which may affect the mechanical properties of plaque tissue.222 For example, certain therapies, such as anabolic parathyroid hormone analogues used to treat osteoporosis, may modify the architecture of calcium deposits and impact calcified plaque vulnerability.219 Research is needed to establish the mechanism linking calcium morphology and plaque vulnerability; the use of 18F-NaF PET scanning offers promise.223 Given the evidence that statins and high-intensity exercise promote calcification without increased risk, these interventions may stabilize mineral morphology. Further studies are needed to better understand these mechanisms in modulating the effects of calcification on plaque vulnerability (Box 7).
Box 7.
Calcification and plaque stability
- Oxidized low-density lipoprotein stimulates vascular calcification by driving osteoblastic differentiation of vascular smooth muscle cells.
- High-density lipoprotein exerts beneficial effects on vascular calcification through effects on bone preosteoclasts.
- The severity of coronary artery calcification is a strong predictor of cardiovascular morbidity and mortality.
- It is still unclear whether coronary artery calcium is simply a marker of advanced disease or whether it increases risk of plaque rupture.
- Clinical studies provide varying results with respect to association of calcification with plaque rupture.
- Statins and high-intensity exercise promote calcification without increasing risk.
References: 192–223
Although the role of LDL in coronary artery calcification remains unclear, 224 it is well-established that an elevated LDL-C level is a strong risk factor for progression of calcification.225 Interestingly, modified LDL stimulates vascular calcification by driving osteoblastic differentiation of vascular SMCs,197 while inhibiting osteoclast differentiation of macrophages.224 In contrast, HDL appears to exert beneficial effects on vascular calcification, as HDL-mediated efflux of cholesterol from bone preosteoclasts inhibits both their maturation and osteoblast RANKL expression, and stimulates their apoptosis.226
In addition, several clinical trials have demonstrated that Lp(a) is an independent risk factor for coronary artery calcification.227 Ongoing research suggests a causal role for Lp(a) in arterial calcification; although the underlying mechanisms remain unclear, oxidized phospholipids in Lp(a) may induce differentiation of valve interstitial cells into a procalcification, osteoblast-like phenotype.228 Ongoing trials with Lp(a)-lowering therapies will provide insight into the potential role of Lp(a) in coronary artery calcification.
Can genes influence the susceptibility of the artery wall to coronary disease?
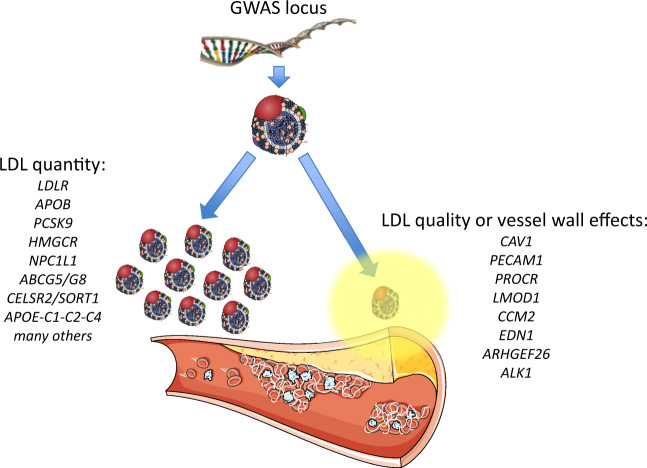
Genome-wide association studies and related research indicate that predisposition to ASCVD is associated with multiple variants in genes that affect plasma LDL concentration (Figure 6).229,230 Indeed, genomic risk scores that predict coronary artery disease (CAD) risk contain a large number of variants that affect LDL particle quantity and LDL-C levels.231 Most GWAS loci governing LDL-C levels and CAD risk occur in noncoding regions and predominantly alter gene expression that affects uptake and metabolism of LDL in the hepatic cell. Other genomic loci affect qualitative attributes of LDL (Figure 6) including arterial wall susceptibility to LDL infiltration, transcytosis, retention, and modification (Box 8).229
Figure 6.
Genomic loci associated with atherosclerosis. Loci identified by genome-wide association studies (GWAS) can have different effects on low-density lipoprotein (LDL). On the left are shown selected GWAS loci associated with LDL-cholesterol (LDL-C) levels, several of which are associated with atherosclerosis events and are incorporated in predictive risk scores. Many have also been independently validated in Mendelian randomization studies and in studies of rare families. Some are proven drug targets to reduce clinical events. On the right are shown loci that do not primarily affect LDL-C levels, but may instead underlie qualitative changes in either the particle itself or in the vessel wall to locally promote atherogenesis.
Box 8.
New concepts in genetic determinants of arterial wall biology and susceptibility to atherosclerotic cardiovascular disease
- Genome-wide association studies (GWAS) reveal causal associations of coronary artery disease with loci for several genes regulating arterial wall susceptibility to infiltration, transcytosis, retention and modification of low-density lipoprotein (LDL).
- The interconnectedness of gene-regulatory networks means that virtually any expressed gene can modulate the function of a ‘core’ disease-related gene.
- Atherosclerosis heritability will ultimately be explained in large part by genes acting outside core mechanistic pathways, as exemplified by non-canonical, LDL-associated genes.
- ‘Omnigenic’ models of disease are being vigorously explored in large-scale GWAS.
A few early GWAS hits for lipid levels and CAD have mechanistic links to LDL transcytosis across the endothelium, including SRB1 encoding SR-B1 and LDLR encoding the LDL receptor.32,232,233 Low-density lipoprotein transcytosis requires caveolin 1,32 encoded by CAV1, in which the single-nucleotide polymorphism (SNP) rs3807989 is associated with increased CAV1 expression from leucocytes, altered plasma LDL-C levels and increased CAD risk.234
More recent GWAS and sequencing efforts further support a causal role for such qualitative local pathways. For instance, a GWAS of 88 192 CAD cases and 162 544 controls found 25 new SNP-CAD associations from 15 genomic regions, including rs1867624 at PECAM1 (encoding platelet and endothelial cell adhesion molecule 1), rs867186 at PROCR (encoding protein C receptor), and rs2820315 at LMOD1 (encoding SMC-expressed leiomodin 1).235 Another GWAS of 34 541 CAD cases and 261 984 controls from the UK Biobank, with replication in 88 192 cases and 162 544 controls, identified 64 novel CAD risk loci, including several loci implicated by network analysis in arterial wall biology, such as CCM2 encoding cerebral cavernous malformation scaffolding protein and EDN1 encoding endothelin 1.236
Next-generation DNA sequencing of 4831 CAD cases and 115 455 controls identified 15 new CAD loci, which included rs12483885, a common p.Val29Leu polymorphism in ARHGEF26 encoding Rho guanine nucleotide exchange factor 26.237 The ARHGEF26 Leu29 isoform had an allele frequency of 0.85 and increased CAD risk by ∼8%, 237 a finding that was confirmed by an independent GWAS in the UK Biobank.238 ARHGEF26 activates Rho guanosine triphosphatase, thereby enhancing formation of endothelial docking structures, and in turn, promoting transendothelial migration of leucocytes.239–241In vitro studies showed the high-risk Leu29 isoform to be degradation-resistant and associated with increased leucocyte transendothelial migration compared with the low-risk Val29 isoform.237_ApoE-_null mice crossed with _Arhgef26_-null mice displayed reduced aortic atherosclerosis without any change in lipid levels,240 supporting a modulatory role for ARHGEF26 in atherogenesis.
Other studies indicate a role for genes governing transcytosis of LDL in CAD. For instance, genome-wide RNA interference screening supplemented by pathway analysis and GWAS data cross-referencing identified ALK1 as a key mediator of LDL uptake into endothelial cells. By directly binding LDL, ALK1 diverts LDL from lysosomal degradation via a unique endocytic pathway and promotes LDL transcytosis.38 Endothelium-specific ablation of Alk1 in _Ldlr_-null mice reduced LDL uptake into cells.38 In studies of highly expressed genes in human carotid endarterectomy samples, lipid metabolism pathways, driven by genes such as ApoE, coincided with known CAD risk-associated SNPs from GWAS.242 Consistent with this mechanism, macrophage-specific re-introduction of apoE in hyperlipidaemic _ApoE_-null mice ameliorated lipid lesion formation independent of LDL levels, indicating a local apoE-related mechanism in the arterial wall.243
Finally, as meta-analyses of GWAS incorporate ever-larger patient cohorts, gene-regulatory networks are recognized as being highly interconnected. For instance, a meta-analysis of GWAS results showed that common CAD-associated variants near COL4A2 encoding collagen type 4 alpha chain, and ITGA1 encoding integrin alpha 9, both of which are important in cell adhesion and matrix biology, were also significant determinants of plasma LDL-C levels.63 For complex traits, such as LDL-C, arterial wall susceptibility, and CAD risk, Boyle et al.244 proposed that gene-regulatory networks are sufficiently interconnected that any gene expressed in disease-relevant cells can modulate the function of core disease-related genes and that most heritability is explained by genes that act outside core mechanistic pathways. This ‘omnigenic model’ of disease is under active investigation in current large-scale genetic studies.
Which plaque components favour a thrombotic reaction upon rupture?
Fibrous cap rupture is defined as a structural defect in the fibrous cap that separates the lipid-rich necrotic core of a plaque from the lumen of the artery.245 The key features of a vulnerable plaque are a thin fibrous cap, a large necrotic core, pronounced inflammation, and low vascular SMC density.246 Both biomechanical and haemodynamic factors contribute to plaque rupture,247 and the exposure of the blood to plaque components initiates the coagulation cascade, promoting thrombus formation at the site of rupture.248 The question is: which plaque components favour this thrombotic reaction?
The initial trigger of thrombus formation is the exposure of tissue factor (TF) in the cell membrane of plaque macrophages and/or lipid-laden vascular SMCs to blood components. Uptake of exogenous non-lipoprotein cholesterol and oxLDL by human monocyte-macrophages and foam cells markedly up-regulates TF synthesis and release of TF+ microvesicles,249,250 with a strong correlation between intracellular cholesterol content and TF production.251,252 Such exogenous cholesterol may be derived from intimally retained atherogenic lipoproteins subsequent to their degradation by macrophage- and SMC-derived foam cells. TF expression may also be induced in endothelial cells by remnant lipoproteins.253 Exposure of the extracellular domain of TF to flowing blood initiates the coagulation cascade,254 and leads to thrombin formation; thrombin then cleaves fibrinogen to fibrin, with ensuing formation of a fibrin monolayer covering the surface of the exposed damaged plaque surface. Thrombosis evolves with a predominance of platelets that are rapidly activated and recruited from the blood to the growing thrombus. In addition, hypercholesterolaemia and oxidized lipids can promote procoagulant activity and propagate the coagulation cascade that is initiated by TF-VIII.249 Moreover, it is established that FH is associated with increased platelet activation and an underlying pro-coagulant state.255 Both native and oxidized forms of LDL may prime platelets and increase platelet activation in response to various agonists, thereby contributing to increased risk of atherothrombosis.256,257 Plasma levels of platelet activation markers (such as thrombin-antithrombin complex, soluble P-selectin, and soluble CD40L) or P-selectin exposure at the surface of platelets can also be enhanced in hypercholesterolaemic patients, and are intimately associated with increased platelet membrane cholesterol.
The healthy endothelium typically exhibits strong anticoagulant, antiplatelet, and fibrinolytic properties that counterbalance prothrombotic factors.258 Upon plaque fissure (or plaque erosion), the local antithrombotic actions of the normal endothelium are lost, as endothelium is absent from the fissured or eroded surface. An important amplifier of the thrombotic reaction upon fissure is the interaction between inflammatory cells and platelets,247 which promotes an autocrine loop stimulating platelet aggregation and adhesion and sustained neutrophil adhesion and recruitment.259 Moreover, both oxLDL and oxidized phospholipids may activate platelets.260 The cardiovascular risk reduction seen with antiplatelet therapy is generally thought to be an effect of platelet inhibition in the event of plaque rupture.261 However, platelets may also have direct involvement in plaque instability.261
Does aggressive low-density lipoprotein lowering positively impact the plaque?
Previous sections in this article have described the complex nature of atherosclerotic plaques, including foam cells, lipid cores, fibrotic caps, necrosis, and calcification, all resulting from the retention and accumulation of LDL in the subendothelial matrix.262 The structural complexity of plaques almost certainly constitutes the basis of the heterogeneous progression of ASCVD from subclinical to clinical, 246,263 as demonstrated in early studies where sites of modest stenosis were observed to rapidly progress to a clinical coronary event upon rupture or erosion of plaques, with subsequent complete occlusion of a vessel.264,265 Recent studies, using a variety of intravascular imaging approaches, show that plaque characteristics can not only predict initial events, but also provide important insights into the course of CHD after an individual’s first episode, lesions with large necrotic cores, and thin fibrous caps being significantly associated with greater risk for subsequent events.266–268
Although the evidence that treatments to reduce LDL-C lead to fewer ASCVD events is unequivocal,4,5 understanding of how the beneficial effects of lower circulating LDL levels translate to changes in the atherosclerotic plaque is less clear. A pioneering investigation of bilateral, biopsied carotid endarterectomy samples at baseline and after 6 months of pravastatin treatment was seminal in demonstrating statin-induced increases in collagen content and reductions in lipid content, inflammatory cells, metalloprotease activity, and cell death, all of which favour plaque stabilization.17 Furthermore, several early studies involving quantitative coronary angiography without269 or with intravascular ultrasound18 demonstrated modest but significant benefits from statin-mediated LDL lowering on the degree of coronary artery stenosis. The magnitude of the effects of statin treatment on plaque volume and composition, particularly the thickness of the fibrous cap and the size of the lipid-rich core have not, however, been uniform among studies, potentially reflecting the differing resolution of the imaging modalities applied and dissimilarities in the underlying substrate.270,271 On the other hand, an open-label study with serial intravascular optical coherence tomographic measurements indicated that efficient LDL lowering can alter the balance between cap formation and degradation, leading to thicker caps and, by inference, lower risk of rupture and thrombosis.272 Of note, reductions in LDL-C by the PCSK9 inhibitor evolocumab in a secondary prevention trial reduced major coronary events273 and plaque volume274 but did not alter the composition of plaques over 76 weeks of treatment.275 However, the validity of virtual histology for plaque composition measurements remains uncertain.275 Moreover, this trial was conducted in patients previously treated with a statin, suggesting that the lesions studied may, in all probability, have been stabilized to a significant degree before the addition of evolocumab.
Can high-density lipoprotein or its components modulate intra-plaque biology driven by low-density lipoprotein?
Our understanding of the putative direct role of HDL and its major apolipoprotein, apoAI, in the pathophysiology of atherogenesis remains unclear, as does the potential modulation of the atherogenicity of LDL by HDL and its components within plaque tissue (Box 9). Nonetheless, we cannot exclude the possibility that the biological activities of functional HDL/apoAI particles may directly or indirectly attenuate the atherogenic drive of LDL particles in plaque progression.276–281
Box 9.
Apolipoprotein AI (apoAI), high-density lipoprotein (HDL), and atherosclerosis
- HDL/apoAI possess diverse functional properties, including cellular cholesterol efflux capacity and anti-oxidative and anti-inflammatory activities.
- Which of these activities may be most relevant to intra-plaque biology is unclear.
- HDL/apoAI may slow plaque progression by lipid efflux and by attenuating both intra-plaque oxidative modification of low-density lipoprotein (LDL) and inflammatory processes driven by modified LDL. For example, HDL plasmalogens attenuate the propagation of lipid peroxidation in LDL particles.
- Abundant apoAI in human atheroma tissue is typically dysfunctional due to extensive oxidative modification.
The finding of abundant dysfunctional, cross-linked apoAI in human atheroma tissue is perhaps relevant.282 Such dysfunction results from chemical modification (oxidation, carbamylation, or glycation) of key amino acid residues in apoAI by macrophage-derived myeloperoxidase;282 moreover, oxidative modification also alters the endothelial effects of HDL.283,284 These observations raise the possibility that a primary function of apoAI/HDL in plaque tissue is anti-inflammatory and anti-oxidative, i.e. apoAI acts to neutralize reactive oxygen species, a central feature of the oxidative stress and inflammation integral to the oxidative modification of LDL and thus to the pathogenesis of accelerated atherosclerosis.285,286 Furthermore, recent data suggest that plasmalogens of the HDL lipidome may also play an antioxidative role by attenuating the propagation of lipid peroxidation in LDL particles.279 These initial insights into the potential actions of HDL/apoAI in counterbalancing the atherogenic effects of LDL particles within plaque tissue require confirmation and extensive additional experimentation.
Missing pieces of the puzzle and their potential translation into innovative therapeutics
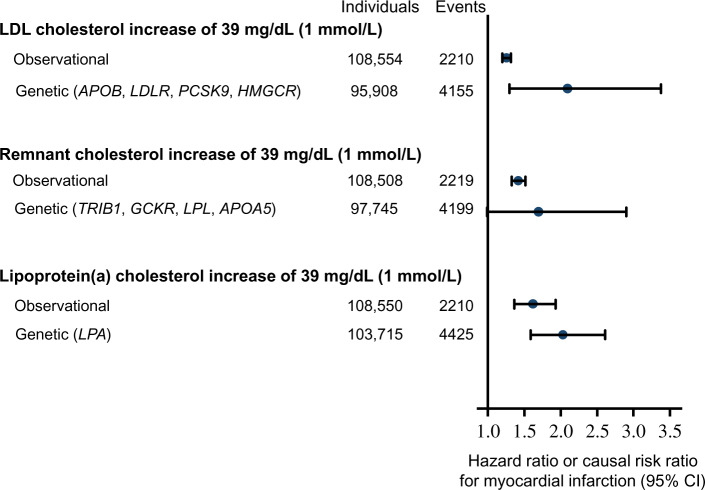
Genetic studies suggest that, in addition to LDL, TG-rich remnants and Lp(a) are directly causal in ASCVD, independent of LDL-C levels.6,7,9,11 Indeed, the hazard ratios for myocardial infarction for a 1 mmol/L (39 mg/dL) cholesterol increment were 1.3-fold for LDL, 1.4-fold for remnants, and 1.6-fold for Lp(a) when tested in parallel in approximately 100 000 individuals in the Copenhagen General Population Study (Figure 7).311 Using Mendelian randomization genetic data, the corresponding causal risk ratios for myocardial infarction were 2.1-fold for LDL, 1.7-fold for remnants, and 2.0-fold for Lp(a).
Figure 7.
Comparison of risk of myocardial infarction by 1 mmol/L (39 mg/dL) higher levels of low-density lipoprotein (LDL) cholesterol, remnant-cholesterol, and lipoprotein(a)-cholesterol from observational and genetic studies. Data from individuals in the Copenhagen General Population Study adapted with permission from Nordestgaard et al.311
These three lipoprotein classes may differ with respect to the mechanisms that underlie their respective contributions to plaque progression (Figure 7 and Box 10). Therefore, combining all three lipoprotein classes as total apoB or non-HDL-C should demand caution. Simplified expressions, such as ‘atherogenic apoB-containing lipoproteins’, may misinform the reader. As described above, LDL-C is a main causal driver of atherosclerosis development and thereby ASCVD, and typically is the most abundant atherogenic particle in the majority of individuals (LDL ∼1 mmol/L; VLDL ∼ 40 µmol/L). Of note, however, HDL particles are some 10-fold more abundant than those of LDL (∼12 mmol/L). Triglyceride-rich lipoproteins or Lp(a) (molar particle concentration range: 0.1–0.7 mmol/L) may be quantitatively more important than LDL in the causation of ASCVD in some individuals as a function of genetic background and metabolic state.
Box 10.
Outstanding questions
- Do the causal mechanisms by which low-density lipoprotein (LDL), lipoprotein(a) [Lp(a)], and remnant particles drive atherosclerotic cardiovascular disease differ?
- Do omega-3 fatty acids influence the mechanisms that underlie the atherogenicity of lipoproteins, including remnants and LDL?
- Will therapeutic modulation of apolipoprotein C-III and/or ANGPTL3 attenuate the impact of LDL on arterial plaque biology?
- To what degree can therapeutic modulation of HDL particles and their components attenuate atherobiology driven by LDL?
References: 6,7,9,11,12,287,311–333
ANGPTL3, angiopoietin-like 3.
As a consequence of their elevated cholesterol content (‘remnant cholesterol’, <4000 cholesterol molecules per particle), TG-rich remnants also contribute to intimal cholesterol deposition. Like LDL, remnants enter the arterial intima, in all likelihood by endothelial transcytosis, and are trapped prior to uptake as native (rather than modified) particles by macrophages to produce foam cells.6,312 In addition, hydrolysis of remnant TG by LPL in the arterial intima will produce tissue-toxic free fatty acids and thereby induce inflammation.313,314
In the REDUCE-IT trial, treatment with icosapent ethyl omega-3 fatty acid (4 g daily) resulted in a 25% reduction in ASCVD concomitant with a 20% reduction in plasma TG levels and 40% reduction in C-reactive protein (Box 10).315 This finding is consistent with genetic studies that indicated a causal role of TG in the aetiology of CAD.287,316,317 However, cardiovascular event reduction in the REDUCE-IT trial was independent of TG levels both at baseline and on treatment. This finding might raise questions about the role of TGRL in eliciting clinical benefit. However, consideration of the area under the curve for TGRL and remnants during the atherogenic postprandial period indicates that levels of TGRL and remnants are considerably amplified in subjects with Type 2 diabetes;78 such individuals represented 58% of participants in the REDUCE-IT trial. It is possible that attenuation of the postprandial response by eicosapentanoic acid, the hydrolytic product of icosapent ethyl, may underlie a significant proportion of clinical benefit in the REDUCE-IT trial.
The results of similar cardiovascular outcome trials using another purified omega-3 fatty acid formulation (STRENGTH; NCT02104817) or pemafibrate, a selective peroxisome proliferator-activated receptor alpha agonist, are eagerly awaited.318 In addition, ongoing phase three trials involving inhibitors of apoC-III319 and of ANGPTL3,320,321 whose action enhances the activity of LPL, should significantly reduce remnant cholesterol and TG levels and may translate into cardiovascular benefit (Box 10).
Implications for future prevention of atherosclerotic cardiovascular disease
Extensive evidence on the pathophysiology of ASCVD presented here supplements and extends our earlier review on the causality of LDL based on epidemiological, GWAS, and Mendelian randomization studies, as well as controlled intervention trials with pharmacological agents targeting the LDL receptor.4 Such evidence, together with the associated molecular mechanisms, has clear implications across the continuum of ASCVD prevention (i.e. primordial, primary, secondary, and tertiary) and is consistent with the central concept derived from genomics that the cumulative arterial burden of LDL-C drives the development and progression of ASCVD and its clinical sequelae.4,334,335
Furthermore, the pathophysiological evidence supports therapeutic strategies aimed at maintaining very low levels of LDL-C (e.g. <1 mmol/L or 40 mg/dL) in patients with established ASCVD at very high risk of recurrent events.336 Such low plasma LDL-C levels are now attainable with the combination of statins and PCSK9 inhibitors (with or without addition of ezetimibe), therapeutic regimens that have proven safety and tolerability.273,337,338 The unequivocal body of evidence for LDL causality in ASCVD will impact on future international recommendations for the management of atherogenic and ASCVD-promoting dyslipidaemias and will guide the rational use of both existing and new therapies.339–342 The success of modern programmes of ASCVD prevention will also rely on the practice of precision medicine and patient-centred approaches.343
Finally, this thesis has highlighted emerging mechanistic features of atherosclerosis that can potentially lead to evaluation of new therapeutic targets integral to arterial wall biology and plaque stability. Prominent amongst these are endothelial transcytosis of atherogenic lipoproteins, monocyte/macrophage and SMC biology, efferocytosis, inflammation, innate and adaptive immune responses to the intimal retention of apoB-containing lipoproteins and calcification (Take home figure). The future holds great promise but will not be lacking in surprises.
Take home figure.
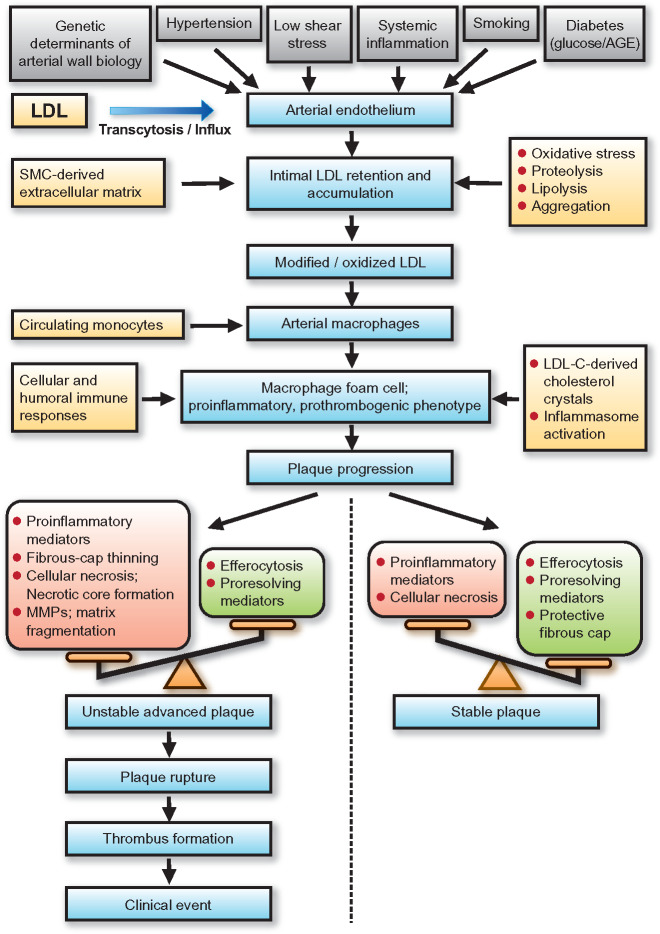
Low-density lipoprotein (LDL) and atherobiology. Summary of the principal mechanisms underlying the entry, retention, and accumulation of LDL particles in the artery wall, and subsequent LDL-driven downstream events that are central to the complex pathogenesis of atherothrombosis. Intermediate fatty streak lesions are characterized by subintimal accumulation of macrophage foam cells. AGE, advanced glcation end-products; LDL-C, LDL-cholesterol; MMPs, matrix metallopeptidases
Acknowledgements
The authors thank Sherborne Gibbs Ltd for logistical support during meetings of the Consensus Panel, Dr Jane Stock (European Atherosclerosis Society Consensus Panel Administration Office, London, UK) for editorial and administrative support and Dr Rosie Perkins for scientific editing and proof reading.
Funding
The European Atherosclerosis Society (EAS) supported travel and accommodation of Panel members and meeting logistics. Funding to pay the open access publication charges for this article was provided by the European Atherosclerosis Society.
Conflict of interest: J.F.B. has received research grants from Regeneron and Ferring Pharmaceuticals and honoraria for consultancy from Novo Nordisk. C.J.B. has received honoraria for consultancy and lectures from Amgen and AOP Pharma. J.B. has received research grants from Amgen, AstraZeneca, NovoNordisk, Pfizer, and Regeneron/Sanofi and honoraria for consultancy and lectures from Amgen, AstraZeneca, Eli Lilly, Merck, Novo-Nordisk, Pfizer, and Regeneron/Sanofi. E.B. has received honoraria from AstraZeneca, Amgen, Genfit, MSD, Sanofi-Regeneron, Unilever, Danone, Aegerion, Chiesi, Rottapharm, Lilly, and Servier and research grants from Amgen, Danone, and Aegerion. A.L.C. has received grants from Pfizer, Sanofi, Regeneron, Merck, and Mediolanum; non-financial support from SigmaTau, Menarini, Kowa, Recordati, and Eli Lilly; and personal honoraria for lectures/speakers bureau or consultancy from AstraZeneca, Genzyme, Menarini, Kowa, Eli Lilly, Recordati, Pfizer, Sanofi, Mediolanum, Pfizer, Merck, Sanofi, Aegerion, and Amgen. M.J.C. has received grants from Amgen, Kowa Europe, and Pfizer; and personal honoraria for lectures/speaker’s bureau from Akcea, Alexion, Amarin, Amgen, AstraZeneca, Daiichi-Sankyo, Kowa Europe, Merck/MSD, Pfizer, Sanofi, Regeneron, and Unilever. M.J.D., L.L.D., G.P., M.-R.T., and B.v.d.S. have no conflict of interest to declare. S.F. discloses compensated consultant and advisory activities with Merck, Kowa, Sanofi, Amgen, Amarin, and Aegerion. B.A.F. has received research grants from Merck, Amgen, and Esperion Therapeutics; and received honoraria for lectures, consulting and/or advisory board membership from Merck, Amgen, Esperion, Ionis, and the American College of Cardiology. H.N.G. has received grants and personal honoraria for consultancy from Merck; grants from Sanofi-Regeneron, Amgen, and Medimmune/AstraZeneca; and personal honoraria for consultancy from Janssen, Sanofi, Regeneron, Kowa, Pfizer, and Resverlogix. I.G. has received speaker fees from MSD and Pfizer relating to cardiovascular risk estimation and lipid guidelines, and consultancy/speaker fee from Amgen. R.A.H. has received grants and personal honoraria for consultancy from Acasti and Akcea/Ionis; grants from Regeneron and Boston Heart Diagnostics; and personal honoraria for consultancy from Aegerion, Amgen, Gemphire, and Sanofi. J.D.H. reports honoraria for consultancy from Gilead, Pfizer, Regeneron, Sanofi Aventis, Merck, Gemphire, BioEnergenix, and stock options from Catabasis. R.M.K. has received research grants, consultancy honoraria, and non-financial support from Quest Diagnostics and is also co-inventor of a licensed patent for measurement of lipoprotein particles by ion mobility. U.L. has received honoraria for lectures and/or consulting from Amgen, Medicines Company, Astra Zeneca, Berlin Chemie, Bayer, Abbott, and Sanofi. U.L. has received honoraria for board membership, consultancy, and lectures from Amgen, MSD, Sanofi, and Servier. L.M. has received honoraria for consultancy and lectures from Amgen, Merck, Sanofi-Regeneron, Mylan, and Daiichi-Sankyo. S.J.N. has received research support from Amgen, AstraZeneca, Anthera, Cerenis, Novartis, Eli Lilly, Esperion, Resverlogix, Sanofi-Regeneron, InfraReDx, and LipoScience and is a consultant for Akcea, Amgen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Merck, Takeda, Pfizer, Roche, Sanofi-Regeneron, Kowa, and Novartis. B.G.N. reports consultancies and honoraria for lectures from AstraZeneca, Sanofi, Regeneron, Amgen, Akcea, Kowa, Novartis, Novo Nordisk. C.J.P. has received research support from MSD and honoraria from Sanofi/Regeneron, Amgen, and Daiichi-Sankyo. F.J.R. has received personal honoraria for consultancy and non-financial support from Amgen, Sanofi/Regeneron, and The Medicines Company. K.K.R. has received grants and personal honoraria for consultancy, advisory boards and/or lectures from Amgen, Sanofi, Regeneron, MSD, and Pfizer personal honoraria for consultancy, advisory boards and/or lectures from Abbvie, AstraZeneca, The Medicines Company, Resverlogix, Akcea, Boehringer Ingelheim, Novo Nordisk, Takeda, Kowa, Algorithm, Cipla, Cerenis, Dr Reddys, Lilly, Zuellig Pharma, Silence Theapeutics, and Bayer. H.S. has received research grants from AstraZeneca and honoraria for speaker fees/consultancy from AstraZeneca, MSD, Amgen, Bayer Vital GmbH, Boehringer Ingelheim, Novartis, Servier, Daiichi Sankyo, Brahms, Bristol-Myers Squibb, Medtronic, Sanofi Aventis, and Synlab. L.T. has received personal honoraria for lectures/speakers bureau or consultancy from MSD, Sanofi, AMGEN, Abbott, Mylan, Bayer, Actelion, Novartis, Astra, Recordati, Pfizer, Servier, and Novo Nordisk. She is also the President, European Atherosclerosis Society (EAS) and an Editorial Board Member, European Heart Journal. G.F.W. has received research support from Sanofi, Regeneron, Arrowhead and Amgen, and honoraria for board membership from Sanofi, Regeneron, Amgen, Kowa, and Gemphire. O.W. has received honoraria for lectures or consultancy from Sanofi and Amgen.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1.Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA.. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650–1656. [DOI] [PubMed] [Google Scholar]
- 2.Newman WP 3rd, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, Williamson GD, Webber LS, Berenson GS.. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med 1986;314:138–144. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Friera L, Penalvo JL, Fernandez-Ortiz A, Ibanez B, Lopez-Melgar B, Laclaustra M, Oliva B, Mocoroa A, Mendiguren J, Martinez de Vega V, Garcia L, Molina J, Sanchez-Gonzalez J, Guzman G, Alonso-Farto JC, Guallar E, Civeira F, Sillesen H, Pocock S, Ordovas JM, Sanz G, Jimenez-Borreguero LJ, Fuster V.. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation 2015;131:2104–2113. [DOI] [PubMed] [Google Scholar]
- 4.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen M-R, Tokgözoğlu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL.. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein JL, Brown MS.. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 2015;161:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–563. [DOI] [PubMed] [Google Scholar]
- 7.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen M-R, Tokgözoglu L, Tybjærg-Hansen A, Watts GF; European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, Borén J, Bruckert E, Catapano AL, Descamps OS, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AFH, Stroes E, Taskinen M-R, Tybjærg-Hansen A, Watts GF, Wiklund O; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014;2:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg-Hansen AEuropean Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG.. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331–2339. [DOI] [PubMed] [Google Scholar]
- 11.Nordestgaard BG, Langsted A.. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 2016;57:1953–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O'Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang S-J, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O'Donnell CJ, Post WS; CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahley RW, Huang Y, Rall SC. Jr. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res 1999;40:1933–1949. [PubMed] [Google Scholar]
- 14.Laufs U, Parhofer KG, Ginsberg HN, Hegele RA.. Clinical review on triglycerides. Eur Heart J 2020;41:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis KL, Boffa MB, Sahebkar A, Koschinsky ML, Watts GF.. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Prog Lipid Res 2017;68:57–82. [DOI] [PubMed] [Google Scholar]
- 16.Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, Moriarty PM, Rader DJ, Remaley AT, Reyes-Soffer G, Santos RD, Thanassoulis G, Witztum JL, Danthi S, Olive M, Liu L.. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018;71:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J.. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 2001;103:926–933. [DOI] [PubMed] [Google Scholar]
- 18.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the trial. JAMA 2006;295:1556–1565. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, Nissen SE.. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078–2087. [DOI] [PubMed] [Google Scholar]
- 20.Di Bartolo BA, Psaltis PJ, Bursill CA, Nicholls SJ.. Translating evidence of HDL and plaque regression. Arterioscler Thromb Vasc Biol 2018;38:1961–1968. [DOI] [PubMed] [Google Scholar]
- 21.Tabas I, Williams KJ, Borén J.. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–1844. [DOI] [PubMed] [Google Scholar]
- 22.Williams KJ, Tabas I.. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995;15:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwenke DC, Carew TE.. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis 1989;9:908–918. [DOI] [PubMed] [Google Scholar]
- 24.Schwenke DC, Carew TE.. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. I. Focal increases in arterial LDL concentration precede development of fatty streak lesions. Arteriosclerosis 1989;9:895–907. [DOI] [PubMed] [Google Scholar]
- 25.Stender S, Zilversmit DB.. Transfer of plasma lipoprotein components and of plasma proteins into aortas of cholesterol-fed rabbits. Molecular size as a determinant of plasma lipoprotein influx. Arteriosclerosis 1981;1:38–49. [DOI] [PubMed] [Google Scholar]
- 26.Nordestgaard BG, Zilversmit DB.. Large lipoproteins are excluded from the arterial wall in diabetic cholesterol-fed rabbits. J Lipid Res 1988;29:1491–1500. [PubMed] [Google Scholar]
- 27.Nordestgaard BG, Tybjaerg-Hansen A, Lewis B.. Influx in vivo of low density, intermediate density, and very low density lipoproteins into aortic intimas of genetically hyperlipidemic rabbits. Roles of plasma concentrations, extent of aortic lesion, and lipoprotein particle size as determinants. Arterioscler Thromb 1992;12:6–18. [DOI] [PubMed] [Google Scholar]
- 28.Nordestgaard BG, Wootton R, Lewis B.. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol 1995;15:534–542. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen LB, Stender S, Jauhiainen M, Nordestgaard BG.. Preferential influx and decreased fractional loss of lipoprotein(a) in atherosclerotic compared with nonlesioned rabbit aorta. J Clin Invest 1996;98:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML, De Caterina R.. Endothelial permeability, LDL deposition, and cardiovascular risk factors—a review. Cardiovasc Res 2018;114:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanoni P, Velagapudi S, Yalcinkaya M, Rohrer L, von Eckardstein A.. Endocytosis of lipoproteins. Atherosclerosis 2018;275:273–295. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Sessa WC, Fernandez-Hernando C.. Endothelial transcytosis of lipoproteins in atherosclerosis. Front Cardiovasc Med 2018;5:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordestgaard BG. The vascular endothelial barrier–selective retention of lipoproteins. Curr Opin Lipidol 1996;7:269–273. [DOI] [PubMed] [Google Scholar]
- 34.Frank PG, Lisanti MP.. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Current Opin Lipidol 2004;15:523–529. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, Sessa WC.. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab 2009;10:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank PG, Pavlides S, Lisanti MP.. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res 2009;335:41–47. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong SM, Sugiyama MG, Levy A, Neculai D, Roufaiel M, Bolz S-S, Cybulsky M, Heit B, Lee WL.. Novel assay for detection of LDL transcytosis across coronary endothelium reveals an unexpected role for SR-B1. Circulation 2014;130(Suppl_2):A11607. [Google Scholar]
- 38.Kraehling JR, Chidlow JH, Rajagopal C, Sugiyama MG, Fowler JW, Lee MY, Zhang X, Ramírez CM, Park EJ, Tao B, Chen K, Kuruvilla L, Larriveé B, Folta-Stogniew E, Ola R, Rotllan N, Zhou W, Nagle MW, Herz J, Williams KJ, Eichmann A, Lee WL, Fernández-Hernando C, Sessa WC.. Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells. Nat Commun 2016;7:13516.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R.. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J Cell Biol 1997;138:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armstrong SM, Sugiyama MG, Fung KY, Gao Y, Wang C, Levy AS, Azizi P, Roufaiel M, Zhu SN, Neculai D, Yin C, Bolz SS, Seidah NG, Cybulsky MI, Heit B, Lee WL.. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc Res 2015;108:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L, Chambliss KL, Gao X, Yuhanna IS, Behling-Kelly E, Bergaya S, Ahmed M, Michaely P, Luby-Phelps K, Darehshouri A, Xu L, Fisher EA, Ge WP, Mineo C, Shaul PW.. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019;569:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghaffari S, Naderi Nabi F, Sugiyama MG, Lee WL.. Estrogen inhibits LDL (Low-Density Lipoprotein) transcytosis by human coronary artery endothelial cells via GPER (G-Protein-Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arterioscler Thromb Vasc Biol 2018;38:2283–2294. [DOI] [PubMed] [Google Scholar]
- 43.Gordon T, Kannel WB, Hjortland MC, McNamara PM.. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med 1978;89:157–161. [DOI] [PubMed] [Google Scholar]
- 44.Sessa WC. Estrogen reduces LDL (low-density lipoprotein) transcytosis. Arterioscler Thromb Vasc Biol 2018;38:2276–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bian F, Yang XY, Xu G, Zheng T, Jin S.. CRP-induced NLRP3 inflammasome activation increases LDL transcytosis across endothelial cells. Front Pharmacol 2019;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai X, Yang X, Jia X, Rong Y, Chen L, Zeng T, Deng X, Li W, Wu G, Wang L, Li Y, Zhang J, Xiong Z, Xiong L, Wang Y, Zhu L, Zhao Y, Jin S.. CAV1-CAVIN1-LC3B-mediated autophagy regulates high glucose-stimulated LDL transcytosis. Autophagy 2019;doi: 10.1080/15548627.2019.1659613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartels ED, Christoffersen C, Lindholm MW, Nielsen LB.. Altered metabolism of LDL in the arterial wall precedes atherosclerosis regression. Circ Res 2015;117:933–942. [DOI] [PubMed] [Google Scholar]
- 48.Williams KJ, Tabas I, Fisher EA.. How an artery heals. Circ Res 2015;117:909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boren J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL.. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest 1998;101:2658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J.. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002;417:750–754. [DOI] [PubMed] [Google Scholar]
- 51.Camejo G, Lalaguna F, Lopez F, Starosta R.. Characterization and properties of a lipoprotein-complexing proteoglycan from human aorta. Atherosclerosis 1980;35:307–320. [DOI] [PubMed] [Google Scholar]
- 52.Melchior JT, Sawyer JK, Kelley KL, Shah R, Wilson MD, Hantgan RR, Rudel LLL. LDL particle core enrichment in cholesteryl oleate increases proteoglycan binding and promotes atherosclerosis. J Lipid Res 2013;54:2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, Lopez R, Kirk EA, Chait A, Wight TN, deBeer FC, LeBoeuf RC.. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol 2005;25:785–790. [DOI] [PubMed] [Google Scholar]
- 54.Hiukka A, Stahlman M, Pettersson C, Levin M, Adiels M, Teneberg S, Leinonen ES, Hulten LM, Wiklund O, Oresic M, Olofsson SO, Taskinen MR, Ekroos K, Boren J.. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes 2009;58:2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olin-Lewis K, Krauss RM, La Belle M, Blanche PJ, Barrett PH, Wight TN, Chait A.. ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J Lipid Res 2002;43:1969–1977. [DOI] [PubMed] [Google Scholar]
- 56.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K.. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol 2007;27:1159–1165. [DOI] [PubMed] [Google Scholar]
- 57.Nakashima Y, Wight TN, Sueishi K.. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res 2008;79:14–23. [DOI] [PubMed] [Google Scholar]
- 58.Nakashima Y, Chen YX, Kinukawa N, Sueishi K.. Distributions of diffuse intimal thickening in human arteries: preferential expression in atherosclerosis-prone arteries from an early age. Virchows Arch 2002;441:279–288. [DOI] [PubMed] [Google Scholar]
- 59.Velican C, Velican D.. Natural resistance to atherosclerosis exhibited by the first centimeter of left and right coronary arteries. Atherosclerosis 1984;50:173–181. [DOI] [PubMed] [Google Scholar]
- 60.Steffensen LB, Mortensen MB, Kjolby M, Hagensen MK, Oxvig C, Bentzon JF.. Disturbed laminar blood flow vastly augments lipoprotein retention in the artery wall: a key mechanism distinguishing susceptible from resistant sites. Arterioscler Thromb Vasc Biol 2015;35:1928–1935. [DOI] [PubMed] [Google Scholar]
- 61.Peiffer V, Sherwin SJ, Weinberg PD.. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res 2013;99:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalan JM, Roberts WC.. Morphologic findings in saphenous veins used as coronary arterial bypass conduits for longer than 1 year: necropsy analysis of 53 patients, 123 saphenous veins, and 1865 five-millimeter segments of veins. Am Heart J 1990;119:1164–1184. [DOI] [PubMed] [Google Scholar]
- 63.Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, Gagnon DR, DuVall SL, Li J, Peloso GM, Chaffin M, Small AM, Huang J, Tang H, Lynch JA, Ho YL, Liu DJ, Emdin CA, Li AH, Huffman JE, Lee JS, Natarajan P, Chowdhury R, Saleheen D, Vujkovic M, Baras A, Pyarajan S, Di Angelantonio E, Neale BM, Naheed A, Khera AV, Danesh J, Chang KM, Abecasis G, Willer C, Dewey FE, Carey DJGlobal Lipids Genetics ConsortiumMyocardial Infarction Genetics (MIGen) ConsortiumGeisinger-Regeneron DiscovEHR CollaborationVA Million Veteran ProgramConcato J, Gaziano JM, O'Donnell CJ, Tsao PS, Kathiresan S, Rader DJ, Wilson PWF, Assimes TL. Genetics of blood lipids among ∼300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet 2018;50:1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berneis KK, Krauss RM.. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002;43:1363–1379. [DOI] [PubMed] [Google Scholar]
- 65.Segrest JP, Jones MK, De Loof H, Dashti N.. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res 2001;42:1346–1367. [PubMed] [Google Scholar]
- 66.Chapman MJ, Laplaud PM, Luc G, Forgez P, Bruckert E, Goulinet S, Lagrange D.. Further resolution of the low density lipoprotein spectrum in normal human plasma: physicochemical characteristics of discrete subspecies separated by density gradient ultracentrifugation. J Lipid Res 1988;29:442–458. [PubMed] [Google Scholar]
- 67.Tribble DL, van den Berg JJ, Motchnik PA, Ames BN, Lewis DM, Chait A, Krauss RM.. Oxidative susceptibility of low density lipoprotein subfractions is related to their ubiquinol-10 and alpha-tocopherol content. Proc Natl Acad Sci USA 1994;91:1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T, Zeiher AM, Landmesser U, Dimmeler S.. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol 2013;33:1392–1400. [DOI] [PubMed] [Google Scholar]
- 69.Lund-Katz S, Laplaud PM, Phillips MC, Chapman MJ.. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry 1998;37:12867–12874. [DOI] [PubMed] [Google Scholar]
- 70.Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ.. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res 1998;39:1263–1273. [PubMed] [Google Scholar]
- 71.Anber V, Griffin BA, McConnell M, Packard CJ, Shepherd J.. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis 1996;124:261–271. [DOI] [PubMed] [Google Scholar]
- 72.Tselepis AD, Dentan C, Karabina SA, Chapman MJ, Ninio E.. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler Thromb Vasc Biol 1995;15:1764–1773. [DOI] [PubMed] [Google Scholar]
- 73.Chancharme L, Thérond P, Nigon F, Lepage S, Couturier M, Chapman MJ.. Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler Thromb Vasc Biol 1999;19:810–820. [DOI] [PubMed] [Google Scholar]
- 74.Packard CJ, Shepherd J.. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol 1997;17:3542–3556. [DOI] [PubMed] [Google Scholar]
- 75.Diffenderfer MR, Schaefer EJ.. The composition and metabolism of large and small LDL. Curr Opin Lipidol 2014;25:221–226. [DOI] [PubMed] [Google Scholar]
- 76.Tremblay AJ, Lamarche B, Ruel IL, Hogue JC, Bergeron J, Gagne C, Couture P.. Increased production of VLDL apoB-100 in subjects with familial hypercholesterolemia carrying the same null LDL receptor gene mutation. J Lipid Res 2004;45:866–872. [DOI] [PubMed] [Google Scholar]
- 77.Guerin M, Dolphin PJ, Chapman MJ.. Preferential cholesteryl ester acceptors among the LDL subspecies of subjects with familial hypercholesterolemia. Arterioscler Thromb 1994;14:679–685. [DOI] [PubMed] [Google Scholar]
- 78.Taskinen MR, Boren J.. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015;239:483–495. [DOI] [PubMed] [Google Scholar]
- 79.Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004;27:1496–1504. [DOI] [PubMed] [Google Scholar]
- 80.Guerin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ.. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol 2001;21:282–288. [DOI] [PubMed] [Google Scholar]
- 81.Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol 2010;21:305–311. [DOI] [PubMed] [Google Scholar]
- 82.Nigon F, Lesnik P, Rouis M, Chapman MJ.. Discrete subspecies of human low density lipoproteins are heterogeneous in their interaction with the cellular LDL receptor. J Lipid Res 1991;32:1741–1753. [PubMed] [Google Scholar]
- 83.Campos H, Arnold KS, Balestra ME, Innerarity TL, Krauss RM.. Differences in receptor binding of LDL subfractions. Arterioscler Thromb Vasc Biol 1996;16:794–801. [DOI] [PubMed] [Google Scholar]
- 84.Thongtang N, Diffenderfer MR, Ooi EMM, Barrett PHR, Turner SM, Le NA, Brown WV, Schaefer EJ.. Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia: effects of rosuvastatin. J Lipid Res 2017;58:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin MJ, Krauss RM.. Apolipoprotein CIII bound to apoB-containing lipoproteins is associated with small, dense LDL independent of plasma triglyceride levels in healthy men. Atherosclerosis 2010;211:337–341. [DOI] [PubMed] [Google Scholar]
- 86.Krauss RM, Wojnooski K, Orr J, Geaney JC, Pinto CA, Liu Y, Wagner JA, Luk JM, Johnson-Levonas AO, Anderson MS, Dansky HM.. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J Lipid Res 2012;53:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.La Belle M, Blanche PJ, Krauss RM.. Charge properties of low density lipoprotein subclasses. J Lipid Res 1997;38:690–700. [PubMed] [Google Scholar]
- 88.La Belle M, Krauss RM.. Differences in carbohydrate content of low density lipoproteins associated with low density lipoprotein subclass patterns. J Lipid Res 1990;31:1577–1588. [PubMed] [Google Scholar]
- 89.Stahlman M, Pham HT, Adiels M, Mitchell TW, Blanksby SJ, Fagerberg B, Ekroos K, Boren J.. Clinical dyslipidaemia is associated with changes in the lipid composition and inflammatory properties of apolipoprotein-B-containing lipoproteins from women with type 2 diabetes. Diabetologia 2012;55:1156–1166. [DOI] [PubMed] [Google Scholar]
- 90.Krauss RM, Burke DJ.. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res 1982;23:97–104. [PubMed] [Google Scholar]
- 91.Adiels M, Olofsson S-O, Taskinen M-R, Borén J.. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:1225–1236. [DOI] [PubMed] [Google Scholar]
- 92.Adiels M, Taskinen M-R, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Häkkinen A, Olofsson S-O, Yki-Järvinen H, Borén J.. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 2006;49:755–765. [DOI] [PubMed] [Google Scholar]
- 93.Boren J, Watts GF, Adiels M, Soderlund S, Chan DC, Hakkarainen A, Lundbom N, Matikainen N, Kahri J, Verges B, Barrett PH, Taskinen MR.. Kinetic and related determinants of plasma triglyceride concentration in abdominal obesity: multicenter Tracer Kinetic Study. Arterioscler Thromb Vasc Biol 2015;35:2218–2224. [DOI] [PubMed] [Google Scholar]
- 94.Taskinen MR, Boren J.. Why is apolipoprotein CIII emerging as a novel therapeutic target to reduce the burden of cardiovascular disease? Curr Atheroscler Rep 2016;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taskinen M-R, Adiels M, Westerbacka J, Söderlund S, Kahri J, Lundbom N, Lundbom J, Hakkarainen A, Olofsson S-O, Orho-Melander M, Borén J.. Dual metabolic defects are required to produce hypertriglyceridemia in obese subjects. Arterioscler Thromb Vasc Biol 2011;31:2144–2150. [DOI] [PubMed] [Google Scholar]
- 96.Lada AT, Rudel LL.. Associations of low density lipoprotein particle composition with atherogenicity. Curr Opin Lipidol 2004;15:19–24. [DOI] [PubMed] [Google Scholar]
- 97.Ruuth M, Nguyen SD, Vihervaara T, Hilvo M, Laajala TD, Kondadi PK, Gisterå A, Lähteenmäki H, Kittilä T, Huusko J, Uusitupa M, Schwab U, Savolainen MJ, Sinisalo J, Lokki M-L, Nieminen MS, Jula A, Perola M, Ylä-Herttula S, Rudel L, Öörni A, Baumann M, Baruch A, Laaksonen R, Ketelhuth DFJ, Aittokallio T, Jauhiainen M, Käkelä R, Borén J, Williams KJ, Kovanen PT, Öörni K.. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur Heart J 2018;39:2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engström G, Williams PT, Kathiresan S, Melander O, Krauss RM.. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol 2009;29:1975–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griffin BA, Caslake MJ, Yip B, Tait GW, Packard CJ, Shepherd J.. Rapid isolation of low density lipoprotein (LDL) subfractions from plasma by density gradient ultracentrifugation. Atherosclerosis 1990;83:59–67. [DOI] [PubMed] [Google Scholar]
- 100.Li KM, Wilcken DE, Dudman NP.. Effect of serum lipoprotein(a) on estimation of low-density lipoprotein cholesterol by the Friedewald formula. Clin Chem 1994;40:571–573. [PubMed] [Google Scholar]
- 101.Giral P, Simon D, Chapman MJ.. Letter by Giral et al regarding article, “lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin).” Circulation 2014;130:e151. [DOI] [PubMed] [Google Scholar]
- 102.Chapman MJ, Le Goff W, Guerin M, Kontush A.. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur Heart J 2010;31:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bjornson E, Packard CJ, Adiels M, Andersson L, Matikainen N, Soderlund S, Kahri J, Sihlbom C, Thorsell A, Zhou H, Taskinen MR, Boren J.. Investigation of human apoB48 metabolism using a new, integrated non-steady-state model of apoB48 and apoB100 kinetics. J Intern Med 2019;285:562–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boren J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL.. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. J Clin Invest 1998;101:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flood C, Gustafsson M, Pitas RE, Arnaboldi L, Walzem RL, Borén J.. Molecular mechanism for changes in proteoglycan binding on compositional changes of the core and the surface of low-density lipoprotein-containing human apolipoprotein B100. Arterioscler Thromb Vasc Biol 2004;24:564–570. [DOI] [PubMed] [Google Scholar]
- 106.Barter PJ, Brewer HB Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR.. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:160–167. [DOI] [PubMed] [Google Scholar]
- 107.Mahley RW, Huang Y.. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J Clin Invest 2007;117:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kypreos KE, Zannis VI.. LDL receptor deficiency or apoE mutations prevent remnant clearance and induce hypertriglyceridemia in mice. J Lipid Res 2006;47:521–529. [DOI] [PubMed] [Google Scholar]
- 109.Williams KJ. Molecular processes that handle—and mishandle—dietary lipids. J Clin Invest 2008;118:3247–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Austin MA, King MC, Vranizan KM, Krauss RM.. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation 1990;82:495–506. [DOI] [PubMed] [Google Scholar]
- 111.Nordestgaard BG, Zilversmit DB.. Comparison of arterial intimal clearances of LDL from diabetic and nondiabetic cholesterol-fed rabbits. Differences in intimal clearance explained by size differences. Arteriosclerosis 1989;9:176–183. [DOI] [PubMed] [Google Scholar]
- 112.Younis N, Charlton-Menys V, Sharma R, Soran H, Durrington PN.. Glycation of LDL in non-diabetic people: small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 2009;202:162–168. [DOI] [PubMed] [Google Scholar]
- 113.de Queiroz Mello AP, da Silva IT, Oliveira AS, Nunes VS, Abdalla DS, Gidlund M, Damasceno NR.. Electronegative low-density lipoprotein is associated with dense low-density lipoprotein in subjects with different levels of cardiovascular risk. Lipids 2010;45:619–625. [DOI] [PubMed] [Google Scholar]
- 114.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL.. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989;320:915–924. [DOI] [PubMed] [Google Scholar]
- 115.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest 2002;110:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moore KJ, Tabas I.. Macrophages in the pathogenesis of atherosclerosis. Cell 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Witztum JL, Lichtman AH.. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol 2014;9:73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Steinberg D, Witztum JL.. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol 2010;30:2311–2316. [DOI] [PubMed] [Google Scholar]
- 119.Nordestgaard BG, Stender S, Kjeldsen K.. Reduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wall. Arteriosclerosis 1988;8:421–428. [DOI] [PubMed] [Google Scholar]
- 120.Koo C, Wernette-Hammond ME, Garcia Z, Malloy MJ, Uauy R, East C, Bilheimer DW, Mahley RW, Innerarity TL.. Uptake of cholesterol-rich remnant lipoproteins by human monocyte-derived macrophages is mediated by low density lipoprotein receptors. J Clin Invest 1988;81:1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF.. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest 1999;103:1697–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gustafsson M, Levin M, Skalen K, Perman J, Fridén V, Jirholt P, Olofsson S-O, Fazio S, Linton MF, Semenkovich CF, Olivecrona G, Borén J.. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ Res 2007;101:777–783. [DOI] [PubMed] [Google Scholar]
- 123.Ullery-Ricewick JC, Cox BE, Griffin EE, Jerome WG.. Triglyceride alters lysosomal cholesterol ester metabolism in cholesteryl ester-laden macrophage foam cells. J Lipid Res 2009;50:2014–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM.. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol 2014;34:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT.. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM, Glynn RJ, Ridker PM, Krauss RM.. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circulation 2015;132:2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ.. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 2010;466:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang H-Y, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson Å, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen L-P, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen A-K, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney ASF, Döring A, Elliott P, Epstein SE, Ingi Eyjolfsson G, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen A-L, Hayward C, Hernandez D, Hicks AA, Holm H, Hung Y-J, Illig T, Jones MR, Kaleebu P, Kastelein JJP, Khaw K-T, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin S-Y, Lindström J, Loos RJF, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TVM, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen Y-DI, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin M-R, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PEH, Sheu WH-H, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BHR, Ordovas JM, Boerwinkle E, Palmer CNA, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR; Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013;45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Binder CJ, Papac-Milicevic N, Witztum JL.. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol 2016;16:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J.. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal 2010;12:1009–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weber C, Noels H.. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011;17:1410–1422. [DOI] [PubMed] [Google Scholar]
- 132.Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Maatta A, Gaddis DE, Bowden K, Pattison J, MacDonald JG, Yla-Herttuala S, Mellon PL, Hedrick CC, Ley K, Miller YI, Glass CK, Peterson KL, Binder CJ, Tsimikas S, Witztum JL.. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018;558:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moore KJ, Koplev S, Fisher EA, Tabas I, Bjorkegren JLM, Doran AC, Kovacic JC.. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD series (Part 2). J Am Coll Cardiol 2018;72:2181–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, Combs CA, Malide D, Zhang WY.. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem 2005;280:2352–2360. [DOI] [PubMed] [Google Scholar]
- 135.Anzinger JJ, Chang J, Xu Q, Buono C, Li Y, Leyva FJ, Park BC, Greene LE, Kruth HS.. Native low-density lipoprotein uptake by macrophage colony-stimulating factor-differentiated human macrophages is mediated by macropinocytosis and micropinocytosis. Arterioscler Thromb Vasc Biol 2010;30:2022–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Williams JW, Giannarelli C, Rahman A, Randolph GJ, Kovacic JC.. Macrophage biology, classification, and phenotype in cardiovascular disease: JACC macrophage in CVD series (Part 1). J Am Coll Cardiol 2018;72:2166–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pourcet B, Staels B.. Alternative macrophages in atherosclerosis: not always protective! J Clin Invest 2018;128:910–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chinetti-Gbaguidi G, Colin S, Staels B.. Macrophage subsets in atherosclerosis. Nat Rev Cardiol 2015;12:10–17. [DOI] [PubMed] [Google Scholar]
- 139.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ.. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 2010;11:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van der Vorst EPC, Döring Y, Weber C.. Chemokines. Arterioscler Thromb Vasc Biol 2015;35:e52–6. [DOI] [PubMed] [Google Scholar]
- 141.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK.. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E.. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ.. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 2013;14:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baumer Y, McCurdy S, Weatherby TM, Mehta NN, Halbherr S, Halbherr P, Yamazaki N, Boisvert WA.. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat Commun 2017;8:1129.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kovanen PT, Bot I.. Mast cells in atherosclerotic cardiovascular disease—activators and actions. Eur J Pharmacol 2017;816:37–46. [DOI] [PubMed] [Google Scholar]
- 146.Tabas I, Lichtman AH.. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017;47:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wolf D, Ley K.. Immunity and inflammation in atherosclerosis. Circ Res 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A.. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res 2014;114:1640–1660. [DOI] [PubMed] [Google Scholar]
- 149.Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH.. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 2013;127:1028–1039. [DOI] [PubMed] [Google Scholar]
- 150.Sage AP, Tsiantoulas D, Binder CJ, Mallat Z.. The role of B cells in atherosclerosis. Nat Rev Cardiol 2019;16:180–196. [DOI] [PubMed] [Google Scholar]
- 151.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ.. B cells and humoral immunity in atherosclerosis. Circ Res 2014;114:1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, Nietzsche S, Westermann M, Peng L, Hu D, Bontha SV, Srikakulapu P, Beer M, Megens RTA, Steffens S, Hildner M, Halder LD, Eckstein HH, Pelisek J, Herms J, Roeber S, Arzberger T, Borodovsky A, Habenicht L, Binder CJ, Weber C, Zipfel PF, Skerka C, Habenicht AJR.. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med 2019;25:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jukema RA, Ahmed TAN, Tardif JC.. Does low-density lipoprotein cholesterol induce inflammation? If so, does it matter? Current insights and future perspectives for novel therapies. BMC Med 2019;17:197.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJCANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 155.Arandjelovic S, Ravichandran KS.. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 2015;16:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poon IK, Lucas CD, Rossi AG, Ravichandran KS.. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 2014;14:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ.. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016;536:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I.. Immunosuppressive effects of apoptotic cells. Nature 1997;390:350–351. [DOI] [PubMed] [Google Scholar]
- 159.Dalli J, Serhan CN.. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012;120:e60–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yurdagul A Jr, Doran AC, Cai B, Fredman G, Tabas IA.. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med 2018;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z.. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol 2008;28:1429–1431. [DOI] [PubMed] [Google Scholar]
- 162.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I.. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arterioscler Thromb Vasc Biol 2008;28:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G, Tabas I.. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest 2017;127:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z.. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 2007;115:2168–2177. [DOI] [PubMed] [Google Scholar]
- 165.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO.. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2009;120:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF, Fazio S.. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol 2010;30:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yancey PG, Ding Y, Fan D, Blakemore JL, Zhang Y, Ding L, Zhang J, Linton MF, Fazio S.. Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation 2011;124:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S.. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res 2007;100:670–677. [DOI] [PubMed] [Google Scholar]
- 169.Gruber S, Hendrikx T, Tsiantoulas D, Ozsvar-Kozma M, Goderle L, Mallat Z, Witztum JL, Shiri-Sverdlov R, Nitschke L, Binder CJ.. Sialic Acid-binding immunoglobulin-like Lectin G promotes atherosclerosis and liver inflammation by suppressing the protective functions of B-1 cells. Cell Rep 2016;14:2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA.. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 2015;35:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, Fong R, Woo YJ, Liu B, Montgomery SB, Wu JC, Zhu K, Chang R, Alamprese M, Tallquist MD, Kim JB, Quertermous T.. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med 2019;25:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abela GS, Aziz K, Vedre A, Pathak DR, Talbott JD, Dejong J.. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol 2009;103:959–968. [DOI] [PubMed] [Google Scholar]
- 173.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R.. Update on acute coronary syndromes: the pathologists' view. Eur Heart J 2013;34:719–728. [DOI] [PubMed] [Google Scholar]
- 174.Dai J, Xing L, Jia H, Zhu Y, Zhang S, Hu S, Lin L, Ma L, Liu H, Xu M, Ren X, Yu H, Li L, Zou Y, Zhang S, Mintz GS, Hou J, Yu B.. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J 2018;39:2077–2085. [DOI] [PubMed] [Google Scholar]
- 175.Iannaccone M, Quadri G, Taha S, D'Ascenzo F, Montefusco A, Omede P, Jang IK, Niccoli G, Souteyrand G, Yundai C, Toutouzas K, Benedetto S, Barbero U, Annone U, Lonni E, Imori Y, Biondi-Zoccai G, Templin C, Moretti C, Luscher TF, Gaita F.. Prevalence and predictors of culprit plaque rupture at OCT in patients with coronary artery disease: a meta-analysis. Eur Heart J Cardiovasc Imaging 2016;17:1128–1137. [DOI] [PubMed] [Google Scholar]
- 176.Pasterkamp G, den Ruijter HM, Libby P.. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat Rev Cardiol 2017;14:21–29. [DOI] [PubMed] [Google Scholar]
- 177.Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P.. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ Res 2017;121:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Virmani R, Burke AP, Farb A, Kolodgie FD.. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47(8 Suppl):C13–C18. [DOI] [PubMed] [Google Scholar]
- 179.Kwak BR, Back M, Bochaton-Piallat ML, Caligiuri G, Daemen MJ, Davies PF, Hoefer IE, Holvoet P, Jo H, Krams R, Lehoux S, Monaco C, Steffens S, Virmani R, Weber C, Wentzel JJ, Evans PC.. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 2014;35:3013–320, 3020a–3020d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vervueren PL, Elbaz M, Dallongeville J, Arveiler D, Ruidavets JB, Montaye M, Wagner A, Amouyel P, Haas B, Bongard V, Ferrieres J.. Relationships between chronic use of statin therapy, presentation of acute coronary syndromes and one-year mortality after an incident acute coronary event. Int J Cardiol 2013;163:102–104. [DOI] [PubMed] [Google Scholar]
- 181.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW.. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–1374. [DOI] [PubMed] [Google Scholar]
- 182.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R.. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276–1282. [DOI] [PubMed] [Google Scholar]
- 183.Kataoka Y, Hammadah M, Puri R, Duggal B, Uno K, Kapadia SR, Murat Tuzcu E, Nissen SE, Nicholls SJ.. Plaque microstructures in patients with coronary artery disease who achieved very low low-density lipoprotein cholesterol levels. Atherosclerosis 2015;242:490–495. [DOI] [PubMed] [Google Scholar]
- 184.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jorgensen HF.. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res 2016;119:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jacobsen K, Lund MB, Shim J, Gunnersen S, Fuchtbauer EM, Kjolby M, Carramolino L, Bentzon JF.. Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight 2017;2:e95890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chung IM, Schwartz SM, Murry CE.. Clonal architecture of normal and atherosclerotic aorta: implications for atherogenesis and vascular development. Am J Pathol 1998;152:913–923. [PMC free article] [PubMed] [Google Scholar]
- 187.Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer G.. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 2018;114:622–634. [DOI] [PubMed] [Google Scholar]
- 188.Johnson JL. Metalloproteinases in atherosclerosis. Eur J Pharmacol 2017;816:93–106. [DOI] [PubMed] [Google Scholar]
- 189.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM.. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016;354:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM.. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 2017;16:718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Postmus AC, Sturmlechner I, Jonker JW, van Deursen JM, van de Sluis B, Kruit JK.. Senescent cells in the development of cardiometabolic disease. Curr Opin Lipidol 2019;30:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abedin M, Tintut Y, Demer LL.. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 2004;24:1161–1170. [DOI] [PubMed] [Google Scholar]
- 193.Liu Y, Shanahan CM.. Signalling pathways and vascular calcification. Front Biosci (Landmark Ed) 2011;16:1302–1314. [DOI] [PubMed] [Google Scholar]
- 194.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS.. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–1870. [DOI] [PubMed] [Google Scholar]
- 195.Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, Budoff MJ.. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging 2012;5:990–999. [DOI] [PubMed] [Google Scholar]
- 196.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL.. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol 1997;17:680–687. [DOI] [PubMed] [Google Scholar]
- 197.Proudfoot D, Davies JD, Skepper JN, Weissberg PL, Shanahan CM.. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation 2002;106:3044–3050. [DOI] [PubMed] [Google Scholar]
- 198.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL.. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 2002;105:650–655. [DOI] [PubMed] [Google Scholar]
- 199.Tintut Y, Patel J, Parhami F, Demer LL.. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000;102:2636–2642. [DOI] [PubMed] [Google Scholar]
- 200.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA.. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol 2007;27:2589–2596. [DOI] [PubMed] [Google Scholar]
- 201.Morris TG, Borland SJ, Clarke CJ, Wilson C, Hannun YA, Ohanian V, Canfield AE, Ohanian J.. Sphingosine 1-phosphate activation of ERM contributes to vascular calcification. J Lipid Res 2018;59:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cholesterol Treatment Trialists Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dykun I, Lehmann N, Kalsch H, Mohlenkamp S, Moebus S, Budde T, Seibel R, Gronemeyer D, Jockel KH, Erbel R, Mahabadi AA.. Statin medication enhances progression of coronary artery calcification: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 2016;68:2123–2125. [DOI] [PubMed] [Google Scholar]
- 204.Puri R, Libby P, Nissen SE, Wolski K, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Raichlen JS, Uno K, Kataoka Y, Tuzcu EM, Nicholls SJ.. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging 2014;15:380–388. [DOI] [PubMed] [Google Scholar]
- 205.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE.. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273–1282. [DOI] [PubMed] [Google Scholar]
- 206.Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Lee BK, Chun EJ, Cademartiri F, Maffei E, Marques H, Leipsic JA, Shin S, Choi JH, Chinnaiyan K, Raff G, Virmani R, Samady H, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK.. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging 2018;11:1475–1484. [DOI] [PubMed] [Google Scholar]
- 207.Aengevaeren VL, Mosterd A, Braber TL, Prakken NHJ, Doevendans PA, Grobbee DE, Thompson PD, Eijsvogels TMH, Velthuis BK.. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 2017;136:138–148. [DOI] [PubMed] [Google Scholar]
- 208.Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, David S, Yeo TJ, Narain R, Malhotra A, Papadakis M, Wilson MG, Tome M, AlFakih K, Moon JC, Sharma S.. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation 2017;136:126–137. [DOI] [PubMed] [Google Scholar]
- 209.DeFina LF, Radford NB, Barlow CE, Willis BL, Leonard D, Haskell WL, Farrell SW, Pavlovic A, Abel K, Berry JD, Khera A, Levine BD.. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol 2019;4:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol 2009;297:H802–H810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Duer MJ, Friscic T, Proudfoot D, Reid DG, Schoppet M, Shanahan CM, Skepper JN, Wise ER, Mineral surface in calcified plaque is like that of bone: further evidence for regulated mineralization. Arterioscler Thromb Vasc Biol 2008;28:2030–2034. [DOI] [PubMed] [Google Scholar]
- 212.Mauriello A, Servadei F, Zoccai GB, Giacobbi E, Anemona L, Bonanno E, Casella S. Coronary calcification identifies the vulnerable patient rather than the vulnerable plaque. Atherosclerosis 2013;229:124–129. [DOI] [PubMed] [Google Scholar]
- 213.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319–326. [DOI] [PubMed] [Google Scholar]
- 214.Nerlekar N, Ha FJ, Cheshire C, Rashid H, Cameron JD, Wong DT, Seneviratne S, Brown AJ. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2018;11:e006973.. [DOI] [PubMed] [Google Scholar]
- 215.Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, Shah ASV, Pawade T, Weir-McCall JR, Roditi G, van Beek EJR, Newby DE, Nicol ED. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.van der Wall EE, de Graaf FR, van Velzen JE, Jukema JW, Bax JJ, Schuijf JD. IVUS detects more coronary calcifications than MSCT; matter of both resolution and cross-sectional assessment? Int J Cardiovasc Imaging 2011;27:1011–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pontone G, Bertella E, Mushtaq S, Loguercio M, Cortinovis S, Baggiano A, Conte E, Annoni A, Formenti A, Beltrama V, Guaricci AI, Andreini D. Coronary artery disease: diagnostic accuracy of CT coronary angiography—a comparison of high and standard spatial resolution scanning. Radiology 2014;271:688–694. [DOI] [PubMed] [Google Scholar]
- 218.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hsu JJ, Lu J, Umar S, Lee JT, Kulkarni RP, Ding Y, Chang CC, Hsiai TK, Hokugo A, Gkouveris I, Tetradis S, Nishimura I, Demer LL, Tintut Y. Effects of teriparatide on morphology of aortic calcification in aged hyperlipidemic mice. Am J Physiol Heart Circ Physiol 2018;314:H1203–H1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW, Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR, Newby DE. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–713. [DOI] [PubMed] [Google Scholar]
- 221.Creager MD, Hohl T, Hutcheson JD, Moss AJ, Schlotter F, Blaser MC, Park MA, Lee LH, Singh SA, Alcaide-Corral CJ, Tavares AAS, Newby DE, Kijewski MF, Aikawa M, Di Carli M, Dweck MR, Aikawa E. (18)F-fluoride signal amplification identifies microcalcifications associated with atherosclerotic plaque instability in Positron emission tomography/computed tomography images. Circ Cardiovasc Imaging 2019;12:e007835.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging 2018;11:127–142. [DOI] [PubMed] [Google Scholar]
- 223.Raggi P, Senior P, Shahbaz S, Kaul P, Hung R, Coulden R, Yeung R, Abele J. (18)F-sodium fluoride imaging of coronary atherosclerosis in ambulatory patients with diabetes mellitus. Arterioscler Thromb Vasc Biol 2019;39:276–284. [DOI] [PubMed] [Google Scholar]
- 224.Akers EJ, Nicholls SJ, Di Bartolo BA. Plaque calcification: do lipoproteins have a role? Arterioscler Thromb Vasc Biol 2019;39:1902–1910. [DOI] [PubMed] [Google Scholar]
- 225.Pohle K, MäFfert R, Ropers D, Moshage W, Stilianakis N, Daniel WG, Achenbach S. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation 2001;104:1927–1932. [DOI] [PubMed] [Google Scholar]
- 226.Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, Reszka AA. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ 2004;11(Suppl 1):S108–S118. [DOI] [PubMed] [Google Scholar]
- 227.Greif M, Arnoldt T, von Ziegler F, Ruemmler J, Becker C, Wakili R, D'Anastasi M, Schenzle J, Leber AW, Becker A. Lipoprotein (a) is independently correlated with coronary artery calcification. Eur J Intern Med 2013;24:75–79. [DOI] [PubMed] [Google Scholar]
- 228.Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol 2019;16:305–318. [DOI] [PubMed] [Google Scholar]
- 229.Erdmann J, Kessler T, Munoz Venegas L, Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res 2018;114:1241–1257. [DOI] [PubMed] [Google Scholar]
- 230.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Cardiogenics, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ, Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schafer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M, Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WH, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JC, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, Marz W, Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O'Donnell CJ, McPherson R, Erdmann JCARDIoGRAM ConsortiumSamani NJ. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 2011;43:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ntalla I, Kanoni S, Zeng L, Giannakopoulou O, Danesh J, Watkins H, Samani NJ, Deloukas P, Schunkert HUK Biobank CardioMetabolic Consortium CHD Working Group. Genetic risk score for coronary disease identifies predispositions to cardiovascular and noncardiovascular diseases. J Am Coll Cardiol 2019;73:2932–2942. [DOI] [PubMed] [Google Scholar]
- 232.Chen S, Wang X, Wang J, Zhao Y, Wang D, Tan C, Fa J, Zhang R, Wang F, Xu C, Huang Y, Li S, Yin D, Xiong X, Li X, Chen Q, Tu X, Yang Y, Xia Y, Xu C, Wang QK. Genomic variant in CAV1 increases susceptibility to coronary artery disease and myocardial infarction. Atherosclerosis 2016;246:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann H-E, Barrett JH, König IR, Stevens SE, Szymczak S, Tregouet D-A, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Brænne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert HWTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med 2007;357:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brænne I, Civelek M, Vilne B, Di Narzo A, Johnson AD, Zhao Y, Reiz B, Codoni V, Webb TR, Foroughi Asl H, Hamby SE, Zeng L, Trégouët D-A, Hao K, Topol EJ, Schadt EE, Yang X, Samani NJ, Björkegren JLM, Erdmann J, Schunkert H, Lusis AJ; Leducq Consortium CAD Genomics. Prediction of causal candidate genes in coronary artery disease loci. Arterioscler Thromb Vasc Biol 2015;35:2207–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Howson JMM, Zhao W, Barnes DR, Ho WK, Young R, Paul DS, Waite LL, Freitag DF, Fauman EB, Salfati EL, Sun BB, Eicher JD, Johnson AD, Sheu WHH, Nielsen SF, Lin WY, Surendran P, Malarstig A, Wilk JB, Tybjaerg-Hansen A, Rasmussen KL, Kamstrup PR, Deloukas P, Erdmann J, Kathiresan S, Samani NJ, Schunkert H, Watkins HCARDIoGRAMplusC4DDo R, Rader DJ, Johnson JA, Hazen SL, Quyyumi AA, Spertus JA, Pepine CJ, Franceschini N, Justice A, Reiner AP, Buyske S, Hindorff LA, Carty CL, North KE, Kooperberg C, Boerwinkle E, Young K, Graff M, Peters U, Absher D, Hsiung CA, Lee WJ, Taylor KD, Chen YH, Lee IT, Guo X, Chung RH, Hung YJ, Rotter JI, Juang JJ, Quertermous T, Wang TD, Rasheed A, Frossard P, Alam DS, Majumder AAS, Di Angelantonio E, Chowdhury R, Epic CVD, Chen YI, Nordestgaard BG, Assimes TL, Danesh J, Butterworth AS, Saleheen D. Fifteen new risk loci for coronary artery disease highlight arterial-wall-specific mechanisms. Nat Genet 2017;49:1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Klarin D, Zhu QM, Emdin CA, Chaffin M, Horner S, McMillan BJ, Leed A, Weale ME, Spencer CCA, Aguet F, Segre AV, Ardlie KG, Khera AV, Kaushik VK, Natarajan PCARDIoGRAMplusC4D ConsortiumKathiresan S. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet 2017;49:1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, Zeng L, Ntalla I, Lai FY, Hopewell JC, Giannakopoulou O, Jiang T, Hamby SE, Di Angelantonio E, Assimes TL, Bottinger EP, Chambers JC, Clarke R, Palmer CNA, Cubbon RM, Ellinor P, Ermel R, Evangelou E, Franks PW, Grace C, Gu D, Hingorani AD, Howson JMM, Ingelsson E, Kastrati A, Kessler T, Kyriakou T, Lehtimaki T, Lu X, Lu Y, Marz W, McPherson R, Metspalu A, Pujades-Rodriguez M, Ruusalepp A, Schadt EE, Schmidt AF, Sweeting MJ, Zalloua PA, AlGhalayini K, Keavney BD, Kooner JS, Loos RJF, Patel RS, Rutter MK, Tomaszewski M, Tzoulaki I, Zeggini E, Erdmann J, Dedoussis G, Bjorkegren JLMEPIC-CVD Consortium; CARDIoGRAMplusC4D; UK Biobank CardioMetabolic Consortium CHD working groupSchunkert H, Farrall M, Danesh J, Samani NJ, Watkins H, Deloukas P. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet 2017;49:1385–1391. [DOI] [PubMed] [Google Scholar]
- 239.van Rijssel J, van Buul JD. The many faces of the guanine-nucleotide exchange factor trio. Cell Adh Migr 2012;6:482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Samson T, van Buul JD, Kroon J, Welch C, Bakker EN, Matlung HL, van den Berg TK, Sharek L, Doerschuk C, Hahn K, Burridge K. The guanine-nucleotide exchange factor SGEF plays a crucial role in the formation of atherosclerosis. PLoS One 2013;8:e55202.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, García-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol 2007;178:1279–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chai JT, Ruparelia N, Goel A, Kyriakou T, Biasiolli L, Edgar L, Handa A, Farrall M, Watkins H, Choudhury RP. Differential gene expression in macrophages from human atherosclerotic plaques shows convergence on pathways implicated by genome-wide association study risk variants. Arterioscler Thromb Vasc Biol 2018;38:2718–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, Taylor JM, Pitas RE. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J Clin Invest 1995;96:2170–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Boyle EA, Li YI, Pritchard JK. An expanded view of complex traits: from polygenic to omnigenic. Cell 2017;169:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Schaar JA, Muller JE, Falk E, Virmani R, Fuster V, Serruys PW, Colombo A, Stefanadis C, Ward Casscells S, Moreno PR, Maseri A, van der Steen AF. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J 2004;25:1077–1082. [DOI] [PubMed] [Google Scholar]
- 246.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 247.Elia E, Montecucco F, Portincasa P, Sahebkar A, Mollazadeh H, Carbone F. Update on pathological platelet activation in coronary thrombosis. J Cell Physiol 2019;234:2121–2133. [DOI] [PubMed] [Google Scholar]
- 248.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 2014;276:618–632. [DOI] [PubMed] [Google Scholar]
- 249.Owens AP 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, Williams JC, Hubbard BK, Dutton JA, Wang J, Tobias PS, Curtiss LK, Daugherty A, Kirchhofer D, Luyendyk JP, Moriarty PM, Nagarajan S, Furie BC, Furie B, Johns DG, Temel RE, Mackman N. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest 2012;122:558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Obermayer G, Afonyushkin T, Binder CJ. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost 2018;16:418–428. [DOI] [PubMed] [Google Scholar]
- 251.Lesnik P, Rouis M, Skarlatos S, Kruth HS, Chapman MJ. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc Natl Acad Sci USA 1992;89:10370–10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Petit L, Lesnik P, Dachet C, Moreau M, Chapman MJ. Tissue factor pathway inhibitor is expressed by human monocyte-derived macrophages: relationship to tissue factor induction by cholesterol and oxidized LDL. Arterioscler Thromb Vasc Biol 1999;19:309–315. [DOI] [PubMed] [Google Scholar]
- 253.Doi H, Kugiyama K, Oka H, Sugiyama S, Ogata N, Koide S-I, Nakamura S-I, Yasue H. Remnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanism. Circulation 2000;102:670–676. [DOI] [PubMed] [Google Scholar]
- 254.Owens AP 3rd, Mackman N. Tissue factor and thrombosis: the clot starts here. Thromb Haemost 2010;104:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chatterjee M, Rath D, Schlotterbeck J, Rheinlaender J, Walker-Allgaier B, Alnaggar N, Zdanyte M, Müller I, Borst O, Geisler T, Schäffer TE, Lämmerhofer M, Gawaz M. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur Heart J 2017;38:1993–2005. [DOI] [PubMed] [Google Scholar]
- 256.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res 2008;102:1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chan HC, Ke LY, Chu CS, Lee AS, Shen MY, Cruz MA, Hsu JF, Cheng KH, Chan HC, Lu J, Lai WT, Sawamura T, Sheu SH, Yen JH, Chen CH. Highly electronegative LDL from patients with ST-elevation myocardial infarction triggers platelet activation and aggregation. Blood 2013;122:3632–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol 2012;9:439–453. [DOI] [PubMed] [Google Scholar]
- 259.Brown GT, McIntyre TM. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1beta-rich microparticles. J Immunol 2011;186:5489–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ardlie NG, Selley ML, Simons LA. Platelet activation by oxidatively modified low density lipoproteins. Atherosclerosis 1989;76:117–124. [DOI] [PubMed] [Google Scholar]
- 261.Chen YC, Huang AL, Kyaw TS, Bobik A, Peter K. Atherosclerotic plaque rupture: identifying the straw that breaks the Camel's back. Arterioscler Thromb Vasc Biol 2016;36:e63–72. [DOI] [PubMed] [Google Scholar]
- 262.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–671. [DOI] [PubMed] [Google Scholar]
- 263.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 264.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988;12:56–62. [DOI] [PubMed] [Google Scholar]
- 265.Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, Santamore WP. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988;78(5 Pt 1):1157–1166. [DOI] [PubMed] [Google Scholar]
- 266.Vergallo R, Porto I, D’Amario D, Annibali G, Galli M, Benenati S, Bendandi F, Migliaro S, Fracassi F, Aurigemma C, Leone AM, Buffon A, Burzotta F, Trani C, Niccoli G, Liuzzo G, Prati F, Fuster V, Jang I-K, Crea F. Coronary atherosclerotic phenotype and plaque healing in patients with recurrent acute coronary syndromes compared with patients with long-term clinical stability: an in vivo optical coherence tomography study. JAMA Cardiol 2019;4:321.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015;36:1377–1384. [DOI] [PubMed] [Google Scholar]
- 268.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, Investigators P. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 269.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med 1990;323:1289–1298. [DOI] [PubMed] [Google Scholar]
- 270.Almeida SO, Budoff M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med 2019;29:431–455. [DOI] [PubMed] [Google Scholar]
- 271.Andelius L, Mortensen MB, Norgaard BL, Abdulla J. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2018;19:850–858. [DOI] [PubMed] [Google Scholar]
- 272.Hattori K, Ozaki Y, Ismail TF, Okumura M, Naruse H, Kan S, Ishikawa M, Kawai T, Ohta M, Kawai H, Hashimoto T, Takagi Y, Ishii J, Serruys PW, Narula J. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging 2012;5:169–177. [DOI] [PubMed] [Google Scholar]
- 273.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TRFOURIER Steering Committee and Investigators. .Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 274.Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 275.Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJP, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Honda S, Shishikura D, Scherer DJ, Borgman M, Brennan DM, Wolski K, Nissen SE. Effect of evolocumab on coronary plaque composition. J Am Coll Cardiol 2018;72:2012–2021. [DOI] [PubMed] [Google Scholar]
- 276.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res 2004;95:764–772. [DOI] [PubMed] [Google Scholar]
- 277.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med 2011;17:594–603. [DOI] [PubMed] [Google Scholar]
- 278.Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res 2014;55:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Orsoni A, Therond P, Tan R, Giral P, Robillard P, Kontush A, Meikle PJ, Chapman MJ. Statin action enriches HDL3 in polyunsaturated phospholipids and plasmalogens and reduces LDL-derived phospholipid hydroperoxides in atherogenic mixed dyslipidemia. J Lipid Res 2016;57:2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Speer T, Zewinger S. High-density lipoprotein (HDL) and infections: a versatile culprit. Eur Heart J 2018;39:1191–1193. [DOI] [PubMed] [Google Scholar]
- 281.Genest G, Genest J. High-density lipoproteins and inflammatory diseases: full circle ahead. Clin Chem 2019;65:607–608. [DOI] [PubMed] [Google Scholar]
- 282.Huang Y, DiDonato JA, Levison BS, Schmitt D, Li L, Wu Y, Buffa J, Kim T, Gerstenecker GS, Gu X, Kadiyala CS, Wang Z, Culley MK, Hazen JE, Didonato AJ, Fu X, Berisha SZ, Peng D, Nguyen TT, Liang S, Chuang CC, Cho L, Plow EF, Fox PL, Gogonea V, Tang WH, Parks JS, Fisher EA, Smith JD, Hazen SL. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med 2014;20:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Luscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011;121:2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Huang Y, Wu Z, Riwanto M, Gao S, Levison BS, Gu X, Fu X, Wagner MA, Besler C, Gerstenecker G, Zhang R, Li XM, DiDonato AJ, Gogonea V, Tang WH, Smith JD, Plow EF, Fox PL, Shih DM, Lusis AJ, Fisher EA, DiDonato JA, Landmesser U, Hazen SL. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest 2013;123:3815–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 286.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 287.Do R, Willer CJ, Schmidt EM,, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J,, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL,, Chang HY,, Demirkan A,, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM,, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM,, O'Connell JR, Palmer CD,, Perola M,, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G,, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Doring A, Elliott P,, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ,, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ,, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimaki T,, Lin SY, Lindstrom J, Loos RJ,, Mach F, McArdle WL, Meisinger C,, Mitchell BD, Muller G, Nagaraja R,, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A,, Rader DJ,, Reilly MP, Ridker PM,, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stancakova A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB,, Ferrieres J,, Ferrucci L,, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB,, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE,, Hunt SC, Hveem K, Iribarren C,, Jarvelin MR,, Jula A, Kahonen M, Kaprio J, Kesaniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D,, Kuusisto J, Kyvik KO, Laakso M, Lakka TA,, Lind L,, Lindgren CM, Martin NG,, Marz W, McCarthy MI,, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njolstad I, Pedersen NL,, Power C, Pramstaller PP,, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P,, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS,, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013;45:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478–2486. [DOI] [PubMed] [Google Scholar]
- 289.Ko DT, Alter DA, Guo H, Koh M, Lau G, Austin PC, Booth GL, Hogg W, Jackevicius CA, Lee DS, Wijeysundera HC, Wilkins JT, Tu JV. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART Study. J Am Coll Cardiol 2016;68:2073–2083. [DOI] [PubMed] [Google Scholar]
- 290.Gordon DJ, Rifkind BM. High-density lipoprotein–the clinical implications of recent studies. N Engl J Med 1989;321:1311–1316. [DOI] [PubMed] [Google Scholar]
- 291.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC; Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301–1310. [DOI] [PubMed] [Google Scholar]
- 292.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012;380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532. [DOI] [PubMed] [Google Scholar]
- 294.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61:427–436. [DOI] [PubMed] [Google Scholar]
- 295.Badimon JJ, Badimon L, Galvez A, Dische R, Fuster V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest 1989;60:455–461. [PubMed] [Google Scholar]
- 296.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 1990;85:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nicholls SJ, Cutri B, Worthley SG, Kee P, Rye KA, Bao S, Barter PJ. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005;25:2416–2421. [DOI] [PubMed] [Google Scholar]
- 298.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA 1994;91:9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991;353:265–267. [DOI] [PubMed] [Google Scholar]
- 300.Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation 2001;104:2447–2452. [DOI] [PubMed] [Google Scholar]
- 301.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol 2013;33:1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Khera AV, Rader DJ. Cholesterol efflux capacity: full steam ahead or a bump in the road? Arterioscler Thromb Vasc Biol 2013;33:1449–1451. [DOI] [PubMed] [Google Scholar]
- 304.Shea S, Stein JH, Jorgensen NW, McClelland RL, Tascau L, Shrager S, Heinecke JW, Yvan-Charvet L, Tall AR. Cholesterol mass efflux capacity, incident cardiovascular disease, and progression of carotid plaque. Arterioscler Thromb Vasc Biol 2019;39:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007;297:499–508. [DOI] [PubMed] [Google Scholar]
- 306.Cui Y, Watson DJ, Girman CJ, Shapiro DR, Gotto AM, Hiserote P, Clearfield MB. Effects of increasing high-density lipoprotein cholesterol and decreasing low-density lipoprotein cholesterol on the incidence of first acute coronary events (from the Air Force/Texas Coronary Atherosclerosis Prevention Study). Am J Cardiol 2009;104:829–834. [DOI] [PubMed] [Google Scholar]
- 307.Honda S, Sidharta SL, Shishikura D, Takata K, Di Giovanni GA, Nguyen T, Janssan A, Kim SW, Andrews J, Psaltis PJ, Worthley MI, Nicholls SJ. High-density lipoprotein cholesterol associated with change in coronary plaque lipid burden assessed by near infrared spectroscopy. Atherosclerosis 2017;265:110–116. [DOI] [PubMed] [Google Scholar]
- 308.Madsen CM, Varbo A, Tybjærg-Hansen A, Frikke-Schmidt R, Nordestgaard BG. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J 2018;39:1181–1190. [DOI] [PubMed] [Google Scholar]
- 309.Madsen CM, Varbo A, Nordestgaard BG. Low HDL cholesterol and high risk of autoimmune disease: two population-based cohort studies including 117341 individuals. Clin Chem 2019;65:644–652. [DOI] [PubMed] [Google Scholar]
- 310.Stahlman M, Fagerberg B, Adiels M, Ekroos K, Chapman JM, Kontush A, Boren J. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the HDL lipidome in type 2 diabetic subjects in the DIWA cohort: impact on small HDL particles. Biochim Biophys Acta 2013;1831:1609–1617. [DOI] [PubMed] [Google Scholar]
- 311.Nordestgaard BG, Nicholls SJ, Langsted A, Ray KK, Tybjærg-Hansen A. Advances in lipid-lowering therapy through gene-silencing technologies. Nat Rev Cardiol 2018;15:261–272. [DOI] [PubMed] [Google Scholar]
- 312.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 313.Mead JR, Ramji DP. The pivotal role of lipoprotein lipase in atherosclerosis. Cardiovasc Res 2002;55:261–269. [DOI] [PubMed] [Google Scholar]
- 314.Pentikainen MO, Oksjoki R, Oorni K, Kovanen PT. Lipoprotein lipase in the arterial wall: linking LDL to the arterial extracellular matrix and much more. Arterioscler Thromb Vasc Biol 2002;22:211–217. [DOI] [PubMed] [Google Scholar]
- 315.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 316.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators Stitziel NO, Stirrups KE, Masca NG, Erdmann J, Ferrario PG,, Konig IR, Weeke PE, Webb TR, Auer PL, Schick UM, Lu Y, Zhang H, Dube MP,, Goel A, Farrall M,, Peloso GM, Won HH, Do R, van Iperen E, Kanoni S, Kruppa J, Mahajan A,, Scott RA, Willenberg C, Braund PS, van Capelleveen JC, Doney AS, Donnelly LA, Asselta R, Merlini PA,, Duga S, Marziliano N, Denny JC, Shaffer CM, El-Mokhtari NE, Franke A, Gottesman O, Heilmann S, Hengstenberg C, Hoffman P, Holmen OL, Hveem K, Jansson JH, Jockel KH, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, McCarthy MI, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alver M, Moebus S, Morris AD,, Muller-Nurasyid M, Nikpay M, Olivieri O,, Lemieux Perreault A, AlQarawi LP, Robertson NR, Akinsanya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C,, Jackson RD, Stahl E, Strauch K, Varga TV, Waldenberger M, Zeng L, Kraja AT, Liu C, Ehret GB, Newton-Cheh C, Chasman DI, Chowdhury R, Ferrario M, Ford I, Jukema JW, Kee F, Kuulasmaa K, Nordestgaard BG, Perola M, Saleheen D, Sattar N, Surendran P, Tregouet D, Young R, Howson JM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP,, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho-Melander M, Melander O, Metspalu A,, Palmer CN, Peters A, Rader D, Reilly MP,, Loos RJ,, Reiner AP, Roden DM, Tardif JC, Thompson JR, Wareham NJ,, Watkins H, Willer CJ, Kathiresan S, Deloukas P,, Samani NJ, Schunkert H. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med 2016;374:1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Do R, Stitziel NO, Won HH, Jorgensen AB, Duga S, Angelica Merlini P, Kiezun A, Farrall M, Goel A, Zuk O, Guella I, Asselta R, Lange LA, Peloso GM, Auer PLNHLBI Exome Sequencing ProjectGirelli D, Martinelli N, Farlow DN, DePristo MA, Roberts R, Stewart AF, Saleheen D, Danesh J, Epstein SE, Sivapalaratnam S, Hovingh GK, Kastelein JJ, Samani NJ, Schunkert H, Erdmann J, Shah SH, Kraus WE, Davies R, Nikpay M, Johansen CT, Wang J, Hegele RA, Hechter E, Marz W, Kleber ME, Huang J, Johnson AD, Li M, Burke GL, Gross M, Liu Y, Assimes TL, Heiss G, Lange EM, Folsom AR, Taylor HA, Olivieri O, Hamsten A, Clarke R, Reilly DF, Yin W, Rivas MA, Donnelly P, Rossouw JE, Psaty BM, Herrington DM, Wilson JG, Rich SS, Bamshad MJ, Tracy RP, Cupples LA, Rader DJ, Reilly MP, Spertus JA, Cresci S, Hartiala J, Tang WH, Hazen SL, Allayee H, Reiner AP, Carlson CS, Kooperberg C, Jackson RD, Boerwinkle E, Lander ES, Schwartz SM, Siscovick DS, McPherson R, Tybjaerg-Hansen A, Abecasis GR, Watkins H, Nickerson DA, Ardissino D, Sunyaev SR, O'Donnell CJ, Altshuler D, Gabriel S, Kathiresan S. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015;518:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, Ginsberg H, Hiatt WR, Ishibashi S, Koenig W, Nordestgaard BG, Fruchart JC, Libby P, Ridker PM. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J 2018;206:80–93. [DOI] [PubMed] [Google Scholar]
- 319.Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham MJ, Crooke RM, Witztum JL, Brunzell JD, Kastelein JJ. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med 2015;373:438–447. [DOI] [PubMed] [Google Scholar]
- 320.Dewey FE, Gusarova V, Dunbar RL, O'Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D,, Herman GA, Sheu WHH,, Lee IT, Liang KW, Guo X, Rotter JI, Chen YI, Kraus WE, Shah SH, Damrauer S, Small A,, Rader DJ, Wulff AB, Nordestgaard BG, Tybjaerg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ,, Gromada J, Baras A. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017;377:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–232. [DOI] [PubMed] [Google Scholar]
- 322.Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013;128:1298–1309. [DOI] [PubMed] [Google Scholar]
- 323.Utermann G. The mysteries of lipoprotein (a). Science 1989;246:904–910. [DOI] [PubMed] [Google Scholar]
- 324.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med 2013;273:6–30. [DOI] [PubMed] [Google Scholar]
- 325.Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res 2016;57:745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol 2014;63:470–477. [DOI] [PubMed] [Google Scholar]
- 327.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke 2007;38:1959–1966. [DOI] [PubMed] [Google Scholar]
- 328.Beheshtian A, Shitole SG, Segal AZ, Leifer D, Tracy RP, Rader DJ, Devereux RB, Kizer JR. Lipoprotein (a) level, apolipoprotein (a) size, and risk of unexplained ischemic stroke in young and middle-aged adults. Atherosclerosis 2016;253:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M; PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 2009;361:2518–2528. [DOI] [PubMed] [Google Scholar]
- 330.Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J 2019;40:2760–2770. [DOI] [PubMed] [Google Scholar]
- 331.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, Witztum JL, Stroes ES, Tsimikas S. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–2253. [DOI] [PubMed] [Google Scholar]
- 332.Gaudet D, Watts GF, Robinson JG, Minini P, Sasiela WJ, Edelberg J, Louie MJ, Raal FJ. Effect of alirocumab on lipoprotein(a) over ≥1.5 years (from the Phase 3 ODYSSEY Program). Am J Cardiol 2017;119:40–46. [DOI] [PubMed] [Google Scholar]
- 333.O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, Im K, Lira Pineda A, Wasserman SM, Ceska R, Ezhov MV, Jukema JW, Jensen HK, Tokgozoglu SL, Mach F, Huber K, Sever PS, Keech AC, Pedersen TR, Sabatine MS. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 2019;139:1483–1492. [DOI] [PubMed] [Google Scholar]
- 334.Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol 2018;72:1141–1156. [DOI] [PubMed] [Google Scholar]
- 335.Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJ, Hutten BA. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med 2019;381:1547–1556. [DOI] [PubMed] [Google Scholar]
- 336.Landmesser U, Chapman MJ, Farnier M, Gencer B, Gielen S, Hovingh GK, Luscher TF, Sinning D, Tokgozoglu L, Wiklund O, Zamorano JL, Pinto FJ, Catapano AL; European Society of Cardiology (ESC); European Atherosclerosis Society (EAS). European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J 2017;38:2245–2255. [DOI] [PubMed] [Google Scholar]
- 337.Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 2014;168:682–689. [DOI] [PubMed] [Google Scholar]
- 338.Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Ruzza A, Ma Y, Hamer AW, Wasserman SM, Raal FJ. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol 2019;74:2132–2146. [DOI] [PubMed] [Google Scholar]
- 339.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Authors/Task Force Members, Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;253:281–344. [DOI] [PubMed] [Google Scholar]
- 341.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT; ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 342.Mach FESC Scientific Document GroupBaigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Mueller C, Drexel H, Aboyans V, Corsini A, Doehner W, Farnier M, Gigante B, Kayikcioglu M, Krstacic G, Lambrinou E, Lewis BS, Masip J, Moulin P, Petersen S, Petronio AS, Piepoli MF, Pintó X, Räber L, Ray KK, Reiner Ž, Riesen WF, Roffi M, Schmid J-P, Shlyakhto E, Simpson IA, Stroes E, Sudano I, Tselepis AD, Viigimaa M, Vindis C, Vonbank A, Vrablik M, Vrsalovic M, Zamorano JL, Collet J-P, Koskinas KC, Casula M, Badimon L, John Chapman M, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Nibouche D, Zelveian PH, Siostrzonek P, Najafov R, van de Borne P, Pojskic B, Postadzhiyan A, Kypris L, Špinar J, Larsen ML, Eldin HS, Viigimaa M, Strandberg TE, Ferrières J, Agladze R, Laufs U, Rallidis L, Bajnok L, Gudjónsson T, Maher V, Henkin Y, Gulizia MM, Mussagaliyeva A, Bajraktari G, Kerimkulova A, Latkovskis G, Hamoui O, Slapikas R, Visser L, Dingli P, Ivanov V, Boskovic A, Nazzi M, Visseren F, Mitevska I, Retterstøl K, Jankowski P, Fontes-Carvalho R, Gaita D, Ezhov M, Foscoli M, Giga V, Pella D, Fras Z, de Isla LP, Hagström E, Lehmann R, Abid L, Ozdogan O, Mitchenko O, Patel RS. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 343.Currie G, Delles C. Precision medicine and personalized medicine in cardiovascular disease. Adv Exp Med Biol 2018;1065:589–605. [DOI] [PubMed] [Google Scholar]