Plasmacytoid dendritic cells in immunity (original) (raw)
Abstract
Human and mouse plasmacytoid dendritic cells have been shown to correspond to a specialized cell population that produces large amounts of type I interferons in response to viruses, the so-called natural interferon–producing cells. As a result, intensive investigation is now focused on the potential functions of plasmacytoid dendritic cells in both innate and adaptive immunity. Here we review recent progress on the characterization of plasmacytoid dendritic cell origin, development, migration and function in immunity and tolerance, as well as their effect on human diseases.
Similar content being viewed by others
Main
In 1958, Lennert and Remmele first reported a cell type similar to plasma cells but lacking B cell and plasma cell markers that was abundant in the T cell zones of human lymphoid tissue1 (Fig. 1). These cells were called T-associated plasma cells1 and later plasmacytoid T cells2. They were also identified in the thymus and in lymph nodes of patients with various inflammatory and neoplastic diseases2. Because these cells shared some markers with myelomonocytic cells2, they were also called plasmacytoid monocytes, and these different terms were used interchangeably for many years.
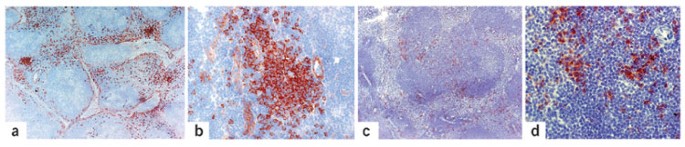
Figure 1: Localization of pDCs.
The pDCs are present mostly in clusters in the T cell–rich area of secondary lymphoid organs. (a,b) In human lymph nodes, they are closely associated with high endothelial venules. (c,d) In mouse spleens, pDCs are found mostly in the periarteriolar lymphoid sheaths, but scattered pDC are present in the marginal zone and red pulp, with a distribution distinct from that of DCs22,23. Inflammatory conditions induce the clustering of pDCs either in the marginal zone or in the T cell area of the spleen (C. Asselin-Paturel, unpublished data) and their recruitment to the sentinel lymph nodes23. Images show pDCs in human lymph nodes (a,b) and mouse spleens (c,d) at various magnifications after staining with antibodies CD123 (a,b) and 440c (c,d). Courtesy of F. Facchetti (Department of Pathology, University of Brescia, Brescia, Italy). Original magnifications, ×50 (a,c), ×400 (b) and ×200 (d).
Human peripheral blood leukocytes are known to be efficient producers of type I interferon after exposure to viruses. In 1978, Trinchieri and colleagues3 showed that type I interferon is a potent enhancer of natural killer (NK) cell–mediated cytotoxicity and that during exposure of peripheral blood leukocytes to viruses or virus-infected cells, only a small subset of cells is responsible for production of most of the interferon antiviral activity. These interferon-producing cells were identified as non-T, non-B, non-NK, non-monocytic cells expressing low-affinity Fcγ receptors and major histocompatibility complex (MHC) class II antigens3,4,5,6. Although interferon-producing cells were rare nonadherent lineage-negative MHC class II–positive mononuclear cells4, they were also clearly distinct from classical peripheral blood dendritic cells (DCs)7,8.
In 1994, O'Doherty et al.9 identified in human peripheral blood a subset of CD11c− immature DCs with low MHC class II expression and poor T cell stimulatory ability. After culture with monocyte-conditioned medium, these cells increased MHC class II expression and acquired a more irregular dendritic-like morphology. Grouard et al.10 subsequently isolated CD11c−CD4+CD45RA+ cells from tonsil and showed that they have characteristics almost identical to those of the peripheral blood CD11c− immature DC subset and corresponded to the plasmacytoid T cells or monocytes described in secondary lymphoid organs. These cells died rapidly in culture, but in presence of interleukin 3 (IL-3) and CD40 ligand (CD40L) they differentiated into cells with mature interdigitating DC morphology10 (Fig. 2). The survival effect of IL-3 on these 'plasmacytoid' DC precursors depended on their high expression of IL-3 receptor α-chain (IL-3Rα). On the basis of the ability of the plasmacytoid cells to induce T helper type 2 (TH2) cell differentiation in response to certain stimuli, the name type 2 DC precursor (pDC2) was proposed. Monocytic precursors of myeloid DCs, which favor a TH1 response, were given the name pDC1 (ref. 11). However, because of the flexibility of both classical DCs and plasmacytoid DCs to give raise to either TH1 or TH2 responses, the descriptive term plasmacytoid DCs (pDCs) is now preferred. In 1999, Siegal et al.12 and Cella et al.13 definitively identified the pDCs of peripheral blood and secondary lymphoid organs as being identical to the interferon-producing cells responsible for type I interferon production in peripheral blood in response to most viruses.
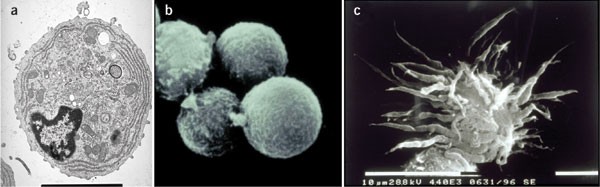
Figure 2: Morphology of pDCs.
(a) By electron microscopy, pDCs appear as lymphoblasts with a medium-to-large diameter, a slightly eccentric, indented, round or oval nucleus, lightly stained perinuclear areas and well developed rough endoplasmic reticulum. (b,c) By scanning electron microscopy, resting pDCs have a spherical shape (b), whereas CD40L-activated pDCs have a dendritic cell–like morphology (c). Original magnifications, ×7,000 (a) and ×3,000 (b,c). Reproduced from ref. 10 by copyright permission of the Rockefeller University Press.
The phenotype of human pDCs
Human pDCs are CD4+CD45RA+IL-3Rα(CD123)+ILT3+ILT1−CD11c− lineage− cells. The low-affinity Fcγ receptor identified in early studies of interferon-producing cells3,4 corresponds to FcγRIIa (CD32), which modulates type I interferon production14. Two additional markers, BDCA-2 and BDCA-4, are restricted to human pDCs in peripheral blood and bone marrow15. BDCA-2 is a C-type lectin transmembrane glycoprotein that can internalize antigen for presentation to T cells, and antibodies to BDCA-2 inhibit type I interferon production from these cells15. BDCA-4 is identical to neuropilin-1, a neuronal receptor of the class 3 semaphorin subfamily that is also a coreceptor for vascular endothelial growth factor A on endothelial and tumor cells. Antibodies to BDCA-4 do not have a substantial effect on pDC function and can be used for the purification of pDCs by positive selection16. A malignant counterpart of pDCs has been identified as a very rare hematological neoplasia17. These tumor cells have a morphology very similar to that of pDCs, they express the same surface markers, including BDCA-2 and BDCA-4, and they produce large amounts of type I interferons in response to viruses17.
Characterization of mouse pDCs
In 2001, three different groups reported the identification of mouse pDCs18,19,20, which share most of the morphological, phenotypic and functional characteristics of their human counterparts. Unlike human pDCs, mouse pDCs express the cell surface antigens B220 and Ly6C. Thus, they react with the RB6-8C5 (GR1) antibody, which recognizes Ly6G on granulocytes but cross-reacts with Ly6C18,19,20. Moreover, mouse pDCs express CD11c, although in low amounts compared with DCs, and, except in mice treated with the cytokine Flt3 ligand (Flt3L), they do not express CD123 (IL-3Rα)19,20. Activation of pDCs improves their viability in culture and upregulates their expression of MHC class II and costimulatory molecules19. In contrast to freshly isolated pDCs, which have low expression of CD8α in a proportion of cells, activated pDCs have high expression of CD8α19,21.
Three additional antibodies recognizing mouse pDC–restricted surface markers have been identified22,23. The 120G8 antibody reacts with an antigen strongly expressed on mouse pDCs but also upregulated by type I interferon on other cells, such as B lymphocytes and endothelial cells22. Use of the 120G8 antibody effectively depletes pDCs in vivo. The 440c antibody also selectively recognizes mouse pDCs and is not upregulated by type I interferon on other cells. Unlike the 120G8 antibody, the 440c antibody directly reduces type I interferon production by pDCs in vitro and in vivo without depleting the cells23. The mPDCA-1 antibody selectively recognizes and depletes mouse pDCs24,25. In experiments using combinations of traditional surface markers or the new specific antibodies, mouse pDCs have been identified in lymphoid organs (Fig. 1) and also in liver, lung and skin22,23. The number of pDCs in the spleen is variable among mouse strains, with the 129 strain having a several-fold greater number of pDCs than most other strains22. Mice in which pDCs had been depleted in vivo using the GR1 antibody or pDC-specific antibodies 120G8 and mPDCA-1 were unable to produce type I interferons in response to mouse cytomegalovirus infection or to CpG oligonucleotides22,24,25,26, demonstrating that pDCs represent in vivo a specialized type I interferon–producing cell.
The development of pDCs
The developmental path and molecular regulation of pDCs are not fully understood. Flt3L is the main cytokine for the development of pDCs from hematopoietic stem cells in humans and mice27,28,29,30,31. The ability of Flt3L to promote pDC development in vivo was demonstrated by experiments showing that administration of Flt3L to human volunteers led to an increase in the number of peripheral blood pDCs31 and that Flt3L-transgenic mice have more pDCs, whereas Flt3L-deficient mice have fewer pDCs30. Another important cytokine in pDC biology is granulocyte colony-stimulating factor, which promotes pDC mobilization from bone marrow31,32.
The hypothesis that pDCs are of lymphoid origin is supported by findings that the gene transcripts of pre–T cell receptor α (pTα), λ5 and Spi-B, as well as immunoglobulin H (IgH) diversity-joining gene rearrangements, are found in pDCs but not in DCs of myeloid origin (mDCs)33,34. Moreover, overexpression of the dominant negative transcription factor Id2 or Id3 blocks development of pDCs, T cells and B cells but not of mDCs35. However, more recent studies have shown that Flt3+ cells among common lymphoid progenitors (CLPs) or common myeloid progenitors (CMPs) can differentiate into both mDCs and pDCs in cultures and in vivo36,37. In addition, studies of mice deficient in interferon-regulatory factor 8 (IRF8), a transcriptional factor essential for the myeloid cell lineage38, demonstrated that the generation of pDCs, CD8α+ DCs, epidermal DCs and dermal DCs were all impaired39. As a result of these seemingly divergent findings, several different hypotheses have been proposed regarding the developmental origin of pDCs. One hypothesis proposes the existence of a common DC precursor in blood that can give rise to all DC subsets40. Another hypothesis suggests that pDCs arise as a branch of the committed lymphoid lineage10,34. The pDCs also have been proposed to be a population of lymphoid cells undergoing an in vivo cell fate conversion from a lymphoid to a myeloid cell type41.
Although CMPs do not express recombination activating gene products and IgH diversity-joining rearrangement, pDCs derived from CMPs express recombination activating gene products and show IgH diversity-joining rearrangement42. Thus, pDCs may represent a unique hematopoietic lineage, whose development may be much more flexible than both conventional lymphoid (B, T and NK) and myeloid (monocyte and granulocyte) cells. Moreover, it has been shown that virus infection can program bone marrow pDCs to differentiate into mDCs43. Thus, pDC development during immune response against pathogens warrants more in-depth analysis.
Localization, migration and life span
The identification of human pDCs in fetal liver, thymus and bone marrow suggests that pDCs develop from hematopoeitic stem cells in these primary lymphoid tissues27. Transfer of human CD34+ hematopoietic progenitor cells into severe combined immunodeficient mice or mice deficient in recombination activating gene 2 and IL-2Rγ leads to the generation of human pDCs in mouse bone marrow and human thymus grafted subcutaneously44,45,46. During adult life, pDCs are produced constantly in the bone marrow and migrate from the bone marrow to lymph nodes, mucosal-associated lymphoid tissues and spleen in steady-state conditions. Kinetic studies indicate that pDCs in mice have an average turnover of about 2 weeks21.
Human blood pDCs express L-selectin13, which is downregulated in lymphoid organs, where pDCs are particularly abundant in the T cell–rich areas around high endothelial venules2,10 (Fig. 1). Thus, pDCs may constitutively emigrate from the blood into lymph nodes through high endothelial venules, like naive T lymphocytes. Consistent with this hypothesis, noninflamed lymph nodes and spleens of L-selectin-deficient mice show a substantial reduction in pDCs18. However, other studies have suggested that circulating pDCs preferentially accumulate in lymph nodes when they are exposed to an inflammatory stimulus13,23,47. In addition, pDC attachment to inflamed high endothelial venules is dominated by E-selectin- rather than L-selectin-mediated interactions47. Thus, the relative importance of constitutive and inflammation-induced pDC migration into the lymph nodes is still controversial.
Chemokines and their receptors mediate leukocyte trafficking. The pDCs express the chemokine receptor CXCR3 (refs. 13,47–50), which promotes migration in response to interferon-γ (IFN-γ)–inducible chemokines such as CXCL9 and CXCL10. CXCR3 is required for most pDC migration into inflamed lymph nodes47. However, pDCs express at least three additional receptors, CCR1, CCR2 and CCR5, for the inflammatory chemokines CCL2, CCL3, CCL4 and CCL5. They also express two receptors, CXCR4 and (after activation) CCR7, for the constitutive chemokines CXCL12 and CCL21 (refs. 47–49). CCR7 can mediate migration of mouse pDCs in in vitro chemotaxis assays47,49, and CXCR4 is involved in pDC migration in vivo, at least into tumors 51. Moreover, pDCs express the G protein–coupled receptor ChemR23, which drives their migration in response to chemerin, a peptide chemoattractant that is released by inflamed tissues and tumors (S. Sozzani, unpublished data). Thus, CXCR3 may not be solely responsible for pDC migration; at least some redundancy seems likely in vivo in steady-state and/or inflammatory conditions.
In vitro studies have shown that CCR2, CCR5, CCR7 and CXCR3 are not functional in human pDCs48. However, CXCR3 and CXCR4 can mediate pDC migration when simultaneously engaged49,50, suggesting that chemokine receptor function may require cooperation between distinct receptors. Studies in T cells have demonstrated that chemokine receptors can heterodimerize, delivering intracellular signals through pertussis toxin–insensitive pathways that trigger cell adhesion but not chemotaxis52. Thus, CXCR3, CXCR4 and other chemokine receptors on pDCs may heterodimerize, stabilizing pDC adhesive interactions with high endothelial venules. A possible requirement for LFA-1 or other integrins expressed on pDCs, such as VLA4 and VLA5, for firm adherence of pDCs to high endothelial venules remains to be explored. However, involvement of VLA5 has already been found in pDC migration into tumors, as tumor microvasculature expresses a counter-ligand for VLA-5, VCAM-1 (ref. 51).
Pathogen sensing
The ability of pDCs to secrete type I interferons depends on cellular sensors that promptly detect the presence of DNA and RNA viruses. Initial clues regarding the identity of pDC viral sensors came from the demonstration that pDCs express a subset of Toll-like receptors (TLRs), including TLR7 and TLR9 (ref. 53). TLR9 expression accounts for pDC responses to CpG oligonucleotides, which mimic bacterial DNA53. TLR7 is responsible for pDC responses to guanosine analogs as well as imidazoquinolines, which are used as antiviral compounds in the treatment of human papilloma virus infections53,54. All TLRs expressed in pDCs signal through the adaptor molecule MyD88, which recruits signaling mediators to activate the transcription factor NF-κB54. Unlike DCs, pDCs do not express TLR2, TLR4, TLR5 or TLR3, which explains why pDCs do not respond to bacterial products such as peptidoglycans, lipopolysaccharide and flagellin, or poly(I:C), which mimics viral double-stranded RNA.
Because DNA viral genomes contain CpG-rich regions, TLR9 was a likely candidate for pDC recognition of viral DNA. The generation of MyD88- and TLR9-deficient mice54 allowed the demonstration that pDCs require TLR9 to respond to DNA viruses, including herpes simplex virus 2 and 1 and mouse cytomegalovirus24,55,56. Although TLR9-deficient pDCs respond to RNA viruses (such as influenza), MyD88-deficient pDCs do not. This observation, along with the structural homology between some TLR7 ligands and ribonucleotides57, indicated that TLR7 is a likely candidate for pDC recognition of RNA viruses. This has been demonstrated with single-stranded RNA (ssRNA) viruses such as influenza58,59 and vesiculostomatitis virus60, as well as synthetic ssRNA sequences rich in uridine that mimic ssRNA viruses58,59.
The identification of pDC viral sensors raises several intriguing questions. One enigma is how viral nucleic acids actually reach the TLRs. Unlike many TLRs, TLR7 and TLR9 are not expressed on the cell surface but instead are expressed in the endosomal compartment of pDCs61. Accordingly, lysosomal inhibitors like cloroquine inhibit pDC responses to viruses55,59,60. Moreover, recognition of DNA and ssRNA viruses through TLR9–TLR7 does not require active viral replication55,56,59,60,62. Thus, it is possible that after binding and/or fusing to cells, DNA and ssRNA viruses are internalized and delivered to the lysosomal compartment, where DNA and ssRNA are released to interact with TLRs.
Another key question concerns the signaling pathway that connects TLR-MyD88 with secretion of type I interferons. Transcriptional regulation of IFN-β and IFN-α genes is controlled mainly by IRF3 and/or IRF7 (ref. 63). IRF3 can be activated by TLR3 and TLR4 through the pathway mediated by the adaptor molecule TRIF but there is no evidence for this pathway in pDCs. IRF7 has constitutively high expression in pDCs but not in DCs64,65,66. Moreover, MyD88 recruits IRF7 but not IRF3 through the adaptor molecule TRAF6 (ref. 67). Thus, this MyD88-TRAF6-IRF7 pathway may explain why TLRs elicit substantial secretion of IFN-α in pDCs while inducing little IFN-α in DCs.
DCs can secrete type I interferons in response to RNA viruses, although less efficiently than pDCs68. The interferon response of DCs is mediated mainly by TLR-independent molecular sensors of double-stranded RNA69, which include double-stranded RNA–activated protein kinase (PKR) and the RNA helicase RIG-I. The function of these molecules in the pDC response to viruses remains to be explored. The picture emerging from various studies25,56,62,69 shows both pDCs and DCs, and possibly other cells, secreting type I interferon and other cytokines in response to viruses through TLR-dependent and TLR-independent pathways. These multiple pathways may provide the innate system with flexibility in antiviral immunity.
Linking innate and adaptive immunity
Because pDCs produce large amounts of cytokines, particularly type I interferons, they regulate inflammation and link innate with adaptive immunity (Fig. 3). Type I interferons released by human pDCs activate NK cell cytolytic activity but protect uninfected cells from NK cell–mediated lysis3,70. Human pDCs can also induce IFN-γ production in NK cells through IL-12 secretion, although less efficiently than activated DCs. In the mouse, mouse cytomegalovirus infection activates pDCs through TLR9, triggering secretion of type I interferons and IL-12 that increase NK cell–mediated cytotoxicity, IFN-γ production and, in vivo, early antiviral resistance24,71. Type I interferons released by pDCs can also affect T cell functions, inducing early activation markers such as CD69, long-term T cell survival, IFN-γ production and TH1 differentiation, although with different molecular mechanisms in humans and mice72. Moreover, type I interferons promote differentiation, maturation and immunostimulatory functions of DCs73. A very important aspect of pDC-mediated regulation of adaptive immunity is the ability, through the production of both type I interferons and IL-6, to induce human B cells to differentiate into plasma cells and produce immunoglobulin74,75. Moreover, B cells activated by pDCs preferentially secrete IgG rather than IgM, suggesting that pDCs may specifically target memory B cells. As pDCs are activated by virus infections, they may be particularly important in inducing antiviral antibodies.
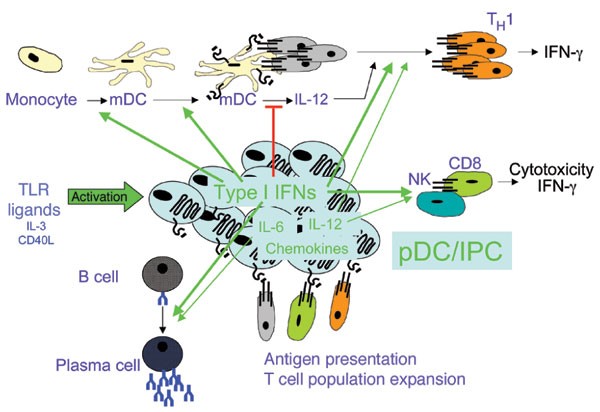
Figure 3: The immunostimulatory functions of activated pDCs.
The pDCs activated by TLRs, CD40L and culture with IL-3 are potent producers of type I interferons, which promote NK and CD8+ T cell cytotoxicity and secretion of IFN-γ; differentiation and maturation of DC; TH1 polarization of uncommitted antigen-experienced CD4+ T helper cell populations expanded by DCs; and differentiation of B cells into plasma cells. Type I interferons also regulate secretion of IL-12 by DCs and other cell types. The pDCs also secrete IL-12, which contributes to TH1 polarization; proinflammatory chemokines such as CXCL9, CXCL10, CCR3, CCR4 and CCR5, which attract activated T cells; and IL-6, which facilitates differentiation of plasma cells. The pDCs can also present antigens and expand memory and/or naive T cell populations, depending on the experimental model. IPC, interferon-producing cell.
Another main effect of type I interferons is the inhibition of IL-12 production, particularly the p40 subunit26 (Fig. 3). In some conditions, however, type I interferons enhance the production of the IL-12 p70 heterodimer72. Moreover, endogeneous type I interferon signaling is required for optimal IL-12 p35 subunit induction and IL-12 p70 heterodimer production by both human and mouse DCs (G. Gautier, unpublished data). Thus, the effects of type I interferon on IL-12 production and the mechanisms underlying these effects are still unclear. The production of IL-12 by human pDCs is controversial, but cells preincubated with IL-3 and then stimulated with CpG oligonucleotides and CD40L are efficient producers of IL-12 (p70 plus p40) while downregulating their ability to produce type I interferon16,76. Mouse pDCs clearly produce IL-12 p70 in response to exposure to viruses or different TLR ligands19. Both human and mouse pDCs are potent producers of chemokines, which may potentiate adaptive responses by attracting activated CD4+ and CD8+ T cells during an immune response49,77,78,79.
pDCs as antigen-presenting cells
Freshly isolated human and mouse pDCs are very poor inducers of T lymphocyte proliferation. The pDCs may present antigens inefficiently because they do not capture, process and load antigens onto MHC molecules as effectively as classical DCs. Supporting this, studies have shown that pDCs do not endocytose antigens as well as DCs10. In addition, pDCs have low expression of two mature cathepsins, cathepsin S and cathepsin D, which are lysosomal proteases involved in antigen processing80. Most importantly, pDCs have minimal expression of costimulatory molecules and low MHC class II expression compared with DCs7,10,19. The transcription factor MHC class II transactivator (CIITA), which regulates MHC class II expression, is driven by different promoters in pDCs (C2ta pIII) and DCs (C2ta pI)81. Although CIITA type I mRNA is silenced shortly after DC maturation, inhibiting de novo MHC class II biosynthesis, CIITA type III mRNA is not silenced by pDC activation. Whether the unique transcriptional regulation of MHC class II in pDCs is the basis for low cell surface amounts of MHC class II remains to be explored.
In contrast to freshly isolated pDCs, activated pDCs augment cell surface expression of MHC class II and costimulatory molecules, increasing their T cell stimulatory ability10,19,82 (Fig. 3). Accordingly, activated pDCs induce expansion of antigen-specific memory CD8+ T cell populations and TH1 CD4+ T cell populations specific for endogenous antigens83 and influenza virus78. Human leukemic pDCs also efficiently present antigens to influenza virus–specific CD4+ and CD8+ T cell clones and tetanus toxoid–specific T cell clones17. Mouse pDCs activated in vivo with mouse cytomegalovirus and in vitro with CpG present pulsed peptides to naive T cells and induce a potent TH1 polarization in vitro71,84. Moreover, CpG-activated and influenza virus-activated mouse pDCs expand naive CD8+ T cell populations in vivo in response to endogenous and exogenous antigens, respectively85,86. Thus, activated pDCs can present antigens and induce considerable expansion of T cell populations, although less efficiently than DCs. Because CD8+ T cell responses against herpes simplex virus 1, vaccinia virus and influenza virus in vivo are primed mainly by CD8α+ DCs87 and not by other DC or pDC subsets, further investigation of pDC antigen presentation during diverse viral infections in vivo is warranted. It is also possible that pDCs cooperate with DCs in vivo, inducing the differentiation of unpolarized antigen-experienced T cell populations that have been expanded by DCs83,88. This independent and coordinated control of T cell proliferation and differentiation may provide the immune system with greater flexibility in regulating immune responses.
pDCs in T cell polarization
The pDCs are considerably flexible in directing T cell responses, depending on the maturation stage and nature and concentration of the antigen84,89. Virus-activated pDCs have been shown to elicit a potent TH1 polarization, inducing T cells to produce large amounts of IFN-γ82 or both IFN-γ and IL-10 (ref. 90). The TH1 polarization mediated by mature pDCs was shown to depend on type I interferon90 or on both type I interferon and IL-12 (ref. 82; Fig. 3). In one study, CD40L-activated pDCs preincubated with IL-3 were also shown to induce TH1 polarization82. However, an earlier study showed that IL-3- and CD40L-activated pDCs induced a modest TH2 response in allogeneic T cells that was not mediated by IL-4 (ref. 11). Moreover, IL-3- and CD40L-activated pDCs that expressed the costimulatory molecule OX40L and produced little type I interferon preferentially primed TH2 cells through an OX40L-dependent mechanism91 (Fig. 3). Virus activation of pDCs for 24 h induced a strong type I interferon response that resulted in the masking of the OX40L effect and development of TH1 cells91. As IL-3 progressively augments production of IL-12 by pDCs16,76, the discrepancies regarding the ability of CD40L-activated pDCs to prime different types of T helper responses and the reciprocal function of type I interferon and IL-12 in T cell priming may be due to variations in the periods of preincubation with IL-3, which was used for maintaining pDC viability11,82.
When CD40L-activated pDCs are cultured with allogeneic CD8+ T cells, the T cells show poor secondary proliferative and cytolytic activity92 (Fig. 4). These pDC-primed CD8+ T cells produce little IFN-γ but substantial IL-10 and inhibit bystander proliferation of naive CD8 T cells92. Both the generation of these CD8+ T cells and their suppression mechanism are IL-10 dependent, consistent with a regulatory function for these cells92. However, production of IL-10 is often found in strongly TH1-polarized T cell clones and it is directly induced by priming with TH1-polarizing cytokines such as IL-12 (ref. 93) and type I interferon94.
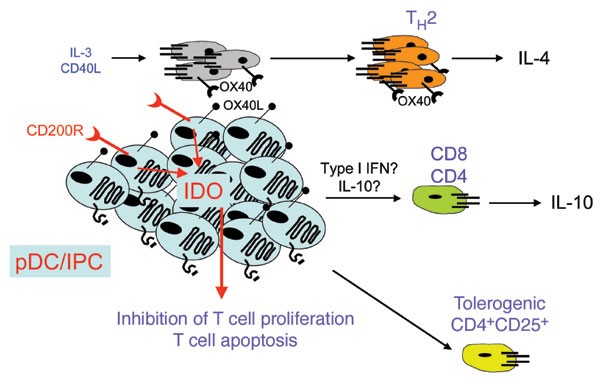
Figure 4: The tolerogenic functions of pDCs.
The pDCs activated in the presence of CD40L, short-term culture in IL-3 and the absence of TLR ligands preferentially prime TH2 cells through an OX40L-dependent mechanism. CD8+ (and CD4+) T cells primed with CD40L-activated pDCs have a regulatory phenotype, with poor proliferative and cytolytic activity and little production of IFN-γ but release of IL-10. Generation of these regulatory T cells may be induced by type I interferon, strong TH1 polarization, IL-10 or as-yet-unknown mechanisms. Stimulation of pDCs through the CD200 receptor (CD200R) induces the production of indoleamine 2,3 dioxygenase (IDO), leading to tryptophan depletion and the generation of toxic metabolites, which have a strong inhibitory effect on T cell proliferation and survival. The pDCs have also been reported to induce CD4+CD25+ T regulatory cells capable of suppressing T cell proliferation in antigen-independent way96.
Tolerogenic functions of immature pDCs
In humans, freshly isolated pDC induce anergy in human CD4+ T cell clones with various antigenic specificities. IL-2 and IFN-γ production by T cells is impaired, whereas IL-10 production is increased. Anergy is reversed by the addition of exogeneous IL-2 (ref. 95). One study has suggested that human pDCs can induce CD4+CD25+ T regulatory cells96 (Fig. 4). In the mouse, freshly isolated antigen-pulsed spleen pDCs induce minimal proliferation and no cytokine polarization in antigen-specific T cell receptor–transgenic T cells84,97. These T cells do not proliferate even if exogenous IL-2 is added but they acquire regulatory activity and suppress antigen-specific T cell proliferation97. Stimulation of pDCs with the inhibitory ligands CTLA4-Ig or OX2(CD200)-Ig induces the production of indoleamine 2,3-dioxygenase, which has a strong inhibitory activity on T cell proliferation and could contribute to the tolerogenic potential of these cells89,98 (Fig. 4). In addition, depletion of pDCs in mice during inhalation of normally inert antigen led to a TH2 response and all the cardinal features of asthma, whereas adoptive transfer of pDCs before sensitization prevented disease in a mouse asthma model, suggesting that pDCs may provide intrinsic protection against inflammatory responses to harmless antigens99. Unlike results with spleen pDCs, CpG oligonucleotide stimulation of cells derived from the mesenteric lymph nodes with characteristics of the pDCs resulted in antigen-presenting cells that were able to differentiate naive T cells into regulatory T cells with suppressive activity100. Thus, pDCs, particularly in their immature state, or in certain anatomic localizations, may have tolerogenic functions, although the exact nature and mechanisms of these functions remain to be explored (Fig. 4).
pDCs and human diseases
The expression of CD4, CXCR4 and CCR5 by human pDCs suggest that they may be the targets of human immunodeficiency virus (HIV) infection48. Immunohistochemical staining of tonsils and thymus from HIV-infected people shows the presence of pDCs containing the HIV p24 antigen, indicating that pDCs are productively infected by HIV101. Earlier studies showed that peripheral blood mononuclear cells from patients with AIDS have a decreased ability to produce IFN-α in response to herpes simplex virus stimulation102. Subsequently, studies demonstrated that peripheral blood pDC numbers are decreased in advanced stages of HIV infection103,104,105. Loss of circulating pDCs correlates with a high HIV viral load and the occurrence of opportunistic infections and Kaposi sarcoma103.
A link between IFN-α and human systemic lupus erythematosus (SLE) was originally made by two clinical observations: the development of SLE during IFN-α therapy in a 23-year-old woman with a metastatic carcinoma106, and the finding that many SLE patients have increased serum concentrations of type 1 interferons107. The numbers of circulating pDCs are decreased in patients with SLE, but large numbers of activated pDCs infiltrate the skin lesions and actively produce type 1 interferon in these patients108,109. The pDCs seem to be activated by immune complexes consisting of double-stranded DNA antibodies and DNA derived from apoptotic cells15,110. The high concentrations of IFN-α in the sera of SLE patients were found to activate DCs to trigger T cell–mediated autoimmunity111 as well as to promote the differentiation of B cells into antibody-secreting plasma cells74. The expression of type 1 interferon genes and interferon-induced genes represent the most salient molecular 'signatures' of SLE peripheral blood cells112,113, and they may be the most important effector molecules in SLE pathology, as well as targets for treating SLE.
Both immature DCs and pDCs infiltrate solid tumors114,115,116. Tumor-infiltrating immature DCs seem to be refractory to stimulation by lipopolysaccharide or CD40L and lack the ability to activate T cells115,117. These tumor-infiltrating immature myeloid DCs probably present tumor antigens continuously and induce tumor-specific regulatory T cells118,119. In breast cancer, large numbers of infiltrating CD123+ pDCs are correlated with an increased risk of tumor dissemination and relapse (I. Treilleux, unpublished data). In ovarian epithelial cell carcinomas, large numbers of pDCs were found in ascites. These pDCs were incapable of activating T cells and instead induced IL-10-producing regulatory T cells51. The pDCs in the tumor-draining lymph nodes may express indoleamine 2,3-dioxygenase, which may create a local microenvironment that is potently suppressive of host antitumor T cell responses120. These findings suggest that both immature DCs and pDCs in the solid tumor are incapable of inducing antitumor immune responses, but instead may induce regulatory T cells that inhibit immunity. TLR agonists may be used to activate tumor-infiltrating pDCs to produce type 1 interferon, which may activate tumor-infiltrating immature DCs to induce antitumor T cell responses or may have antiangiogenetic effects. Indeed, one study has demonstrated that targeting pDCs by imidazoquinolines in cutaneous tumors can induce tumor regression121.
Outstanding questions
Despite extensive characterization of pDCs, particularly in the past few years, there are still unsolved mysteries regarding their origin and function. The relationship between pDCs and DCs, especially whether pDCs are actually precursors that develop into mature DCs fully endowed with antigen-presenting function, will continue to be an important matter of investigation. Analyses of pDCs and DCs subset transcriptomes at distinct stages of differentiation will be essential for delineating the pDC developmental pathway(s) at the molecular level. Identification of previously unknown pDC-specific markers, including 120G8, 440c and mPDCA-1, in conjunction with analysis of pDC differentiation in mice with various genetic defects in leukocyte development will also aid in 'dissecting' pDC development.
A related key issue is the contribution of pDCs to immune responses, especially in comparison with DCs. Classical DCs serve as sentinels in peripheral tissues, where they alert the immune system to pathogens and then initiate immune responses in secondary lymphoid organs122. The pDCs, however, circulate in the blood and access lymph nodes from the blood, driven mainly by inflammatory stimuli. Therefore, pDCs may not be essential for initiating immune responses. In fact, pDCs may specialize in modulating the strength, duration and quality of NK, T and B cell responses by releasing appropriate cytokines and chemokines as well as presenting antigens. Moreover, depending on the stimulus, pDCs may become either immunostimulating or tolerogenic. The availability of depleting antibodies22,23,24,25 will allow investigation of pDC function in vivo in a variety of infectious models. In addition, imaging of pDCs in vivo by two-photon microscopy may provide a direct view of the interaction of pDCs with other cells during immune responses.
Although pDCs have been detected in the mucosa of patients with allergic rhinitis123, in the skin of patients with SLE108,109 and in tumors51,116, the assessment of pDC function in experimental models of allergy, autoimmunity and cancer has just begun. Adoptive transfer of pDCs activated with various stimuli and depletion of pDCs in these models will further the understanding of pDC function in the pathogenesis of human diseases and may also indicate that these cells could be potential targets for therapeutic intervention.
References
- Lennert, K. & Remmele, W. [Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes.]. Acta Haematol. 19, 99–113 (1958).
Article CAS PubMed Google Scholar - Facchetti, F., Vermi, W., Mason, D. & Colonna, M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 443, 703–717 (2003).
Article PubMed Google Scholar - Trinchieri, G., Santoli, D., Dee, R.R. & Knowles, B.B. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation. J. Exp. Med. 147, 1299–1313 (1978).
Article CAS PubMed Google Scholar - Perussia, B., Fanning, V. & Trinchieri, G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro α interferon production in response to viruses. Nat. Immunol. Cell Growth Regul. 4, 120–137 (1985).
CAS Google Scholar - Abb, J., Abb, H. & Deinhardt, F. Phenotype of human α-interferon producing leucocytes identified by monoclonal antibodies. Clin. Exp. Immunol. 52, 179–184 (1983).
CAS PubMed PubMed Central Google Scholar - Ronnblom, L., Ramstedt, U. & Alm, G.V. Properties of human natural interferon-producing cells stimulated by tumor cell lines. Eur. J. Immunol. 13, 471–476 (1983).
Article CAS PubMed Google Scholar - Chehimi, J. et al. Dendritic cells and IFN-α-producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunology 68, 488–490 (1989).
PubMed Central Google Scholar - Feldman, M. & Fitzgerald-Bocarsly, P. Sequential enrichment and immunocytochemical visualization of human interferon-α-producing cells. J. Interferon Res. 10, 435–446 (1990).
Article CAS PubMed Google Scholar - O'Doherty, U. et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 82, 487–493 (1994).
CAS PubMed PubMed Central Google Scholar - Grouard, G. et al. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185, 1101–1111 (1997).
Article CAS PubMed PubMed Central Google Scholar - Rissoan, M.C. et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283, 1183–1186 (1999).
Article CAS PubMed Google Scholar - Siegal, F.P. et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–7 (1999).
Article CAS PubMed Google Scholar - Cella, M. et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5, 919–923 (1999).
Article CAS PubMed Google Scholar - Bave, U. et al. FcγRIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. J. Immunol. 171, 3296–3302 (2003).
Article CAS PubMed Google Scholar - Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194, 1823–1834 (2001).
Article CAS PubMed PubMed Central Google Scholar - Dzionek, A. et al. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum. Immunol. 63, 1133–1148 (2002).
Article CAS PubMed Google Scholar - Chaperot, L. et al. Leukemic plasmacytoid dendritic cells share phenotypic and functional features with their normal counterparts. Eur. J. Immunol. 34, 418–426 (2004).
Article CAS PubMed Google Scholar - Nakano, H., Yanagita, M. & Gunn, M.D. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194, 1171–1178 (2001).
Article CAS PubMed PubMed Central Google Scholar - Asselin-Paturel, C. et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2, 1144–1150 (2001).
Article CAS PubMed Google Scholar - Bjorck, P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98, 3520–3526 (2001).
Article CAS PubMed Google Scholar - O'Keeffe, M. et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J. Exp. Med. 196, 1307–1319 (2002).
Article CAS PubMed PubMed Central Google Scholar - Asselin-Paturel, C., Brizard, G., Pin, J.J., Briere, F. & Trinchieri, G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 171, 6466–6477 (2003).
Article CAS PubMed Google Scholar - Blasius, A. et al. A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-α. Blood 103, 4201–4206 (2004).
Article CAS PubMed Google Scholar - Krug, A. et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21, 107–119 (2004).
Article CAS PubMed Google Scholar - Barchet, W. et al. Dendritic cells respond to Influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. (in the press).
- Dalod, M. et al. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195, 517–528 (2002).
Article CAS PubMed PubMed Central Google Scholar - Blom, B., Ho, S., Antonenko, S. & Liu, Y.J. Generation of interferon α-producing predendritic cell (Pre-DC)2 from human CD34+ hematopoietic stem cells. J. Exp. Med. 192, 1785–1796 (2000).
Article CAS PubMed PubMed Central Google Scholar - Chen, W. et al. Thrombopoietin cooperates with FLT3-ligand in the generation of plasmacytoid dendritic cell precursors from human hematopoietic progenitors. Blood 103, 2547–2553 (2004).
Article CAS PubMed Google Scholar - Gilliet, M. et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195, 953–958 (2002).
Article CAS PubMed PubMed Central Google Scholar - Brawand, P. et al. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J. Immunol. 169, 6711–6719 (2002).
Article CAS PubMed Google Scholar - Pulendran, B. et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 165, 566–572 (2000).
Article CAS PubMed Google Scholar - Arpinati, M., Green, C.L., Heimfeld, S., Heuser, J.E. & Anasetti, C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95, 2484–2490 (2000).
CAS PubMed Google Scholar - Rissoan, M.C. et al. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood 100, 3295–3303 (2002).
Article CAS PubMed Google Scholar - Corcoran, L. et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 170, 4926–4932 (2003).
Article CAS PubMed Google Scholar - Spits, H., Couwenberg, F., Bakker, A.Q., Weijer, K. & Uittenbogaart, C.H. Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 192, 1775–1784 (2000).
Article CAS PubMed PubMed Central Google Scholar - Karsunky, H., Merad, M., Cozzio, A., Weissman, I.L. & Manz, M.G. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 198, 305–313 (2003).
Article CAS PubMed PubMed Central Google Scholar - D'Amico, A. & Wu, L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J. Exp. Med. 198, 293–303 (2003).
Article CAS PubMed PubMed Central Google Scholar - Tamura, T. & Ozato, K. ICSBP/IRF-8: its regulatory roles in the development of myeloid cells. J. Interferon Cytokine Res. 22, 145–152 (2002).
Article CAS PubMed Google Scholar - Aliberti, J. et al. Essential role for ICSBP in the in vivo development of murine CD8α+ dendritic cells. Blood 101, 305–310 (2003).
Article CAS PubMed Google Scholar - del Hoyo, G.M. et al. Characterization of a common precursor population for dendritic cells. Nature 415, 1043–1047 (2002).
Article PubMed Google Scholar - Comeau, M.R., Van der Vuurst de Vries, A.R., Maliszewski, C.R. & Galibert, L. CD123bright plasmacytoid predendritic cells: progenitors undergoing cell fate conversion? J. Immunol. 169, 75–83 (2002).
Article CAS PubMed Google Scholar - Shigematsu, H. et al. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity 21, 43–53 (2004).
Article CAS PubMed Google Scholar - Zuniga, E.I., McGavern, D.B., Pruneda-Paz, J.L., Teng, C. & Oldstone, M.B. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat. Immunol. 5, 1227–1234 (2004).
Article CAS PubMed PubMed Central Google Scholar - Palucka, A.K. et al. Human dendritic cell subsets in NOD/SCID mice engrafted with CD34+ hematopoietic progenitors. Blood 102, 3302–3310 (2003).
- Weijer, K. et al. Intrathymic and extrathymic development of human plasmacytoid dendritic cell precursors in vivo. Blood 99, 2752–2759 (2002).
Article CAS PubMed Google Scholar - Traggiai, E. et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304, 104–107 (2004).
Article CAS PubMed Google Scholar - Yoneyama, H. et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16, 915–928 (2004).
Article CAS PubMed Google Scholar - Penna, G., Sozzani, S. & Adorini, L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J. Immunol. 167, 1862–1866 (2001).
Article CAS PubMed Google Scholar - Krug, A. et al. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J. Immunol. 169, 6079–6083 (2002).
Article CAS PubMed Google Scholar - Vanbervliet, B. et al. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J. Exp. Med. 198, 823–830 (2003).
Article CAS PubMed PubMed Central Google Scholar - Zou, W. et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat. Med. 7, 1339–46 (2001).
Article CAS PubMed Google Scholar - Mellado, M. et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 20, 2497–2507 (2001).
Article CAS PubMed PubMed Central Google Scholar - Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–95 (2004).
Article CAS PubMed Google Scholar - Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Article CAS PubMed Google Scholar - Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 (2003).
Article CAS PubMed PubMed Central Google Scholar - Krug, A. et al. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103, 1433–1437 (2004).
Article CAS PubMed Google Scholar - Lee, J. et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 100, 6646–6651 (2003).
Article CAS PubMed PubMed Central Google Scholar - Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Article CAS PubMed Google Scholar - Diebold, S.S., Kaisho, T., Hemmi, H., Akira, S. & Reis, E.S.C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Article CAS PubMed Google Scholar - Lund, J.M. et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101, 5598–5603 (2004).
Article CAS PubMed PubMed Central Google Scholar - Ahmad-Nejad, P. et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32, 1958–1968 (2002).
Article CAS PubMed Google Scholar - Hochrein, H. et al. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101, 11416–11421 (2004).
Article CAS PubMed PubMed Central Google Scholar - Taniguchi, T., Ogasawara, K., Takaoka, A. & Tanaka, N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19, 623–655 (2001).
Article CAS PubMed Google Scholar - Coccia, E.M. et al. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34, 796–805 (2004).
Article CAS PubMed Google Scholar - Izaguirre, A. et al. Comparative analysis of IRF and IFN-α expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74, 1125–1138 (2003).
Article CAS PubMed Google Scholar - Dai, J., Megjugorac, N.J., Amrute, S.B. & Fitzgerald-Bocarsly, P. Regulation of IFN regulatory factor-7 and IFN-α production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J. Immunol. 173, 1535–1548 (2004).
Article CAS PubMed Google Scholar - Kawai, T. et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5, 1061–1068 (2004).
Article CAS PubMed Google Scholar - Cella, M. et al. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189, 821–829 (1999).
Article CAS PubMed PubMed Central Google Scholar - Diebold, S.S. et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424, 324 (2003).
Article CAS PubMed Google Scholar - Bandyopadhyay, S., Perussia, B., Trinchieri, G., Miller, D.S. & Starr, S.E. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J. Exp. Med. 164, 180–195 (1986).
Article CAS PubMed Google Scholar - Dalod, M. et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon α/β. J. Exp. Med. 197, 885–898 (2003).
Article CAS PubMed PubMed Central Google Scholar - Agnello, D. et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 23, 147–161 (2003).
Article CAS PubMed Google Scholar - Santini, S.M. et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191, 1777–1788 (2000).
Article CAS PubMed PubMed Central Google Scholar - Jego, G. et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19, 225–234 (2003).
Article CAS PubMed Google Scholar - Poeck, H. et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 103, 3058–3064 (2004).
Article CAS PubMed Google Scholar - Krug, A. et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31, 3026–3037 (2001).
Article CAS PubMed Google Scholar - Penna, G. et al. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J. Immunol. 169, 6673–6676 (2002).
Article CAS PubMed Google Scholar - Fonteneau, J.F. et al. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101, 3520–3526 (2003).
Article CAS PubMed Google Scholar - Megjugorac, N.J., Young, H.A., Amrute, S.B., Olshalsky, S.L. & Fitzgerald-Bocarsly, P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 75, 504–514 (2004).
Article CAS PubMed Google Scholar - Fiebiger, E. et al. Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J. Exp. Med. 193, 881–92 (2001).
Article CAS PubMed PubMed Central Google Scholar - LeibundGut-Landmann, S., Waldburger, J.M., Reis e Sousa, C., Acha-Orbea, H. & Reith, W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat. Immunol. 5, 899–908 (2004).
Article CAS PubMed Google Scholar - Cella, M., Facchetti, F., Lanzavecchia, A. & Colonna, M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1, 305–310 (2000).
Article CAS PubMed Google Scholar - Krug, A. et al. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 197, 899–906 (2003).
Article CAS PubMed PubMed Central Google Scholar - Boonstra, A. et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J. Exp. Med. 197, 101–109 (2003).
Article CAS PubMed PubMed Central Google Scholar - Salio, M., Palmowski, M.J., Atzberger, A., Hermans, I.F. & Cerundolo, V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 199, 567–579 (2004).
Article CAS PubMed PubMed Central Google Scholar - Schlecht, G. et al. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood 104, 1808–1815 (2004).
Article CAS PubMed Google Scholar - Belz, G.T. et al. Cutting edge: conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 172, 1996–2000 (2004).
Article CAS PubMed Google Scholar - Iezzi, G., Scheidegger, D. & Lanzavecchia, A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J. Exp. Med. 193, 987–93 (2001).
Article CAS PubMed PubMed Central Google Scholar - Liu, Y.J., Kanzler, H., Soumelis, V. & Gilliet, M. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2, 585–589 (2001).
Article CAS PubMed Google Scholar - Kadowaki, N., Antonenko, S., Lau, J.Y. & Liu, Y.J. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192, 219–226 (2000).
Article CAS PubMed PubMed Central Google Scholar - Ito, T. et al. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J. Immunol. 172, 4253–4259 (2004).
Article CAS PubMed Google Scholar - Gilliet, M. & Liu, Y.J. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 195, 695–704 (2002).
Article CAS PubMed PubMed Central Google Scholar - Gerosa, F. et al. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J. Exp. Med. 183, 2559–2569 (1996).
Article CAS PubMed Google Scholar - Levings, M.K. et al. IFN-α and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166, 5530–5539 (2001).
Article CAS PubMed Google Scholar - Kuwana, M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum. Immunol. 63, 1156–1163 (2002).
Article CAS PubMed Google Scholar - Moseman, E.A. et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 173, 4433–4442 (2004).
Article CAS PubMed Google Scholar - Martin, P. et al. Characterization of a new subpopulation of mouse CD8α+B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood 100, 383–90 (2002).
Article CAS PubMed Google Scholar - Fallarino, F. et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J. Immunol. 173, 3748–3754 (2004).
Article CAS PubMed Google Scholar - De Heer, H.J. et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200, 89–98 (2004).
Article CAS PubMed PubMed Central Google Scholar - Bilsborough, J., George, T.C., Norment, A. & Viney, J.L. Mucosal CD8α+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology 108, 481–492 (2003).
Article CAS PubMed PubMed Central Google Scholar - Fong, L., Mengozzi, M., Abbey, N.W., Herndier, B.G. & Engleman, E.G. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 76, 11033–11041 (2002).
Article CAS PubMed PubMed Central Google Scholar - Lopez, C., Fitzgerald, P.A. & Siegal, F.P. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-α in vitro associated with severe opportunistic infections. J. Infect. Dis. 148, 962–966 (1983).
Article CAS PubMed Google Scholar - Soumelis, V. et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98, 906–912 (2001).
Article CAS PubMed Google Scholar - Feldman, S. et al. Decreased interferon-α production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101, 201–210 (2001).
Article CAS PubMed Google Scholar - Chehimi, J. et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168, 4796–4801 (2002).
Article CAS PubMed Google Scholar - Ronnblom, L.E., Alm, G.V. & Oberg, K.E. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 227, 207–210 (1990).
Article CAS PubMed Google Scholar - Preble, O.T., Black, R.J., Friedman, R.M., Klippel, J.H. & Vilcek, J. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science 216, 429–431 (1982).
Article CAS PubMed Google Scholar - Farkas, L., Beiske, K., Lund-Johansen, F., Brandtzaeg, P. & Jahnsen, F.L. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 (2001).
Article CAS PubMed PubMed Central Google Scholar - Cederblad, B. et al. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-α-producing cells. J. Autoimmun. 11, 465–470 (1998).
Article CAS PubMed Google Scholar - Vallin, H., Perers, A., Alm, G.V. & Ronnblom, L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-α inducer in systemic lupus erythematosus. J. Immunol. 163, 6306–6313 (1999).
CAS PubMed Google Scholar - Blanco, P., Palucka, A.K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Article CAS PubMed Google Scholar - Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Article CAS PubMed PubMed Central Google Scholar - Baechler, E.C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 100, 2610–2615 (2003).
Article CAS PubMed PubMed Central Google Scholar - Bell, D. et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med. 190, 1417–14126 (1999).
Article CAS PubMed PubMed Central Google Scholar - Vicari, A.P. et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 196, 541–549 (2002).
Article CAS PubMed PubMed Central Google Scholar - Vermi, W. et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J. Pathol. 200, 255–268 (2003).
Article PubMed Google Scholar - Chaux, P. et al. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression. A role in the immune tolerance to antigenic tumors. Adv. Exp. Med. Biol. 417, 525–528 (1997).
Article CAS PubMed Google Scholar - Dhodapkar, M.V. & Steinman, R.M. Antigen-bearing immature dendritic cells induce peptide-specific CD8+ regulatory T cells in vivo in humans. Blood 100, 174–177 (2002).
Article CAS PubMed Google Scholar - Mahnke, K., Qian, Y., Knop, J. & Enk, A.H. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101, 4862–4869 (2003).
Article CAS PubMed Google Scholar - Munn, D.H. et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 114, 280–290 (2004).
Article CAS PubMed PubMed Central Google Scholar - Palamara, F. et al. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J. Immunol. 173, 3051–3061 (2004).
Article CAS PubMed Google Scholar - Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Article CAS PubMed Google Scholar - Jahnsen, F.L. et al. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 165, 4062–4068 (2000).
Article CAS PubMed Google Scholar
Acknowledgements
We thank F. Facchetti for pDC images; and S. Sozzani, S. Gilfillan, A. Blasius and W. Barchet for comments.
Author information
Authors and Affiliations
- Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri, USA
Marco Colonna - Laboratory for Immunological Research, Schering-Plough, 27 chemin des peupliers, BP11, Dardilly, 69571, Cedex, France
Giorgio Trinchieri - Department of Immunology, MD Anderson Cancer Center, University of Texas, Houston, 77030, USA
Yong-Jun Liu
Authors
- Marco Colonna
You can also search for this author inPubMed Google Scholar - Giorgio Trinchieri
You can also search for this author inPubMed Google Scholar - Yong-Jun Liu
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toMarco Colonna.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Colonna, M., Trinchieri, G. & Liu, YJ. Plasmacytoid dendritic cells in immunity.Nat Immunol 5, 1219–1226 (2004). https://doi.org/10.1038/ni1141
- Published: 17 November 2004
- Issue Date: 01 December 2004
- DOI: https://doi.org/10.1038/ni1141