Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance (original) (raw)
Abstract
The immune system generally avoids horror autotoxicus or autoimmunity, an attack against the body's own constituents. This avoidance requires that self-reactive T cells be actively silenced or tolerized. We propose that dendritic cells (DCs) play a critical role in establishing tolerance, especially in the periphery, after functioning T cells have been produced in the thymus. In the steady state, meaning in the absence of acute infection and inflammation, DCs are in an immature state and not fully differentiated to carry out their known roles as inducers of immunity. Nevertheless, immature DCs continuously circulate through tissues and into lymphoid organs, capturing self antigens as well as innocuous environmental proteins. Recent experiments have provided direct evidence that antigen-loaded immature DCs silence T cells either by deleting them or by expanding regulatory T cells. This capacity of DCs to induce peripheral tolerance can work in two opposing ways in the context of infection. In acute infection, a beneficial effect should occur. The immune system would overcome the risk of developing autoimmunity and chronic inflammation if, before infection, tolerance were induced to innocuous environmental proteins as well as self antigens captured from dying infected cells. For chronic or persistent pathogens, a second but dire potential could take place. Continuous presentation of a pathogen by immature DCs, HIV-1 for example, may lead to tolerance and active evasion of protective immunity. The function of DCs in defining immunologic self provides a new focus for the study of autoimmunity and chronic immune-based diseases.
The experiments of Paul Ehrlich at the turn of the last century helped establish the science of immunology. In addition to his prescient findings on specific immune receptors (1), Ehrlich used a collection of stains to identify many types of white blood cells, including lymphocytes, the mediators of immunity. Ehrlich's experiments on antibodies led him to conclude that immunity is exclusively directed to foreign materials or antigens; normally there is no reactivity or tolerance to self. For example, he found that a goat made antibodies to red blood cells from other goats but not to its own red blood cells. Thus the body avoids an immune attack on itself. He states: “We pointed out that the organism possesses certain contrivances by means of which the immunity reaction, so easily produced (induced) by all kinds of cells, is prevented from acting against the organism's own elements and so giving rise to autotoxins … so that one might be justified in speaking of a ‘horror autotoxicus’ of the organism” (p. 253, ref. 2). Actually, autoimmunity does develop in many diseases, including systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, psoriasis, and juvenile diabetes.
Ehrlich suggested that self-reactive lymphocytes could be silenced or tolerized by losing their self-specific receptors (p. 208, ref. 2), a prediction that has proven correct for antibody-producing B cells (3,4). Here we propose that one type of white cell, the dendritic cell (DC), has major roles in silencing self-reactive T lymphocytes. These T cells are produced centrally in the thymus, where some self-reactive T cells are tolerized through the aegis of thymic DCs and other antigen-presenting cells (Fig. 1). Then T cells emerge into the periphery to patrol and defend the body against pathogens. Here evidence will be outlined that DCs silence peripheral T cells as well (Fig. 1). Before going over this information, two background topics need to be considered: the limitations of central thymic tolerance and the traditional function of DCs in inducing immunity to foreign antigens especially infections (5,6).
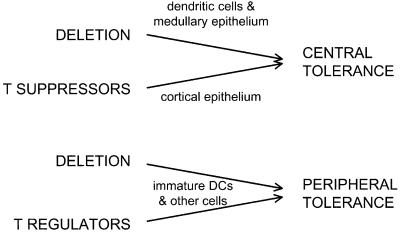
Figure 1.
Central and peripheral mechanisms for avoiding horror autotoxicus via T lymphocytes. In the thymus (central tolerance) and in other parts of the body (peripheral tolerance), self-reactive T cells can either be eliminated (deleted) or regulated (suppressed) by other T cells. Several types of antigen-presenting cells can bring about tolerance as shown by the arrows. DCs play a pervasive role, particularly for dying cells and innocuous self and environmental proteins that have to be captured and processed before presentation (as MHC class I and II–peptide complexes) to antigen receptors on T cells.
Central Tolerance
The Importance of Central Tolerance in Preventing Autoimmunity.
So-called central tolerance is the best-known pathway to silencing self-reactive lymphocytes. Developing B and T cells rearrange Ig and T cell receptor genes to produce clones of lymphocytes with unique antigen receptors. Gene rearrangement is random, so that both self- and nonself-reactive clones are produced in the central lymphoid organs, the bone marrow, and thymus. However, when self antigens are present during development, the autoreactive B and T cells or their receptors can be selectively deleted as envisaged by Burnet (7) and Lederberg (8). Owen first uncovered experimental evidence for this fundamental developmental route to tolerance in his studies on cattle (9). He observed that fraternal twins, although genetically different, failed to mount immune responses to each other's cells. This finding was remarkable in view of Ehrlich's observations that any individual reliably formed antibodies to the cells of another individual. The basis for the tolerance in cattle twins was a shared placental circulation, whereby the twins became hematopoietic chimeras during development. Billingham, Brent, and Medawar exploited Owen's finding when they made the dramatic discovery that an injection of foreign white blood cells into neonatal mice could induce tolerance to transplantation antigens, especially products of the foreign major histocompatibility complex (MHC) (10). The underlying mechanisms for central tolerance of developing lymphocytes were then appreciated once methods were developed to identify these lymphocytes. It was noted that self-reactive T and B cells were deleted centrally (11–14) or, in the case of B cells, their receptors could be edited and replaced by receptors for foreign antigens (3, 4).
DCs and Central Tolerance.
DCs play an important role in the self/nonself distinction imposed by the thymus. Located almost entirely in circumscribed medullary regions (15, 16), DCs present self antigens to developing T cells and delete lymphocytes with autoreactivity (17–20) (Fig. 1).
Limitations of Central Tolerance.
Despite its effectiveness for some classes of antigens, central tolerance has major limitations (Table1). Self-reactive T and B cells can escape deletion and editing (21) or, as Nossal vividly summarized, “The immunological self exerts its purgative mastery on lymphocytes only to a degree” (22). Many self antigens may not access the thymus (23), whereas others are expressed later in life, after the lymphocyte repertoire has been formed (24). Furthermore, the body is constantly exposed to innocuous nonpathogenic environmental antigens to which it remains tolerant, e.g., proteins and commensal organisms within our airways and intestines. The chance that lymphocyte receptors for foreign antigens crossreact with self proteins is also substantial (25). These limitations of central tolerance necessitate effective peripheral silencing mechanisms (26, 27). Indeed, T lymphocytes can be tolerized in peripheral tissues (28). Here we propose that DCs function to control antigen-specific peripheral tolerance (Fig.2), which may seem counterintuitive, because DCs have many critical roles in inducing immunity. We will propose that the tolerizing function of DCs occurs in the steady state, i.e., before an acute infection, and is essential to their subsequent function in generating antimicrobial immunity.
Table 1.
The limitations of central tolerance in avoiding horror autotoxicus
| Self-reactive lymphocytes escape negative selection. |
|---|
| Certain self antigens may not gain sufficient access to thymic antigen-presenting cells. |
| Many self antigens are expressed only after the T cell repertoire has been formed. |
| Many innocuous environmental proteins enter the body postnatally. |
| Lymphocyte receptors for foreign antigens can crossreact with self. |
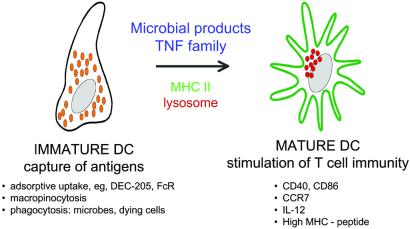
Figure 2.
DC maturation, a control point for regulating tolerance and immunity. Immature DCs capture antigens by several pathways, whereas mature DCs stimulate T cell immunity, i.e., helper and cytolytic effector lymphocytes as well as memory. Maturation stimuli act via TLRs (wherein distinct microbial products act although distinct TLR) and TNF family receptors (such as TNF itself and CD40L). Maturation leads to several changes, including: the redistribution of MHC class II molecules and MHC–peptide complexes from within the endocytic system to the cell surface as diagrammed here, the production of several cytokines and membrane associated T cell stimulatory molecules, and the remodeling of expressed chemokine receptors.
DC Maturation: The Risk of Autoimmunity and Chronic Inflammation During the Defense Against Pathogens
DC Maturation as a Control Point for Initiating Immunity.
DCs are specialized to process antigens, presenting them as peptides bound to MHC products and initiating immunity. However, the capture of antigens and the initiation of immune responses are distinct functions carried out by DCs at different stages of development, termed immature and mature (Fig. 2). These terms have some imprecision (see_Questions_), because they encompass cells found in different organs and pathologic settings as well as DC subsets and DCs generated in culture by different methods. Nevertheless, most types of immature DCs are known to capture antigens, both soluble and particulate, and have a number of receptors and intracellular compartments appropriate for the task (29). During maturation, additional functions develop that enhance the ability of DCs to induce immunity (Fig. 2) (30–41). Some changes that take place on maturation and enhance immunogenicity include: (i) increased formation of stable MHC–peptide complexes (36, 42–44); (ii) higher expression of membrane molecules like CD86 and other B7 family members for T cell binding and activation (45–47); (iii) new synthesis of cytokines that influence T cell proliferation and differentiation (48, 49); and (iv) altered production of chemokines and chemokine receptors that intensify movement of DCs into lymphatic vessels and lymphoid organs (50–53).
Janeway has independently emphasized a theme that parallels DC maturation (54, 55). He reasoned that a key component to immunogenicity, distinct from antigen processing, is the capacity of pathogens to activate antigen-presenting cells through pattern recognition receptors. These receptors induce expression of costimulatory functions required for immunity. The changes associated with pathogen recognition are encompassed by the events of DC maturation. Yet DC maturation also occurs in the absence of infection, during such powerful T cell immune responses as transplantation (56), contact allergy (57), and autoimmunity (58).
The Risks of DC Maturation.
To initiate T cell immunity to pathogens, DCs must accomplish two things: process the pathogen to form MHC–peptide complexes (antigen presentation) and differentiate or mature as summarized above. However, maturation creates a problem with respect to self/nonself discrimination. Consider influenza infection of the lung as an example: DCs not only capture the virus but also are likely to be taking up dying influenza-infected cells (59, 60). Furthermore, DCs capture airway proteins continuously, even without the provocation of a pathogenic infection (61, 62) (Fig. 3). As Ehrlich would have predicted, the development of autoimmunity is the exception, not the rule, during recovery from respiratory and other infections. He pointed out: “During the individual's life, even under physiological although especially under pathological conditions, the absorption of all material of its own body can and must occur very frequently. The formation of tissue autotoxins would therefore constitute a danger threatening the organism more frequently and much more severely than all exogenous injuries” (p. 253, ref. 2). Even during the influenza pandemic of World War I, most infected people recovered without residual chronic reactivity to their airways or airway proteins. How, then, do DCs stimulate immunity to influenza but at the same time avoid stimulation of T cells reactive to self and innocuous environmental antigens?
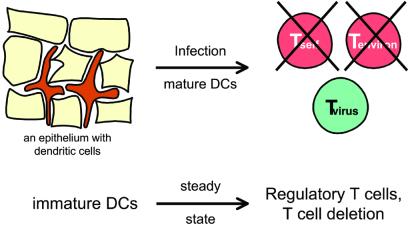
Figure 3.
Overcoming the risk of autoimmunity and horror autotoxicus inherent to the maturation of DCs on exposure to pathogens. During infection, DCs mature, e.g., in response to pathogen signals via TLRs (Fig. 2). However, the maturing DCs will likely be presenting peptides not only from the pathogen but also from dying self tissue and innocuous environmental proteins. To overcome this risk, it is proposed that immature DCs induce antigen-specific peripheral tolerance in the steady state, before DC maturation during inflammation and infection. DCs can do so by deleting naïve T cells or inducing regulatory T cells. The tolerized T cells can either be self-reactive lymphocytes that have escaped central tolerance or T cells reactive to innocuous proteins in the environment.
A Proposal: Immature DCs in the Steady State Define Immunologic Self and Tolerize T Cells Peripherally, Avoiding the Risks Associated with DC Maturation During Infection.
We suggest that DCs in the steady state, before infection or inflammation, critically define immunologic self and prevent the induction of both autoimmunity and chronic inflammation against environmental proteins (Fig. 3). According to this theory, proteins captured and processed by DCs in the steady state are tolerogenic, i.e., the DCs silence the corresponding antigen-specific T cells. As a result, when the same proteins are presented during infection, the immune response is able to focus on the pathogen, not on self or environmental antigens that are presented along with the pathogen (Fig.3). Reciprocally, chronic inflammatory diseases against otherwise nonpathogenic antigens would be directed primarily to proteins that are not presented by DCs in the steady state.
Immature DC Function in the Steady State: Migration and Uptake of Self and Environmental Proteins
The Distribution, Migration, and Turnover of DCs in the Steady State.
DCs are located at body surfaces, especially the skin (30) and airways (61, 63), in the interstitial spaces of many organs (64), lymphoid tissues (65, 66), blood (67), and, importantly, afferent lymphatics (68–71), the conduits between peripheral tissues and immunologically active lymph nodes. DCs can insinuate themselves into epithelia (61, 72), possibly after the interaction of CCR6 receptors on DCs (73) with epithelial MIP-3α/CCL20 (74). By expressing important molecular components of intercellular junctions, DCs at body surfaces may even insinuate through tight epithelia, extending their processes into the environment to capture proteins without breaking the epithelial barrier; this phenomenon can be enhanced by microbial stimuli (75). In mucosal associated lymphoid tissues, DCs lie beneath the antigen-transporting epithelia, again in the perfect niche to capture antigens transported through epithelial M cells (76).
In the steady state, immature DCs circulate between nonlymphoid and lymphoid tissues at a rapid rate. At least some, and perhaps most, peripheral DCs enter afferent lymphatics and then migrate to the T cell area, where they die, because few DCs are present in efferent lymphatics that leave the lymph node (70, 77). In mice, the life span of most DCs in the lung and lymphoid tissues is <2 days (78–81). DC migration from epithelial surfaces and deeper tissues to lymphoid organs can be further increased by applying a contact allergen (68) or by administering inflammatory cytokines or microbial products (82, 83). Another group of DCs, termed plasmacytoid cells (84, 85), enter the lymphoid tissues directly from the blood (86). Thus, DCs patrol most tissues continuously in the steady state. They are perfectly positioned to capture self and environmental antigens and to access the corresponding specific T cells (reviewed in ref. 87).
DCs Capture Antigens in the Steady State.
The proposal under consideration here is that immature DCs in the steady state are vital to defining self in the periphery. Appropriately, DCs efficiently pick up and process proteins, e.g., from the airway (61, 62), blood (88–90), muscle (71), and intestine (91). The experimental approach is to inject the antigen without any other stimulus or adjuvant, isolate DCs a day later, and then test whether the DCs can present the antigen to specific T cells in culture. In every case, DCs show high levels of antigen presentation to specific T cells, whereas other cells exhibit little if any activity (89). Likewise DCs continually capture particulates, including dying cells_in vivo_. DCs that traffic through the liver and into hepatic lymphatics can pick up latex particles and colloidal carbon (92), Langerhans cells in skin-draining lymph nodes contain melanin granules acquired from cells in the skin (93), and DCs capture intestinal epithelial cells before entry into the mesenteric lymphatics (94). Thus, the normal process of cellular turnover in nonlymphoid tissues appears to provide circulating DCs with a constant supply of self antigens for processing and presentation (94, 95), an important prerequisite for peripheral tolerance (96). Together, efficient antigen capture, rapid turnover, and widespread circulation through tissues allow DCs to perpetually sample self and environmental antigens.
Two New Lines of Evidence for Peripheral Tolerance via Immature DCs
Peripheral T Cell Deletion via Immature DCs.
Although antigen uptake by DCs in the steady state is well documented_in vivo_ (above), the immunologic consequences have not been pursued. It turns out that peripheral tolerance can ensue. One mechanism involves deletion of specific T cells, a consequence that parallels central or thymic tolerance. A recent experiment, which revealed this role of DCs in situ, involved the targeting of antigens to DCs through an adsorptive endocytosis receptor, DEC-205 (97, 98). This receptor is abundantly expressed on many DCs in the T cell areas of peripheral lymphoid tissues, i.e., in the ideal place to present captured antigens to T cells circulating through lymphoid organs (99). We chose DEC-205 for targeting antigens to DCs, because it mediates uptake of bound ligand, and its cytosolic domain contains an EDE triacidic amino acid targeting sequence that delivers ligands to MHC class II containing compartments 30–100 times more effectively than homologous receptors (100). The proteins delivered by DEC-205 to such compartments are processed and loaded onto MHC class II molecules (100). Because natural ligands for DEC-205 are not yet known, anti-DEC-205 antibodies were engineered to carry antigenic peptides from a model antigen hen egg lysozyme (HEL). The anti-DEC/HEL antibody, in fact, targeted selectively to DCs in situ in the steady state**,** and when corresponding TCR transgenic T cells were exposed to these targeted DCs, the T cells proliferated vigorously at first (98). Within a week, however, the majority of the responding T cells were deleted, and the mice became tolerant, unable to be primed by injection of peptide with the powerful Freund's adjuvant. This peripheral tolerance could be converted to immunity if the anti-DEC-205/HEL were given together with a DC maturation stimulus.
In these experiments, the doses of injected protein antigen were low (<1 μgm of antibody or <15 ngm of peptide), and the dose of antigen-specific T cells high (2 × 106 were tolerized; the total number of T cells in a mouse is estimated to be ≈2 × 108, of which <2 × 104 typically respond to any one antigen). Therefore, through the use of DEC-205 to target DCs in the steady state, small amounts of an intact protein can lead to either tolerance or immunity. These results contrast with the prior literature on peripheral tolerance where much higher doses of preprocessed peptides (100 μgm or more) have been used (101, 102). The tolerance observed after targeting of proteins to DCs in situ also bears on striking observations that bone marrow-derived cells—presumably DCs (103)—can mediate either peripheral deletion (104, 105) or anergy (106, 107) in situ. The DEC-205 targeting experiments specifically implicate DCs as the inducers of peripheral tolerance by T cell deletion. This outcome can be converted to immunity if the DCs additionally receive an appropriate maturation stimulus.
Induction of Regulatory T Cells by Immature DCs.
The induction of regulatory T cells by DCs is another mechanism for peripheral tolerance. There may be different types of regulatory or suppressor T cells, e.g., those formed in the thymus and in the periphery. Regulatory cells are found as a small fraction (<5%) of the T cells in blood, and they are able to suppress the responses of other T cells to powerful stimuli (108–112). Because these regulatory cells dampen the responses of other effector (helper and killer) lymphocytes, they give rise to functional tolerance. The regulatory mechanisms are at this time unclear but, in addition, how are these cells induced in the first place?
The concept is that immature DCs are responsible for the formation of peripheral regulatory T cells, which has emerged during studies with DCs in humans. The approach is to isolate precursors from blood [either CD34 + proliferating progenitors (113) or CD14 + nonproliferating monocytes (39, 40)] and convert these to DCs ex vivo before reinfusion. The field is still in its early stages (reviewed in refs. 114 and 115), but one of the goals is to use DCs as “nature's adjuvant” to immunize patients against antigens in their tumors. The ex vivo approach is valuable, because DCs can be loaded with large arrays of antigens, including those expressed by tumor cells (116, 117), and because DC maturation can be regulated.
The initial studies of humans in situ were carried out with mature DCs and a model MHC (HLA-A2.1) binding influenza viral peptide. A single injection of peptide-charged DCs rapidly expanded peptide specific immunity an average of 5-fold (118), whereas a booster dose enhanced T cell functional affinity 30- to 100-fold (119). In contrast, when immature peptide-pulsed DCs were injected, influenza-specific CD8 + interferon (IFN)γ-secreting T cells virtually disappeared from the blood stream; in their place, peptide-specific IL-10-secreting T cells appeared (41). At least some regulatory T cells are known to produce high levels of IL-10 (120, 121). When tested, the peptide-specific T cells induced by immature DCs were indeed able to suppress the effector function of IFNγ-secreting cells (M. V. Dhodapkar and R.M.S., unpublished work). The induction of regulatory cells was transient and reversed within 1–3 months, with a return of the IFNγ-secreting T cells. These in vivo experiments in humans, coupled with additional studies in tissue culture (122), demonstrate the capacity of immature DCs to rapidly induce regulatory T cells. The latter, it is known, are able to silence effector T cells including autoaggressive ones in mice (27, 110, 123–127). Possibly regulatory T cells operate by changing the function of DCs (128). In any case, the induction of these suppressive T cells provides another mechanism whereby DCs could induce antigen-specific peripheral tolerance.
In summary, we propose that immature DCs define immunologic self, silencing the T cell repertoire to self and environmental antigens captured during the steady state. When the DCs subsequently mature in response to infection, the preexisiting tolerance nullifies the development of reactivity to innocuous antigens and focuses the immune response on the pathogen. In the thymus, DCs delete self-reactive T cell clones, whereas in the periphery, DCs delete T cells and induce the formation of regulatory T cells. Our proposal draws on the known migratory and antigen-capturing activities of immature DCs in the steady state and is supported by recent evidence that DCs tolerize_in situ_.
Questions and Challenges That Arise from This Concept of Peripheral Tolerance
How Does the Idea that DCs Control Tolerance Differ from Other Theories?
Mechanisms of peripheral tolerance have relied on what is termed the two-signal notion of acquired immunity (reviewed in ref. 129). The proposal is that the presentation of self antigens or “signal one,” in the absence of costimulation, or “signal two,” induces T cell anergy or deletion. However, it is difficult to tolerize an animal with antigen alone, i.e., by the injection of intact proteins or even preprocessed peptides (101, 102), possibly because antigens need to be captured in sufficient amounts by immature DCs in order for tolerance to ensue. There also is information that antigens on non-DCs are ignored and not truly tolerogenic (130, 131). In other instances, antigens expressed by non-DCs are able to tolerize but only after processing by bone marrow derived cells, possibly DCs (105, 132). The “signal one” theory of tolerance therefore seems to be oversimplified and suffers from a dearth of evidence with intact soluble and cell-associated proteins in vivo.
We are instead proposing that the MHC peptide complexes produced by antigen processing become effective tolerogens when presented by DCs in the steady state. Also, the induction of tolerance by immature DCs likely requires a number of special features of these cells, not just “signal one”. Already evident are: (i) the efficient capture of antigens, including the exogenous pathway whereby DCs are specialized to form MHC class I-peptide complexes from soluble proteins, immune complexes, and dying cells (reviewed in ref. 29); (ii) the potential to bind T cells to be tolerized via receptors like DC-SIGN, a newly recognized lectin that interacts with intercellular adhesion molecule-3 on resting T cells (133); (iii) the production of IL-10 and possibly other regulatory cytokines (see below); and (iv) the ability to migrate to positions that optimizes access to antigens and T cells in situ. Likewise, the alterations that convert DCs to the immunogenic state are beginning to be unraveled. On microbial challenge, it is known that maturing DCs: (i) secrete cytokines like IL-12 that cause the differentiation of T cells to IFNγ−producing effectors (134); (ii) express increased levels of the CD80 and CD86 costimulatory molecules (45, 46), particularly in coclusters with MHC–peptide complexes (36); and (iii) regulate other costimulatory B7 family members, e.g., a molecule called B7-DC is induced (47). Despite progress in this area, there is a great deal to be learned about the features of DCs that regulate the balance between T cell immunity and tolerance.
Are There Different Types of Immature DCs?
There is not simply one discrete immature and mature type of DCs. Instead, there is a differentiation pathway triggered by a spectrum of external stimuli (microbial products, members of the tumor necrosis factor (TNF) family, other cytokines, heat shock proteins), possibly with distinct outcomes. In addition there are subsets of immature DCs, which can differ in their receptors for antigen uptake, the cytokines produced on stimulation, and the microbial products to be recognized (135). Currently, a perplexing area is the relationship between immature DCs produced in tissue culture and the tolerizing, DEC-205 positive, DCs within peripheral lymphoid organs. Immature DCs developing in culture go through a stage where antigens are captured, but MHC class II–peptide formation is weak (44). In contrast, the immature DCs in peripheral lymphoid organs (targeted with our anti-DEC antibodies, above) efficiently process and present antigens to induce tolerance (98, 136).
The precise physiologic counterpart of the frequently studied immature DC, produced with cytokines in culture, is not yet obvious. It may be the Langerhans cell and its homologues in other surface epithelia and/or the monocyte-derived DCs migrating from tissues after encountering antigen (137) or lymphatic endothelial cells (138). Understanding the relationships between these DCs is important, because different immature cells may induce peripheral tolerance by distinct mechanisms, such as deletion and induction of regulatory T cells.
Another key variable may be the capacity of immature DCs to produce IL-10 or other suppressive cytokines like transforming growth factor β. MHC class II bearing, IL-10 producing cells can regulate experimental autoimmune encephalomyelitis in mice (139). High amounts of IL-10 are made by DCs isolated from lung (140) and intestine (141) and by DCs developing ex vivo from monocytes (142–144). This IL-10 may lead to tolerance in several ways: IL-10 can itself suppress T cells (145); IL-10 may be required to differentiate regulatory T cells (125, 146); or IL-10 can act on DCs to decrease their function (147, 148) or make them tolerogenic (149). In contrast, DCs in the T cell areas of lymphoid tissue are not yet known to be producing IL-10 in the steady state. These DCs nonetheless can efficiently form MHC class II–peptide complexes and delete T cells in the steady state (98), but they lack other features of maturing DCs, such as IL-12 production and high levels of CD86 and CD40 (150, 151).
What Controls DC Maturation?
Microbial signaling through toll-like receptors (TLRs) is an effective way to mature DCs to their immunogenic state (150–153). However, DC maturation can also be induced under sterile circumstances, as in the cases of transplantation (56) and contact allergy (154). In these intense T cell-mediated immune responses, the requisite receptors for DC maturation have yet to be identified. A recent proposal is that TLRs are engaged by endogenous ligands, such as heparin sulfates and hyaluronans (155, 156). Many cell types produce cytokines when signaled through TLRs and the associated MyD88 adaptor protein (157). However, in DCs there is an additional MyD88 independent TLR-dependent pathway that leads to maturation and the capacity to initiate immunity (158, 159). TNF family members, e.g., CD40L on mast cells and platelets, and hematopoietin families, e.g., granulocyte macrophage–colony-stimulating factor, IL-4, IL-13, additionally influence DC development and maturation. These non-TLR stimuli may produce DCs with different functions or, alternatively, they may be required in concert with TLR signaling for full DC activity. For example, DCs require both a microbial and a TNF family stimulus to make large amounts of IL-12, a key cytokine for strong cell-mediated immunity (160). Increased understanding of DC maturation should yield new ways to manipulate this critical control point in immunity and tolerance.
Do Other Antigen-Presenting Cells Contribute to the Induction of Tolerance?
Other cells can contribute to tolerance in important ways (Fig. 1). Thymic medullary epithelial cells can induce central tolerance (161–163) (reviewed in ref. 164), possibly to epithelial and neuroendocrine antigens that they synthesize and that are not otherwise available to thymic DCs. Thymic cortical epithelial cells recently have been shown to induce suppressor T cells (165, 166), which seem related functionally to the peripheral regulatory T cells induced by immature DCs (reviewed in refs. 126, 127, and 167). Liver sinusoidal endothelial cells also can silence antigen reactive T cells, perhaps those specific for intestinal proteins that continually enter the portal circulation (168). B cells have been implicated in T cell tolerance, but, in the case of a B cell lymphoma, tolerance is induced only after the B cells are processed by other bone marrow-derived cells, possibly DCs (107). At this time, DCs cannot be regarded as exclusive mediators of tolerance or as exclusive mediators of immunity. Instead, DCs are specialized and efficient controllers of immunity, particularly when proteins (self, environmental, pathogenic) must be internalized and processed before presentation to quiescent peripheral T cells.
Implications
Monitoring and Manipulating Tolerance at the Level of Antigen-Presenting DCs.
Dozens of chronic inflammatory diseases are considered to be autoimmune in origin, and several autoantigens are known (169). When initiated, autoimmunity can selectively destroy tissue targets. For example, in juvenile diabetes, T cells attack insulin-producing cells in the pancreatic islets of Langerhans, and in multiple sclerosis, T cells attack the glial elements of the central nervous system. Other chronic inflammatory diseases may represent a failure of tolerance mechanisms toward normally innocuous environmental proteins and microorganisms rather than self. Inflammatory bowel disease, for example, may be directed to nonpathogenic bacteria in the intestine.
We have reasoned (Fig. 3) that the function of DCs in tolerance is most important for those self and environmental proteins that can be processed during an infection. Other self antigens could evade DC-mediated peripheral tolerance because of a low level of expression in the steady state or poor access to DCs (105, 132). The antigens that are not efficiently presented by DCs in the steady state might be good candidates to elicit autoimmune diseases. If these proteins begin to be processed de novo under conditions compatible with DC maturation, e.g., during an infection when proteases are released by inflammatory cells or from microbes themselves, the previously ignored self proteins may be presented by mature DCs and autoimmunity could ensue (170, 171).
The standard experimental and therapeutic approaches to the induction of tolerance are to use antigen-nonspecific agents, which impede the function of all T cells, or T cells responding to any antigen. As in the case of protective immunity, DC biology opens up the possibility for antigen-specific monitoring and manipulation of autoimmunity. Mature DCs might be used experimentally to identify disease-producing autoantigens, as recently shown for an autoimmune disease called primary biliary cirrhosis (172), whereas immature DCs might be used to dampen the autoimmune response in patients. One implication of our proposal is that the targeting of antigens to DCs in specific states of maturation may provide novel strategies for vaccination and immune therapies (41, 98, 173) (M. V. Dhodapkar and R.M.S., unpublished work).
Tolerance Induction by Persistent Pathogens.
The most challenging aspect of DC-induced peripheral tolerance relates to persistent pathogens, both infectious agents and tumors, which are captured by DCs. Some persistent infections, like herpes simplex virus (174), cytomegalovirus (175), and plasmodium falciparum (176), may inhibit DC maturation and decrease the efficacy of the host immune response. We would like to propose that an additional strategy on the part of the pathogen is to actively induce tolerance by virtue of continuous capture and presentation by immature DCs.
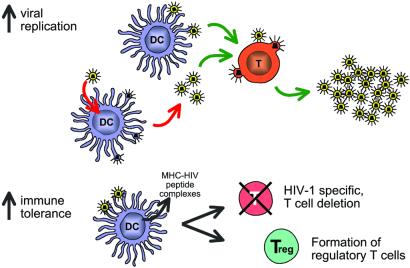
In the case of HIV, a very large number of virions are produced continuously in infected individuals (177, 178). Tissue culture experiments indicate that DCs can drive the replication of virus in T cells (Fig. 4 Upper) (179,180). DCs can either replicate HIV, which then infects T cells in large numbers, or simply capture and directly transmit HIV to permissive T cells. During chronic infection, patients are essentially asymptomatic. Their DCs may well be in an immature or steady state and may take up virions in sizable quantities. Furthermore, immature DCs express several HIV receptors, such as CD4, CCR5, and DC-SIGN (181–184), and support virus replication (182, 185). HIV may therefore exploit immature DCs in an immunologic sense and not just a virologic one (Fig.4 Lower). The virus becomes a very efficient form of “self,” possibly inducing regulatory T cells and/or deleting HIV reactive T cells from the repertoire. Other chronic infections may likewise induce regulatory T cells (186). However, in the case of HIV, the amount of antigen and the targeting of virus to receptors on immature DCs should intensify immune evasion through the induction of tolerance. Many serious infections, such as tuberculosis and influenza, can be lethal, but the immune system assists the majority of infected individuals (>90%) in recovering without residual disease. Yet in HIV infection, the immune system is unable to defeat the pathogen in the vast majority of people, consistent with some built-in restraint mechanism such as tolerance. The theory proposed here may help to explain this ominous property of the AIDS epidemic.
Figure 4.
Potential sites for involvement of DCs in HIV pathogenesis. In the virologic pathway (Upper), emphasized in the past, DCs catalyze HIV replication in T cells. In the immunologic pathway (Lower) proposed here, immature DCs continually capture and even replicate HIV virions, which induces peripheral tolerance, including regulatory T cells, thereby blocking the effector or protective limbs of the immune response.
Abbreviations
DC
dendritic cell
MHC
major histocompatibility complex
TLR
toll-like receptors
TNF
tumor necrosis factor
IFN
interferon
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 1, 2001.
References
- 1.Silverstein A M. Cell Immunol. 1999;194:213–221. doi: 10.1006/cimm.1999.1505. [DOI] [PubMed] [Google Scholar]
- 2.Himmelweit F. Collected Papers of Paul Ehrlich. London: Pergamon; 1956–1960. [Google Scholar]
- 3.Gay D, Saunders T, Camper S, Weigert M. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiegs S L, Russell D M, Nemazee D. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Curr Opin Immunol. 2001;13:291–298. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 7.Burnet F M. Austr J Sci. 1957;20:67–69. doi: 10.1038/icb.1957.8. [DOI] [PubMed] [Google Scholar]
- 8.Lederberg J. Science. 1959;129:1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- 9.Owen R D. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 10.Billingham R E, Brent L, Medawar P B. Nature (London) 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 11.Kappler J W, Roehm N, Marrack P. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 12.Kisielow P, Bluthmann H, Staerz U D, Steinmetz M, von Boehmer H. Nature (London) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 13.Schneider R, Lees R K, Fedrazzini T, Zinkernagel R M, Hengartner H, MacDonald H R. J Exp Med. 1989;169:2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodnow C C, Crosbie J, Adelstein S, Lavoie T B, Smith-Gill S J, Brink R A, Pritchard-Briscoe H, Wotherspoon J S, Loblay R H, Raphael K, et al. Nature (London) 1988;334:676–681. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 15.Barclay A N, Mayrhofer G. J Exp Med. 1981;153:1666–1671. doi: 10.1084/jem.153.6.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agger R, Witmer-Pack M, Romani N, Stössel H, Swiggard W J, Metlay J P, Storozynsky E, Freimuth P, Steinman R M. J Leukcyte Biol. 1992;52:34–42. doi: 10.1002/jlb.52.1.34. [DOI] [PubMed] [Google Scholar]
- 17.Matzinger P, Guerder S. Nature (London) 1989;338:74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- 18.Volkmann A, Zal T, Stockinger B. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 19.Brocker T, Riedinger M, Karjalainen K. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zal T, Volkmann A, Stockinger B. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouneaud C, Kourilsky P, Bousso P. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 22.Nossal G J V. Nature (London) 2001;412:685–686. doi: 10.1038/35089152. [DOI] [PubMed] [Google Scholar]
- 23.Lo D, Burkly L C, Flavell R A, Palmiter R D, Brinster R L. J Exp Med. 1989;170:87–104. doi: 10.1084/jem.170.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzinger P. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 25.Mason D. Immunologist. 1998;6:220–222. [Google Scholar]
- 26.Kamradt T, Mitchison N A. N Engl J Med. 2001;344:655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 27.Maloy K J, Powrie F. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 28.Rocha B, von Boehmer H. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 29.Mellman I, Steinman R M. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 30.Schuler G, Steinman R M. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inaba K, Schuler G, Witmer M D, Valinsky J, Atassi B, Steinman R M. J Exp Med. 1986;164:605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witmer-Pack M D, Olivier W, Valinsky J, Schuler G, Steinman R M. J Exp Med. 1987;166:1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romani N, Inaba K, Pure E, Crowley M, Witmer-Pack M, Steinman R M. J Exp Med. 1989;169:1153–1168. doi: 10.1084/jem.169.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone A M, Fathman C G, Inaba K, Steinman R M. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inaba K, Metlay J P, Crowley M T, Steinman R M. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turley S J, Inaba K, Garrett W S, Ebersold M, Untermaehrer J, Steinman R M, Mellman I. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 37.Stumbles P A, Thomas J A, Pimm C L, Lee P T, Venaille T J, Proksch S, Holt P G. J Exp Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Doherty U, Steinman R M, Peng M, Cameron P U, Gezelter S, Kopeloff I, Swiggard W J, Pope M, Bhardwaj N. J Exp Med. 1993;178:1067–1078. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romani N, Reider D, Heuer M, Ebner S, Eibl B, Niederwieser D, Schuler G. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 40.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 41.Dhodapkar M V, Steinman R M, Krasovsky J, Munz C, Bhardwaj N. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Nature (London) 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 43.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Nature (London) 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 44.Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, Germain R N, Mellman I, Steinman R M. J Exp Med. 2000;191:927–936. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caux C, Vanbervliet B, Massacrier C, Azuma M, Okumura K, Lanier L L, Banchereau J. J Exp Med. 1994;180:1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inaba K, Witmer-Pack M, Inaba M, Hathcock K S, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley P S, Ikehara S, et al. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tseng S-Y, Otsugi M, Gorski K, Huang X, Slanksy J E, Pai S I, Shalabi A, Shin T, Iwai Y, Honjo T, et al. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 49.Ebner S, Ratzinger G, Krosbacher B, Schmuth M, Weiss A, Reider D, Kroczek R A, Herold M, Heufler C, Fritsch P, Romani N. J Immunol. 2001;166:633–641. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- 50.Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. J Immunol. 1998;161:3096–3102. [PubMed] [Google Scholar]
- 51.Dieu M-C, Vanbervliet B, Vicari A, Bridon J-M, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay C R, Qin S, Lanzavecchia A. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 53.Sozzani S, Allavena P, Vecchi A, Mantovani A. J Leukcyte Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Janeway C A., Jr Cold Spring Harb Symp Quant Biol. 1989;54:1–13. [PubMed] [Google Scholar]
- 55.Janeway C A., Jr Immunol Today. 1992;13:11. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 56.Larsen C P, Steinman R M, Witmer-Pack M, Hankins D F, Morris P J, Austyn J M. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enk A, Katz S I. Proc Natl Acad Sci USA. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abrams J R, Kelley S L, Hayes E, Kikuchi T, Brown M J, Kang S, Lebwohl M G, Guzzo C A, Jegasothy B V, Linsley P S, Krueger J G. J Exp Med. 2000;192:681–694. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albert M L, Sauter B, Bhardwaj N. Nature (London) 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 60.Albert M L, Pearce S F A, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holt P G, Schon-Hegrad M A, Oliver J. J Exp Med. 1987;167:262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vermaelen K Y, Carro-Muino I, Lambrecht B N, Pauwels R A. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holt P G, Schon-Hegrad M A, Oliver J, Holt B J, McMenamin P G. Intl Arch Allergy Appl Immunol. 1990;91:155–159. doi: 10.1159/000235107. [DOI] [PubMed] [Google Scholar]
- 64.Hart D N J, Fabre J W. J Exp Med. 1981;154:347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinman R M, Cohn Z A. J Exp Med. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hart D N, McKenzie J L. J Exp Med. 1988;168:157–170. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Voorhis W C, Valinsky J, Hoffman E, Luban J, Hair L S, Steinman R M. J Exp Med. 1983;158:174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drexhage H A, Mullink H, de Groot J, Clarke J, Balfour B M. Cell Tissue Res. 1979;202:407–430. doi: 10.1007/BF00220434. [DOI] [PubMed] [Google Scholar]
- 69.Knight S C, Balfour B M, O'Brien J, Buttifant L, Sumerska T, Clark J. Eur J Immunol. 1982;12:1057–1060. doi: 10.1002/eji.1830121214. [DOI] [PubMed] [Google Scholar]
- 70.Pugh C W, MacPherson G G, Steer H W. J Exp Med. 1983;157:1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bujdoso R, Hopkins J, Dutia B M, Young P, McConnell I. J Exp Med. 1989;170:1285–1302. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maric I, Holt P G, Perdue M H, Bienenstock J. J Immunol. 1996;156:1408–1414. [PubMed] [Google Scholar]
- 73.Greaves D R, Wang W, Dairaghi D J, Dieu M C, de Saint-Vis B, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, et al. J Exp Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dieu-Nosjean M C, Massacrier C, Homey B, Vanbervliet B, Pin J J, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, Caux C. J Exp Med. 2000;192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl J P, Ricciardi-Castagnoli P. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 76.Cook D N, Prosser D M, Forster R, Zhang J, Kuklin N A, Abbondanzo S J, Niu X D, Chen S C, Manfra D J, Wiekowski M T, et al. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 77.Matsuno K, Kudo S, Ezaki T, Miyakawa K. Transplantation. 1995;60:765–768. doi: 10.1097/00007890-199510150-00027. [DOI] [PubMed] [Google Scholar]
- 78.Holt P G, Haining S, Nelson D J, Sedgwick J D. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 79.Steinman R M, Lustig D S, Cohn Z A. J Exp Med. 1974;139:1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamath A T, Pooley J, O'Keeffe M A, Vremec D, Zhan Y, Lew A, D'Amico A, Wu L, Tough D F, Shortman K S. J Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 81.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 82.Roake J A, Rao A S, Morris P J, Larsen C P, Hankins D F, Austyn J M. J Exp Med. 1995;181:2237–2248. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MacPherson G G, Jenkins C D, Stein M J, Edwards C. J Immunol. 1995;154:1317–1322. [PubMed] [Google Scholar]
- 84.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegal F P, Kadowaki N, Shodell M, Fitzgerald-Bocarsly P A, Shah K, Ho S, Antonenko S, Liu Y J. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 86.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 87.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 88.Kyewski B A, Fathman C G, Kaplan H S. Nature (London) 1984;308:196–199. doi: 10.1038/308196a0. [DOI] [PubMed] [Google Scholar]
- 89.Crowley M, Inaba K, Steinman R M. J Exp Med. 1990;172:383–386. doi: 10.1084/jem.172.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kyewski B A, Fathman C G, Rouse R V. J Exp Med. 1986;163:231–246. doi: 10.1084/jem.163.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu L M, MacPherson G G. J Exp Med. 1993;177:1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsuno K, Ezaki T, Kudo S, Uehara Y. J Exp Med. 1996;183:1865–1878. doi: 10.1084/jem.183.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemmi H, Yoshino M, Yamazaki H, Naito M, Iyoda T, Omatsu Y, Shimoyama S, Letterio J J, Nakabayashi T, Tagaya H, et al. Int Immunol. 2001;13:695–704. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- 94.Huang F-P, Platt N, Wykes M, Major J R, Powell T J, Jenkins C D, MacPherson G G. J Exp Med. 2000;191:435–442. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steinman R M, Turley S, Mellman I, Inaba K. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garza K M, Agersborg S S, Baker E, Tung K S. J Immunol. 2000;164:3982–3989. doi: 10.4049/jimmunol.164.8.3982. [DOI] [PubMed] [Google Scholar]
- 97.Jiang W, Swiggard W J, Heufler C, Peng M, Mirza A, Steinman R M, Nussenzweig M C. Nature (London) 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 98.Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch J V, Steinman R M, Nussenzweig M C. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kraal G, Breel M, Janse M, Bruin G. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahnke K, Guo M, Lee S, Sepulveda H, Swain S L, Nussenzweig M, Steinman R M. J Cell Biol. 2000;151:673–683. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liblau R S, Tisch R, Shokat K, Yang X-D, Dumont N, Goodnow C C, McDevitt H O. Proc Natl Acad Sci USA. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aichele P, Brduscha-Riem K, Zinkernagel R M, Hengartner H, Pircher H. J Exp Med. 1995;182:261–266. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heath W R, Carbone F R. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 104.Kurts C, Kosaka H, Carbone F R, Miller J F A P, Heath W R. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morgan D J, Kreuwel H T, Sherman L A. J Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- 106.Adler A J, Marsh D W, Yochum G S, Guzzo J L, Nigam A, Nelson W G, Pardoll D M. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sotomayor E M, Borrello I, Rattis F M, Cuenca A G, Abrams J, Staveley-O'Carroll K, Levitsky H I. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 108.Shevach E M. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk A H. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levings M K, Sangregorio R, Roncarolo M G. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ng W F, Duggan P J, Ponchel F, Matarese G, Lombardi G, Edwards A D, Isaacs J D, Lechler R I. Blood. 2001;98:2736–2744. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 113.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steinman R M, Dhodapkar M. Int J Cancer. 2001;94:459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 115.Nestle F O, Banchereau J, Hart D. Nat Med. 2001;7:761–765. doi: 10.1038/89863. [DOI] [PubMed] [Google Scholar]
- 116.Berard F, Blanco P, Davoust J, Neidhart-Berard E-M, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini J C, Banchereau J, Palucka A K. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dhodapkar, K. M., Krasovsky, J., Williamson, B. & Dhodapkar, M. V. (2002) J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 118.Dhodapkar M, Steinman R M, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe S M, Dunbar P R, Cerundolo V, Nixon D F, Bhardwaj N. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dhodapkar M V, Krasovsky J, Steinman R M, Bhardwaj N. J Clin Invest. 2000;105:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roncarolo M-G, Levings M K, Traversari C. J Exp Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asseman C, Mauze S, Leach M W, Coffman R L, Powrie F. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk A H. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mason D, Powrie F. Curr Opin Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 124.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. Nature (London) 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 125.Piccirillo C A, Shevach E M. J Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 126.Sakaguchi S. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 127.Shevach E M. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 128.Suciu-Foca Cortesini N, Piazza F, Ho E, Ciubotariu R, LeMaoult J, Dalla-Favera R, Cortesini R. Hum Immunol. 2001;62:1065–1072. doi: 10.1016/s0198-8859(01)00310-x. [DOI] [PubMed] [Google Scholar]
- 129.Schwartz R H. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott–Raven; 1999. pp. 701–739. [Google Scholar]
- 130.Ohashi P S, Oehen S, Buerki K, Pircher H, Ohashi C T, Odermatt B, Malissen B, Zinkernagel R M, Hengartner H. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 131.Oldstone M, Nerenberg M, Southern P, Price J, Lewicki H. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 132.Kurts C, Miller J F A P, Subramaniam R M, Carbone F R, Heath W R. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Geijtenbeek T B H, Torensma R, van Vliet S J, van Duijnhoven G C F, Adema G J, van Kooyk Y, Figdor C G. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 134.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kadowaki N, Ho S, Antonenko S, de Waal Malefyt R, Kastelein R A, Bazan F, Liu Y-J. J Exp Med. 2001;194:863–870. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman R M. J Exp Med. 1997;186:665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Randolph G J, Inaba K, Robbiani D F, Steinman R M, Muller W A. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 138.Randolph G J, Beaulieu S, Steinman R M, Muller W A. Science. 1998;282:480–483. [PubMed] [Google Scholar]
- 139.Cua D J, Groux H, Hinton D R, Stohlman S A, Coffman R L. J Exp Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akbari O, DeKruyff R H, Umetsu D T. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 141.Iwasaki A, Kelsall B L. J Exp Med. 1999;190:229–240. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bouloc A, Bagot M, Delaire S, Bensussan A, Boumsell L. Eur J Immunol. 2000;30:3132–3139. doi: 10.1002/1521-4141(200011)30:11<3132::AID-IMMU3132>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 143.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Eur J Immunol. 1997;27:756–762. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 144.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. J Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 145.Groux H, Bigler M, DeVries J E, Roncarolo M-G. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Levings M K, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo M G. J Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 147.Fiorentino D F, Zlotnik A, Vieira P, Mosmann T R, Howard M, Moore K W, O'Garra A. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 148.Groux H, Bigler M, de Vries J E, Roncarolo M G. J Immunol. 1998;160:3188–3193. [PubMed] [Google Scholar]
- 149.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk A H. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 150.Sparwasser T, Vabulas R M, Villmow B, Lipford G B, Wagner H. Eur J Immunol. 2000;30:3591–3597. doi: 10.1002/1521-4141(200012)30:12<3591::AID-IMMU3591>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 151.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. J Immunol. 2000;166:5688–5694. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 153.Horng T, Barton G M, Medzhitov R. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 154.Enk A H, Angeloni V L, Udey S I. J Immunol. 1993;150:3698–3704. [PubMed] [Google Scholar]
- 155.Kodaira Y, Nair S K, Wrenshall L E, Gilboa E, Platt J L. J Immunol. 2000;165:1599–1604. doi: 10.4049/jimmunol.165.3.1599. [DOI] [PubMed] [Google Scholar]
- 156.Termeer, C., Benedix, F., Sleeman, J., Fieber, C., Voith, U., Ahrens, T., Miyaki, K., Freudenberg, M., Galanos, C. & Simon, J. C. (2002)J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 157.Akira S, Takeda K, Kaisho T. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 158.Kaisho T, Akira S. Trends Immunol. 2001;22:78–83. doi: 10.1016/s1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 159.Alexopoulou L, Holt A C, Medzhitov R, Flavell R A. Nature (London) 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 160.Schulz O, Edwards A D, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 161.Hoffmann M W, Allison J, Miller J F A P. Proc Natl Acad Sci USA. 1992;89:2526–2530. doi: 10.1073/pnas.89.7.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oukka M, Cohen-Tannoudji M, Tanaka Y, Babinet C, Kosmatopoulos K. J Immunol. 1996;156:968–975. [PubMed] [Google Scholar]
- 163.Oukka M, Colucci-Guyon E, Tran P L, Cohen-Tannoudji M, Babinet C, Lotteau V, Kosmatopoulos K. Immunity. 1996;4:545–553. doi: 10.1016/s1074-7613(00)80481-1. [DOI] [PubMed] [Google Scholar]
- 164.Lo D, Reilly C R, Burkly L C, DeKoning J, Laufer T M, Glimcher L H. Immunol Res. 1997;16:3–14. doi: 10.1007/BF02786320. [DOI] [PubMed] [Google Scholar]
- 165.Jordan M S, Boesteanu A, Reed A J, Petrone A L, Holenbeck A E, Lerman M A, Naji A, Caton A J. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 166.Bensinger S J, Bandeira A, Jordan M S, Caton A J, Laufer T M. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saoudi A, Seddon B, Heath V, Fowell V, Mason D. Immunol Rev. 1996;149:195–216. doi: 10.1111/j.1600-065x.1996.tb00905.x. [DOI] [PubMed] [Google Scholar]
- 168.Limmer A, Ohl J, Kurts C, Ljunggren H G, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle P A. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 169.Lernmark A. J Clin Invest. 2001;108:1091–1096. doi: 10.1172/JCI14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel R M. J Exp Med. 1998;188:1493–1501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dittel B N, Visintin I, Merchant R M, Janeway C A., Jr J Immunol. 1999;163:32–39. [PubMed] [Google Scholar]
- 172.Kita, H., Lian, Z.-X., Van de Water, J., He, X.-S., Matsumura, S., Kaplan, M., Luketic, V., Coppel, R. L., Ansari, A. A. & Gershwin, M. E. (2002) J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 173.Lu L, Thomson A W. In: Dendritic Cells. Biology and Clinical Applications. Lotze M T, Thomson A W, editors. New York: Academic; 2001. pp. 587–607. [Google Scholar]
- 174.Salio M, Cella M, Suter M, Lanzavecchia A. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 175.Andrews D M, Andoniou C E, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti M A. Nat Immunol. 2001;2:1077–1084. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- 176.Urban B C, Ferguson D J, Pain A, Willcox N, Plebanski M, Austyn J M, Roberts D J. Nature (London) 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 177.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Nature (London) 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 178.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Nature (London) 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 179.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 180.Pope M, Betjes M G H, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 181.Delgado E, Finkel V, Baggiolini M, Clark-Lewis I, Mackay C R, Steinman R M, Granelli-Piperno A. Immunobiology. 1998;198:490–500. doi: 10.1016/s0171-2985(98)80073-9. [DOI] [PubMed] [Google Scholar]
- 182.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 183.Zhang L, He T, Talal A, Wang G, Frankel S S, Ho D D. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geijtenbeek T B H, Kwon D S, Torensma R, van Vliet S J, van Duijnhoven G C F, Middel J, Cornelissen I L, Nottet H S, KewalRamani V N, Littman D R, et al. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 185.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman R M. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Iwashiro M, Messer R J, Peterson K E, Stromnes I M, Sugie T, Hasenkrug K J. Proc Natl Acad Sci USA. 2001;98:9226–9230. doi: 10.1073/pnas.151174198. . (First Published July 17, 2001; 10.1073/pnas.151174198) [DOI] [PMC free article] [PubMed] [Google Scholar]