In vivo reprogramming of adult pancreatic exocrine cells to β-cells (original) (raw)
. Author manuscript; available in PMC: 2022 Apr 15.
Published in final edited form as: Nature. 2008 Aug 27;455(7213):627–632. doi: 10.1038/nature07314
Abstract
One goal of regenerative medicine is to instructively convert adult cells into other cell types for tissue repair and regeneration. Although isolated examples of adult cell reprogramming are known, there is no general understanding of how to turn one cell type into another in a controlled manner. Here, using a strategy of re-expressing key developmental regulators in vivo, we identify a specific combination of three transcription factors (Ngn3, Pdx1 and MafA) that reprogram differentiated pancreatic exocrine cells in adult animals into cells that closely resemble β-cells. The induced β-cells are indistinguishable from endogenous islet β-cells in size, shape, and ultrastructure. They express genes essential for β-cell function and can ameliorate hyperglycemia by remodeling local vasculature and secreting insulin. This study provides an example of cellular reprogramming using defined factors in an adult organ and suggests a general paradigm for directing adult cell reprogramming without reversion to a pluripotent stem cell state.
Cells of adult organisms arise from sequential differentiation steps that are generally thought to be irreversible1. Biologists often describe this process of development as proceeding from an undifferentiated (embryonic) cell to a terminally differentiated cell that forms part of an adult tissue or organ. There are rare examples, however, in which cells of one type can be converted to another type in a process called cellular reprogramming or lineage reprogramming2,3. Various forms of cellular reprogramming are referred to in the literature as transdifferentiation, dedifferentiation, or transdetermination4. For example, cellular reprogramming occurs in amphibian limb regeneration and fly imaginal disc identity switches5,6, and it may be central to certain types of pathological metaplasia4. There is long standing interest and fascination in reprogramming studies, in part because of the promise of harnessing this phenomenon for regenerative medicine whereby abundant adult cells that can be easily harvested would be converted to other medically important cell types to repair diseased or damaged tissues.
Somatic cell nuclear transfer (SCNT), developed in the 1960s, demonstrated that nuclei from differentiated adult cells could be reprogrammed to a totipotent state following injection into enucleated eggs2,7. More recently, it was shown that a small number of transcription factors can reprogram cultured adult skin cells to pluripotent stem cells8-13. These studies point to the possibility of regenerating mammalian tissues by first reverting skin or other adult cells to pluripotent stem cells and then redifferentiating these into various cell types. Alternatively, it should be possible to directly convert one cell type into another, without the need to first revert the cell to an undifferentiated pluripotent state. Indeed, there are examples in the literature which suggest that this approach is feasible. For example, studies with embryonic cells have shown that dermal fibroblasts and retinal epithelial cells can be converted into muscle-like cells14, and pancreatic tissue to liver15. In adult animals, mature B lymphocytes have been reprogrammed into macrophages16 or pro-B cells17. Today, well documented examples of cellular reprogramming, especially in adult animals, remain rare and have generally been restricted to cases where a single inducing factor is involved. The recent works on stem cell reprogramming suggest that a specific combination of multiple factors, instead of a single one, might be the most effective way to reprogram adult cells8-13.
We developed a strategy to identify adult cell reprogramming factors by re-expressing multiple embryonic genes in living adult animals. Our focus on embryonic genes is based in part on regeneration studies in newts, frogs and fish, where it has been shown that dedifferentiation of adult cells to progenitors, a form of cellular reprogramming, is accompanied by reactivation of embryonic regulators5,18,19. These studies suggest that re-expression of appropriate embryonic genes may reprogram differentiated cells.
To search for factors that could reprogram adult cells into β-cells, we focused on transcription factors (TF), a class of genes enriched for factors that regulate cell fates during embryogenesis. An in situ hybridization screen of more than 1,100 TFs identified groups of TFs with cell type specific expressions in the embryonic pancreas20. There are at least twenty TFs expressed in mature β-cells and their immediate precursors, the endocrine progenitors (Supplemental table. 1). Of these, nine genes exhibit β-cell developmental phenotypes when mutated21,22, and these were selected for initial reprogramming experiments.
We chose mature exocrine cells of the adult pancreas as target cells for reprogramming. Exocrine cells derive from pancreatic endoderm, as do β-cells23, and exocrine cells can turn on endocrine programs when dissociated and cultured in vitro24,25. We carried out our experiments in vivo so that any reprogrammed β-cells would reside in their native environment which might promote their survival and/or maturation. In addition, this approach allows for a direct comparison of endogenous and induced β-cells. The transcription factors were delivered into the pancreas in adenoviral vectors. It has been shown that adenovirus preferentially infects pancreatic exocrine cells, but not islet cells26, and since most endogenous β-cells reside within islets (Fig. 1b), any newly formed (reprogrammed) β-cells could be easily detected as extra-islet insulin+ cells.
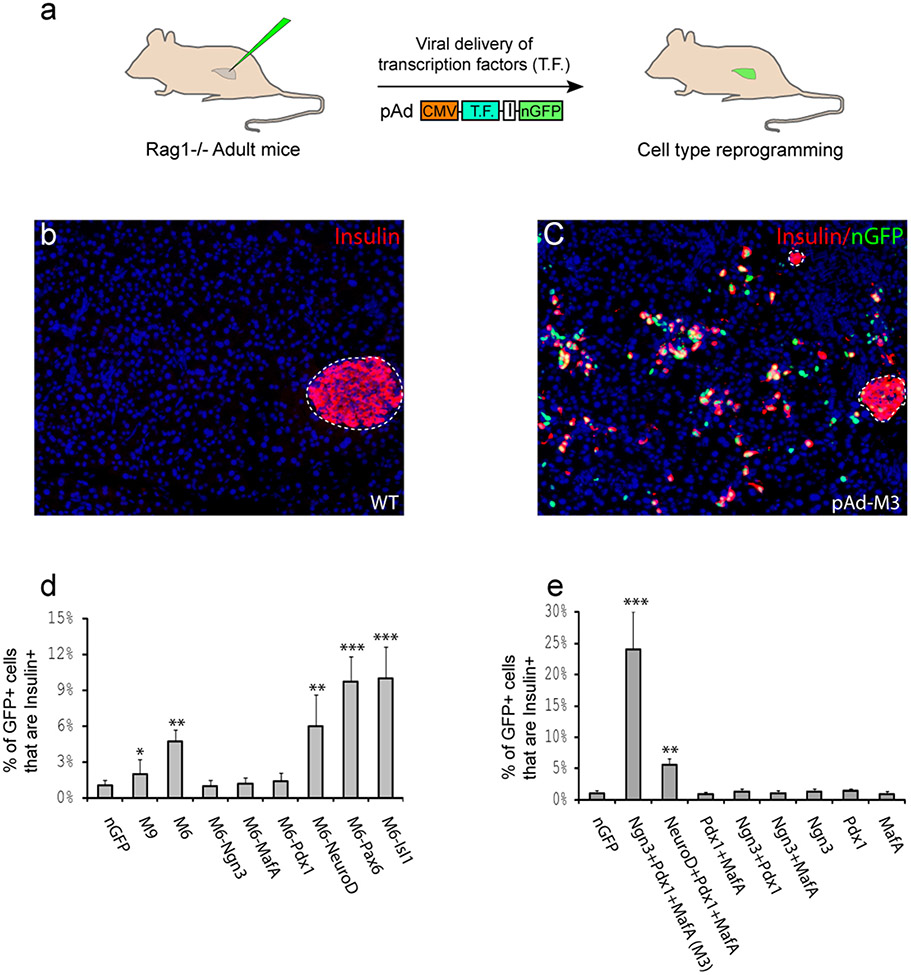
Figure 1. A combination of three transcription factors induces insulin+ cells in adult mouse pancreas in vivo.
a, Schematic diagram of experimental strategy. Adenoviruses encoding bicistronic transcription factor (T.F.) and nuclear GFP (nGFP) via an IRES element (I) were injected into the pancreas of an adult mouse (Rag−/−). b, Wild type (WT) pancreas is predominantly exocrine tissue with insulin+ β-cells in islet (outlined). Nuclei stained blue with DAPI. c, One month after infection with a combination of Ngn3, Pdx1, and MafA viruses (pAd-M3), numerous insulin+ cells appear outside islets. d, e, Quantification of induction one month after infection. M9, M6: mixture of 9 and 6 different viruses, respectively. Data presented as mean ± s.d. n=3 animals. ~1,000 nGFP+ cells/animal. Asterisk, P < 0.05; two asterisks, p < 0.01; three asterisks, p < 0.001.
Induction of Insulin+ cells in adult mice
Adenovirus that co-expresses each TF together with nuclear GFP (nGFP) was purified. All nine viruses were pooled and injected as a mixture (referred to as M9, for mixture of 9) into the pancreata of 2 month old adult mice (Fig. 1a). The immune-deficient Rag1−/− strain was used to avoid complications associated with viral elicited immune response27. One month after viral delivery, immunohistochemistry revealed a modest increase of extra-islet insulin+ cells among viral infected cells (nGFP+) in 2 out of 3 animals (Fig. 1d). To determine which of the nine factors are required, individual factors were removed from the pool one at a time. Pools lacking Nkx2.2, Nkx6.1, or Pax4 continued to produce increased extra-islet insulin+ cells (data not shown), suggesting that these genes are dispensable. Results for the other six genes were inconclusive. We conducted another round of factor withdrawal with mixtures of the remaining six genes (M6) and three of them, Ngn3, Pdx1, and MafA, proved to be absolutely required (Fig. 1d). The combination of these three factors (referred to as M3) converts >20% of infected cells to insulin+ cells (red cells with green nuclei, Fig. 1c, e). Notably, single factors or combinations of any two factors do not elicit this effect (Fig. 1e). Antibody labeling confirms that these three inducing factors are co-expressed in the induced insulin+ cells (Supplemental Fig. 1). NeuroD can functionally replace Ngn3 in M3, but the resulting cocktail has reduced induction efficiency (Fig. 1e).
We noticed that the percentage of insulin+ cells among infected cells increases with progressive removal of factors from the pool such that M3 induces more insulin+ cells than M6, whereas M6 is better than M9 (Fig 1d, e). This is likely due to the fact that a constant volume of virus was injected into each animal, regardless of the viral combinations. The effective concentration of Ngn3, Pdx1 and MafA viruses in a cocktail, therefore, increases when fewer factors are included. New insulin+ cells are detected 3 days after injection, but the expression level is low. The intensity of insulin staining increases gradually so that by day 10, the level is comparable to that of endogenous β-cells (Supplemental Fig. 2). These new insulin+ cells are still present after 3 months, the longest time point we analyzed, and remain as scattered individual cells or small clusters and do not form islets (Fig. 1c). The reprogramming effect of the three factors appears to be rather specific for pancreatic exocrine cells: infection of skeletal muscle in vivo or fibroblasts in vitro with M3 did not induce insulin expression, despite extensive co-expression of the three factors in the target cells (Supplemental Fig. 1).
The new insulin+ cells originate from differentiated exocrine cells
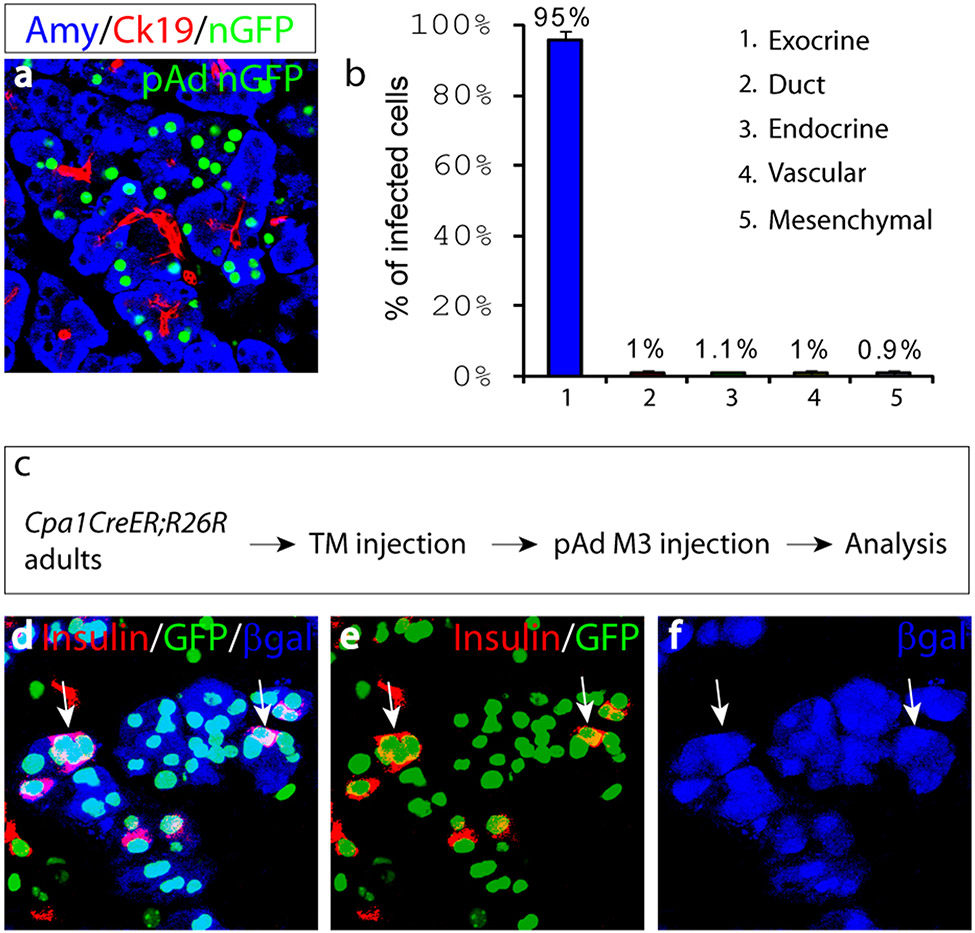
Lineage analysis was performed to determine the origin of the new insulin+ cells. The five major cell types in the adult pancreas can be detected with lineage-specific molecular markers: exocrine (Amylase), duct (Ck19), endocrine (insulin, glucagon, somatostatin, pancreatic polypeptide), vascular (PECAM), and mesenchymal cells (Nestin, Vimentin). Upon injection with a control nGFP virus, the vast majority of infected cells (>95%) were found to be mature amylase+ exocrine cells (Fig. 2a, b), consistent with prior reports26. Non-exocrine cells together account for approximately 5% of infected population. Since more than 20% of M3 infected cells become insulin+ 10 days after viral delivery, this suggests that non-exocrine cells can contribute, at most, to a minor fraction of these new insulin+ cells. As there is little cell death and no enhanced proliferation during this reprogramming (Supplemental Fig. 3), the majority of insulin+ cells would thus appear to originate from mature exocrine cells. To confirm the exocrine origin of the new insulin+ cells, we genetically labeled mature exocrine cells with a mouse line (Cpa1CreERT2) that expresses an inducible form of Cre recombinase (_CreERT_2) specifically in adult exocrine cells20 (Fig. 2c). When crossed with the R26R reporter line, tamoxifen induction in double heterozygous Cpa1CreERT2;R26R adults indelibly labels 5-10% of mature exocrine cells with β-galactosidase (βgal) (Fig. 2d, f); no label is found in other cell types. Following pAd-M3 injection, many βgal+ cells become insulin+ (Fig. 2d-f, pink cells), providing direct evidence that mature exocrine cells give rise to new insulin+ cells.
Figure 2. Induced new β-cells originate from differentiated exocrine cells.
a, 10 days after nGFP viral infection, most infected cells are Amylase+ (Amy) mature exocrine cells, not duct cells (Ck19+). b, Quantification of nGFP infected cell types. Data presented as mean ± s.d. n=3 animals. ~1,000 nGFP+ cells/animal. c, Mature exocrine cells are labeled with βgalactosidase (βgal) in double heterozygous Cpa1CreER T2;R26R adults with tamoxifen injection, reprogramming is subsequently induced by infection with pAd-M3. d-f, 10 days after infection, many βgal+insulin+ cells (arrows) are present. e and f are red (insulin)/green(GFP) and blue(βgal) channels of d, respectively.
Induced β-cells closely resemble islet β-cells
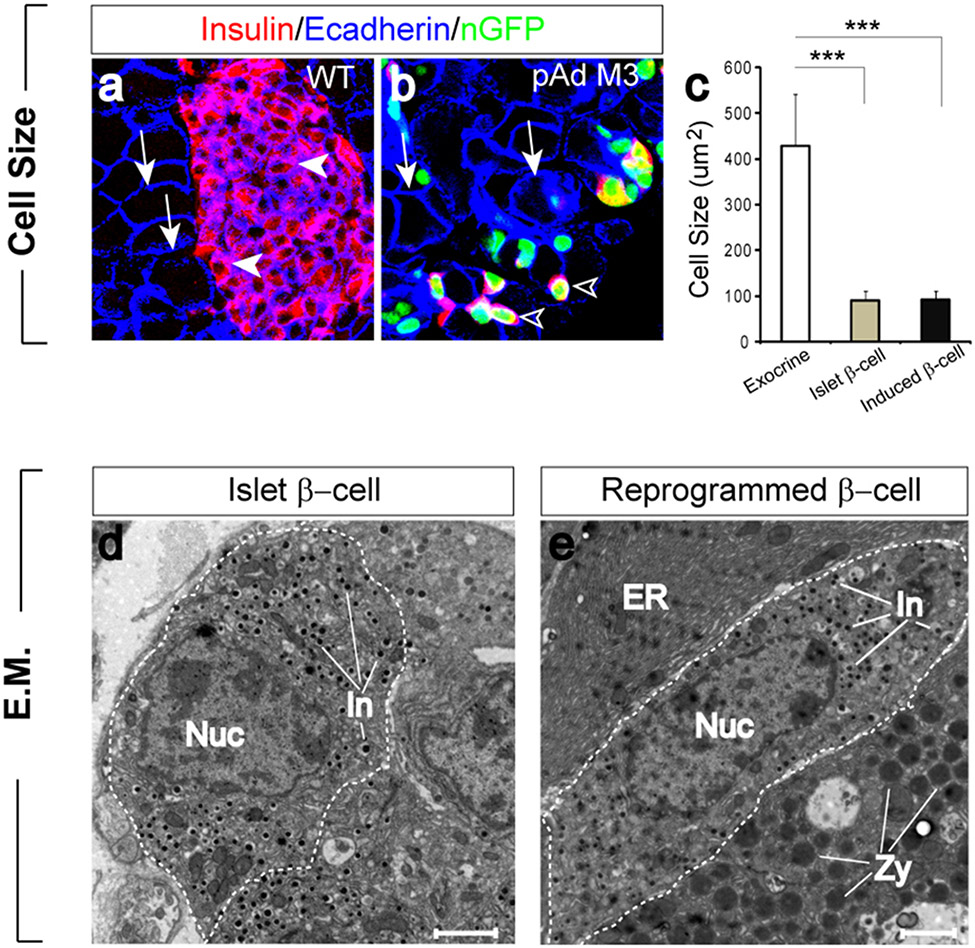
We next examined the new insulin+ cells to determine the extent to which they have been reprogrammed. Morphologically, exocrine cells are large with a cobble stone appearance (Fig. 3a, b) whereas islet β-cells are much smaller and spindle shaped (Fig. 3a). When dissociated into single cells, the diameter of Amylase+ exocrine cells range from 25-17um whereas insulin+ β-cells range from 9-15um. The induced cells are indistinguishable from islet β-cells in size and shape (Fig. 3b, c).
Figure 3. Endogenous and induced β-cells are indistinguishable in morphology and ultrastructure.
a, b, Islet β-cells (a, arrowheads) and induced β-cells (b, arrowheads) are similar in size and shape but distinctly different from exocrine cells (a, b, arrows). Ecadherin staining was used to visualize cell boundaries. c, Size comparison of exocrine cells (white bar), islet β-cells (gray bar), and induced β-cells (black bar). Data presented as mean ± s.d. n=3 animals. >100 cells/animal. Three asterisks: p < 0.001. d, Electron micrograph of a β-cell (outlined) in an islet. e, Example of an induced β-cell situated between two exocrine cells. Endogenous and induced β-cells contain small insulin granules (In) and lack zymogen granules (Zy) of exocrine cells and extensive endoplasmic reticulum (ER). Nuc: nucleus. Scale bar: 2um.
At the ultrastructural level, the reprogrammed cells have all the hallmarks of islet β-cells (Fig. 3d, e). They possess the small dense secretory granules characteristic of insulin granules, and lack the large zymogen granules and dense assemblies of endoplasmic reticulum (ER) that are characteristic of exocrine cells (Fig. 3d, e). Immuno-electron microscopy further showed that the induced β-cells express both GFP in the nucleus and abundant insulin in the granules (Supplemental Fig. 4). Interestingly, the induced β-cells often appear on the electron micrograph as intercalated within exocrine acinar rosettes (Fig. 3e). In wild type pancreatic samples, rare single or small clusters of β-cells reside outside islets, but they often associate with duct but not exocrine cells. The unique position of induced cells likely reflects their exocrine origin.
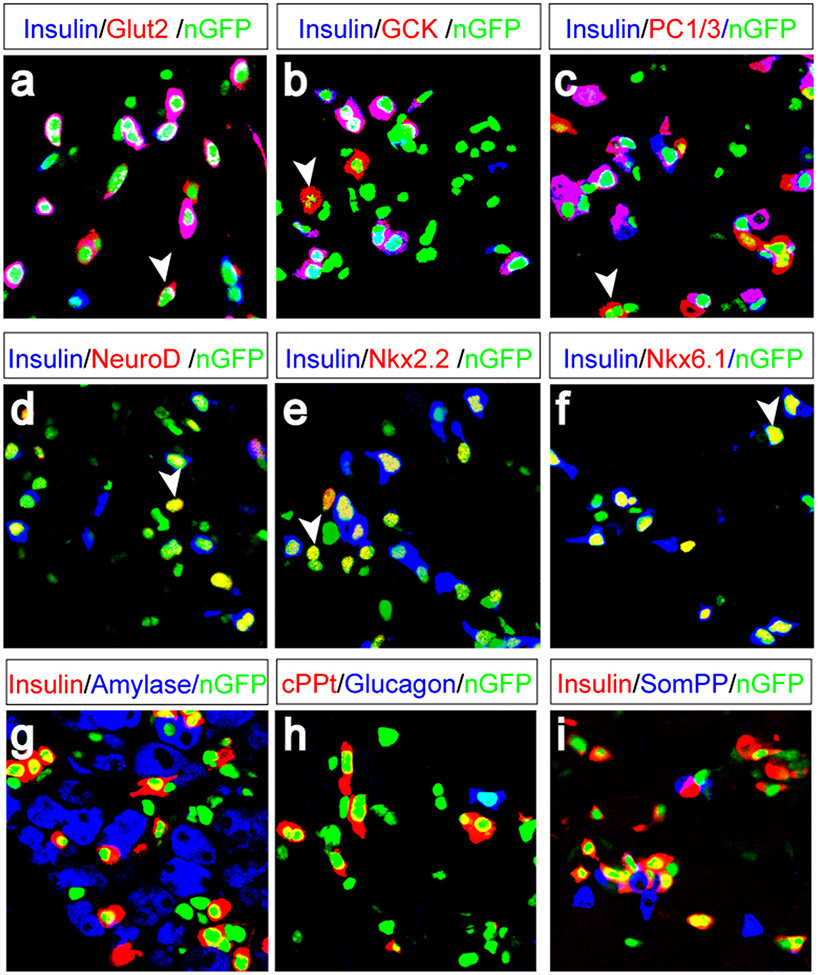
Molecular marker analysis reveals that most of the insulin+ cells coexpress genes essential for β-cell endocrine function including glucose transporter 2 (Glut2, expressed in 92.8% of the new insulin+ cells), glucokinase (GCK, 96.7%), prohormone convertase (PC1/3, 86.7%) (Fig. 4a-c, supplemental Fig. 5), and key β-cell transcription factors NeuroD (88.9%), Nkx2.2 (85.3%), and Nkx6.1 (85.9%) (Fig. 4d-f, supplemental fig. 5). The induced insulin+ cells express C-peptide (Fig. 4h). Expression profile analysis of the reprogrammed cells further indicates a strong overlap of endocrine enriched genes between reprogrammed cells and islet cells, suggesting a high degree of similarity between their endocrine programs (Supplemental fig. 6).
Figure 4. Molecular marker characterization of induced β-cells.
a-f, One month after infection with pAd-M3, most Insulin+ induced β-cells coexpress endocrine genes: glucose transporter 2 (Glut2, a), glucokinase (GCK, b), prohormone convertase 1/3 (PC1/3, c), and β-cell transcription factors NeuroD (d), Nkx2.2 (e), and Nkx6.1 (f). Arrowheads indicate examples of cells that express marker genes but not insulin. g-i, Induced β-cells do not express Amylase (g), glucagon (h), or somatostatin/pancreatic polypeptide (j, SomPP). cPPt: c-peptide.
The new β-cells do not express exocrine genes such as Amylase or Ptf1a, the duct marker Ck19, mesenchymal markers Nestin and Vimentin, nor the neuronal marker Tuji (Fig. 4g, supplemental fig. 5, and data not shown). Nor do the new β-cells express any other pancreatic hormones such as glucagon, somatostatin or pancreatic polypeptide (Fig. 4h, I, and supplemental fig. 5). Thus, the new β-cells do not exhibit a hybrid or mixed phenotype, indicating silencing of non-β-cell programs.
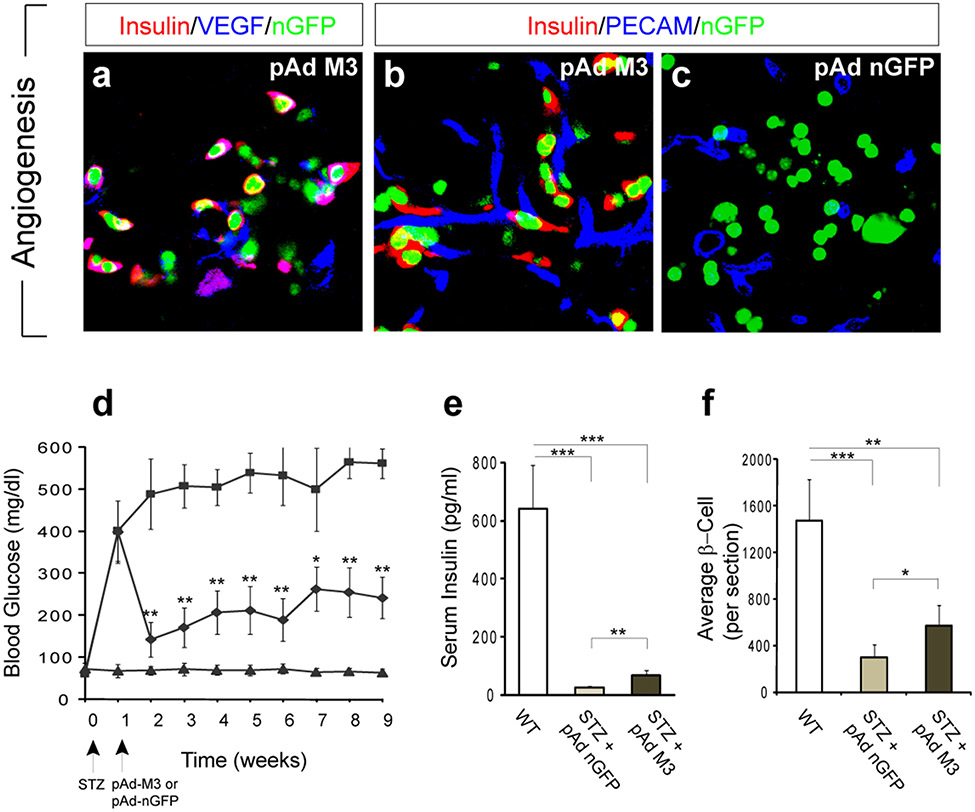
The primary function of β-cells is to synthesize and release insulin. To facilitate the release of insulin into the circulation, β-cells, unique among pancreatic cell types, synthesize vascular endothelial growth factor (VEGF) which promotes local angiogenic remodeling28. Notably, reprogrammed β-cells similarly synthesize VEGF and induce angiogenesis so that blood vessels form next to these new cells (Fig. 5a, b). Quantification indicates that in nGFP controls, 32% of infected cells lie adjacent to blood vessels whereas 61% and 83% of reprogrammed β-cells are directly juxtaposed to blood vessels 10 days and 30 days after induction, respectively.
Figure 5. Induced new β-cells remodel vasculature and ameliorate hyperglycemia.
a-c, New β-cells synthesize vascular endothelial growth factor (VEGF) (a), and induce local angiogenic remodeling (b). Note the close proximity of blood vessels (PECAM+) with the reprogrammed β-cells (b) versus control infected cells (c). d, Improvement of fasting blood glucose level in diabetic mice after injection with pAd-M3 (diamond), compared with controls with nGFP virus (square). Triangle: non-diabetic controls. STZ: streptozotocin. Arrows indicate timing of injection. n= 6-8 animals. e, non-fasting serum insulin levels 6 weeks after injection. n= 6-8 animals. f, average insulin+ β-cell number per section 8 weeks after injection. n= 3 animals. Both islet and reprogrammed β-cells were counted for the pAd-M3 samples. One asterisk, p < 0.05; two asterisks, p < 0.01; three asterisks, p < 0.001. Data presented as mean ± s.d.
To test whether reprogrammed β-cells release insulin, mice were rendered diabetic by streptozotocin (STZ) injection which specifically ablates islet β-cells. When subsequently injected with pAd-M3, fasting blood glucose levels of hyperglycemic animals show a significant and long lasting improvement compared to animals injected with control (nGFP) virus (Fig. 5d). In addition, the pAd-M3 animals show increased glucose tolerance (Supplemental fig. 7), have increased insulin levels in the serum (non-fasting, P<0.01, Fig. 5e), and possess large numbers of reprogrammed β-cells (Fig. 5f). RT-PCR analysis and direct observation revealed that virus injected into the pancreas does not spread to other internal organs such as liver and intestine that, theoretically, could modulate insulin secretion and/or response (supplemental Fig. 8). In addition, we found no evidence that STZ treated animals show spontaneous conversion of exocrine cells to β-cells (Supplemental Fig. 8). As the data in Fig 5 show, the total number of reprogrammed β-cells is rather small compared to the number of β-cells in normal animals and this may account for the limitation to the effectiveness in restoring glucose homeostasis. Alternatively, as the new β-cells are not reorganized into islet structures, this may limit their effectiveness. All together, these data show that reprogrammed β-cells can produce and secrete insulin in vivo.
Transient expression of inducing factors results in a long-lasting β-cell phenotype
Our results thus far support the contention that a combination of three transcription factors fully reprograms exocrine cells to β-cells in vivo. To determine whether continued presence of these factors is required to maintain the phenotype of reprogrammed cells, we used RT-PCR and primers specific to viral transgenes to detect their presence. Transgene expression from all three viruses was substantially diminished after one month and was undetectable after 2 months (Supplemental Fig. 9). Ngn3 protein was undetectable by antibody staining one month after infection (Supplemental Fig. 9). Pdx1 and MafA protein expression in the reprogrammed β-cells, however, remains consistently strong even after 2 months, indicating the activation of endogenous genes (Supplemental Fig. 9). These results are consistent with the fact that endogenous islet β-cells do not express Ngn3, but do express Pdx1 and MafA21,22. Thus, a transient expression of the inducing factors is sufficient to convert exocrine cells to a stable new β-cell state.
β-cell reprogramming does not involve dedifferentiation
In principle, the conversion of exocrine cells to β-cells could be direct or involve dedifferentiation to common progenitors which then redifferentiate into β-cells. Indeed, exocrine and β-cells share a common progenitor during embryogenesis that is characterized by rapid division and expression of genes including Sox9 and Hnf620. Continuous BrdU labeling over the first 10 days of reprogramming, however, shows that few reprogrammed β-cells (3.2%) have divided (supplemental fig. 3). In comparison, 12.9% of endogenous islet β-cells in the same animals incorporated BrdU (supplemental fig. 3). In addition, we detected no induction of Sox9 or Hnf6 (data not shown). These results suggest that in vivo reprogramming of exocrine to β-cells is a direct conversion of cell types and does not involve dedifferentiation. We can not formally exclude the possibility that a very transient or partial dedifferentiation may occur, but our results indicate that extensive replication and reversion to a dedifferentiated cell for an appreciable time does not occur.
Discussion
Our results provide evidence that fully differentiated exocrine cells can be reprogrammed into cells that closely resemble β-cells in adult animals by a combination of just three transcription factors. The three reprogramming factors, Ngn3, Pdx1, and MafA, are known to be important in the embryonic development of pancreas and β-cells21,22. On the other hand, many additional factors are also required for β-cell development21,22. Further studies will be necessary to understand why this particular combination is sufficient for adult β-cell reprogramming.
The reprogrammed β-cells do not organize into islet structures and remain as single cells or small clusters. Signaling between β-cells inhibits basal insulin secretion and enhances glucose stimulated insulin secretion29. The lack of organization of reprogrammed β-cells undoubtedly impairs their function. Strategies that promote aggregation of the induced β-cells in adult should help restore full glucose responsiveness.
There have been prior attempts to convert adult liver cells to β-cells in vivo by expressing pancreatic transcription factors27,30-32. These factors were able to induce expression of some pancreatic genes, but not phenotypic nor morphological conversion into functional β-cells27,30-32. Mature exocrine cells can turn on endocrine programs when dissociated and cultured in vitro24,25,33,34. Interestingly, dissociation itself is apparently sufficient to initiate endocrine programs whereas the addition of growth factors is necessary for cell survival24,25,33,34. However, the molecular mechanisms of this process remain largely unknown. Other studies have shown that pancreatic duct cells and liver cells could be induced to express certain β-cell gene products in culture35-37. Most of these studies, however, did not address whether these cells possess a hybrid phenotype. In addition, RT-PCR on populations of cells, instead of single cell resolution immunohistochemistry, was routinely used to evaluate the expression of β-cells markers. It is unclear how many cells actually expressed these markers or at what level. Finally, β-cells exhibit highly unstable phenotypes when cultured and appear to transform into fibroblast-like cells38,39. In vitro generation of β-cells will likely require suitable culture conditions that have yet to be discovered.
It is surprising that the reprogramming of exocrine cells to β-cells does not involve multiple rounds of cell proliferation. It is generally thought that epigenetic changes that underlie reprogramming events are most easily made during cell division2. It may be the case that many reprogramming events do indeed involve obligatory proliferation steps4. On the other hand, reprogramming of B lymphocytes to macrophages appears to be cell cycle independent16. Early somatic cell nuclear transfer (SCNT) experiments also provided evidence for reprogramming without DNA replication40.
Reprogramming of exocrine cells to β-cells occurs at a relatively fast speed, with the first insulin+ cells appear at day 3, and with efficiency of up to 20%. This is in contrast with recent reports of reprogramming fibroblasts to embryonic stem cells8-13, where it takes considerably longer time (7-30 days) and with much reduced efficiency (typically less than 0.1%). This may be due to the fact that pancreatic exocrine and β-cells are closely related cell types and share much of their epigenomes whereas the epigenomes of fibroblasts and ES cells are largely dissimilar. Conversion between exocrine and β-cell may therefore require much less epigenetic changes.
Recent advances in mammalian cellular reprogramming with defined genes collectively point to the possibility that a limited number of factors could reprogram any given adult cell to a different type of cell such as a stem cell, a committed progenitor, or another mature cell type. All these studies relied on knowledge of the normal development of these cell types which enabled the manipulation of key developmental regulators in adult cells. This approach may prove to be a general strategy for directing adult cell reprogramming. The recent reprogramming of human skin cells to pluripotent stem cells (iPS cells) raises the possibility of generating patient-specific hES lines for therapies9,10,13. This would be the first step in a process that will then require directed differentiation of the iPS cells to produce therapeutically important cell types such as neurons, cardiomyocytes, or pancreatic β-cells. In principle, patient-specific cell therapies could be achieved more directly by reprogramming abundant and easily accessible patient-specific human cells such as fibroblasts, blood cells or adipocytes.
METHODS SUMMARY
Adenovirus construction and purification.
Genes of interest were first cloned into a shuttle vector containing an IRES-nGFP, then into the pAd/CMV/V5-DEST adenoviral vector (invitrogen). High titer virus (>1 x 1010 pfu/ml) was obtained by purification with the AdEasy Kit (Stratagene).
Animals, surgery, physiological studies.
Rag1−/− and Rag1−/−;NOD animals were obtained from Jackson Labs. Adult animals (>2 month) were injected with 100ul (>1 x 109 pfu) of purified adenovirus directly into the splenic lobe of the dorsal pancreas. Blood glucose was measured with Ascensia Elite Blood Glucose Meter. Insulin levels were determined with an Ultrasensitive Insulin ELISA kit (Alpco).
Immunohistochemistry, BrdU labeling, TUNEL analysis.
This was performed as previously described41. 1mg/ml BrdU was provided in drinking water for BrdU labeling following surgery. Apoptotic cells were recognized by TUNEL labeling with a TMR red Cell Death kit (Roche).
Electron microscopy.
Dissected pancreas was fixed in 4% paraformaldehyde and 0.1% glutaraldehyde for 2 hours at room temperature. For conventional transmission electron microscopy, samples were further fixed by osmium tetroxide, embedded in Epon resin and sectioned at 60-80nm. For immuno-gold labeling, ultrathin sections were cut at −120°C and stained with gold conjugated antibodies. Images were obtained with a Tecnai™ G2 Spirit BioTWIN transmission electron microscope.
FACS analysis, gene profiling.
Pancreas was digested with liberase and Elastase (Roche) to single cells. GFP+ cells were isolated by FACS with FACSaria (BD Bioscience). Biotin labeled cRNA probes synthesized with the Illumina TotalPrep RNA Amplification kit (Ambion). Gene profiling performed with Sentrix BeadChip Array MouseRef-8 v1.1 (illumina). Data analyzed with the BeadStudio software.
Supplementary Material
Supplemental material
Acknowledgements
We are grateful to Maria Ericsson for expert assistance on electron microscopy, Renate Hellmiss-Peralta for advice on graphics, and Brian Tilton and Patricia Rogers for FACS. We thank Rita Martinez and George Kenty for technical assistance; Helena Edlund for gift of ptf1a antiserum; Anastasie Kweudjeu for microarray analysis; members of the Melton lab for advice and feedback; and Julie Sneddon, Justin Annes, William Anderson for critical reading of the manuscript. Q.Z. was supported by a Damon-Runyon Cancer Research Foundation Postdoctoral Fellowship and a Pathway to Independence (PI) Award from the National Institute of Health. D.A.M. is an HHMI investigator and this work was supported in part by the Harvard Stem Cell Institute and the NIH.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
The microarray data are available from GEO (Gene Expression Omnibus) with the accession number GSE 12025.
The authors declare no competing financial interests.
Reference
- 1.Weissman IL Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–68 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Hochedlinger K & Jaenisch R Nuclear reprogramming and pluripotency. Nature 441, 1061–7 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Orkin SH & Zon LI Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–44 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slack JM Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol 8, 369–78 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Brockes JP & Kumar A Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol 3, 566–74 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Hadorn E Transdetermination in cells. Sci Am 219, 110–4 passim (1968). [DOI] [PubMed] [Google Scholar]
- 7.Gurdon JB From nuclear transfer to nuclear reprogramming: the reversal of cell differentiation. Annu Rev Cell Dev Biol 22, 1–22 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–76 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–72 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Yu J et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science (2007). [DOI] [PubMed] [Google Scholar]
- 11.Meissner A, Wernig M & Jaenisch R Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol 25, 1177–81 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Wernig M et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–24 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Park IH et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–6 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Choi J et al. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A 87, 7988–92 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen CN, Slack JM & Tosh D Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol 2, 879–87 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Xie H, Ye M, Feng R & Graf T Stepwise reprogramming of B cells into macrophages. Cell 117, 663–76 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Cobaleda C, Jochum W & Busslinger M Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 449, 473–7 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Whitehead GG, Makino S, Lien CL & Keating MT fgf20 is essential for initiating zebrafish fin regeneration. Science 310, 1957–60 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Tanaka EM Cell differentiation and cell fate during urodele tail and limb regeneration. Curr Opin Genet Dev 13, 497–501 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13, 103–14 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Murtaugh LC & Melton DA Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol 19, 71–89 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Jensen J Gene regulatory factors in pancreatic development. Dev Dyn 229, 176–200 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Gu G, Dubauskaite J & Melton DA Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–57 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Baeyens L et al. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 48, 49–57 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Minami K et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci U S A 102, 15116–21 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang AY, Peng PD, Ehrhardt A, Storm TA & Kay MA Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther 15, 405–13 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Wang AY, Ehrhardt A, Xu H & Kay MA Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther 15, 255–63 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Lammert E et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol 13, 1070–4 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Konstantinova I et al. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129, 359–70 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Ferber S et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 6, 568–72 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Kaneto H et al. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes 54, 1009–22 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Miyatsuka T et al. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun 310, 1017–25 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Minami K & Seino S Pancreatic acinar-to-beta cell transdifferentiation in vitro. Front Biosci 13, 5824–37 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Okuno M et al. Generation of insulin-secreting cells from pancreatic acinar cells of animal models of type 1 diabetes. Am J Physiol Endocrinol Metab 292, E158–65 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Sapir T et al. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A 102, 7964–9 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heremans Y et al. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol 159, 303–12 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasa R et al. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc Natl Acad Sci U S A 101, 13245–50 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morton RA, Geras-Raaka E, Wilson LM, Raaka BM & Gershengorn MC Endocrine precursor cells from mouse islets are not generated by epithelial-to-mesenchymal transition of mature beta cells. Mol Cell Endocrinol 270, 87–93 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gershengorn MC et al. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science 306, 2261–4 (2004). [DOI] [PubMed] [Google Scholar]
- 40.De Robertis EM & Gurdon JB Gene activation in somatic nuclei after injection into amphibian oocytes. Proc Natl Acad Sci U S A 74, 2470–4 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dor Y, Brown J, Martinez OI & Melton DA Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–6 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material