Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages (original) (raw)
Abstract
Monocytes are considered to be precursor cells of the mononuclear phagocytic system, and macrophages are one of the leading members of this cellular system. Macrophages play highly diverse roles in maintaining an organism’s integrity by either directly participating in pathogen elimination or repairing tissue under sterile inflammatory conditions. There are different subpopulations of macrophages and each one has its own characteristics and functions. In this review, we summarize present knowledge on the polarization of macrophages that allows the generation of subpopulations called classically activated macrophages or M1 and alternative activated macrophages or M2. Furthermore, there are macrophages that their origin and characterization still remain unclear but have been involved as main players in some human pathologies. Thus, we also review three other categories of macrophages: tumor-associated macrophages, CD169+ macrophages, and the recently named TCR+ macrophages. Based on the literature, we provide information on the molecular characterization of these macrophage subpopulations and their specific involvement in several human pathologies such as cancer, infectious diseases, obesity, and asthma. The refined characterization of the macrophage subpopulations can be useful in designing new strategies, supplementing those already established for the treatment of diseases using macrophages as a therapeutic target.
Introduction
The professional phagocytic cells have an efficient ability to ingest particles that have penetrated our innate immunity barriers. This function provides us protection against what would be potentially hazardous to health, whether origin pathogen or not. The monocytes are considered to be precursor cells of the mononuclear phagocytic system that comprises monocytes, macrophages, and dendritic cells (DCs). However, DC population may also originate directly from the DC precursor as revised by Collin et al. (1).
The existence of this cellular network as part of the immune system is required to remove microorganisms through their phagocytic activity and the subsequent induction of immune responses mediated by T cells. But their presence is not only limited to counteracting pathogens with their antimicrobial activity but are also fundamental in repairing tissue under sterile inflammatory situations (2–4).
Thus, macrophage function is not limited to phagocytosis and degradation of pathogens, these cells are also able to discriminate self from non-self, aspect that emphasizes their importance in the development of some tissue. For example, using a murine model carrying a loss-of-function in the gene encoding the transcription factor PU.1 (regulating myeloid protein expression), there was a reduction in the number of vessel intersection was observed leading to abnormal vasculature process and suggesting that macrophage contact with vessel junctions are required to promote vessel fusion (5, 6).
The current macrophage nomenclature is complex and sometimes leads to confusion due to versatile cells, and, as it will be discussed, macrophages possess functional plasticity mediated by microenvironment signals. In this review, we give an overview on the two main macrophage subsets, named according to stimuli inducing their polarization and cytokine profile that they deliver: (a) classically activated macrophages (M1 macrophages) and (b) alternatively activated macrophages (M2 macrophages). We included a discussion on three other subpopulations of monocyte-derived macrophages: (c) tumor-associated macrophages (TAM), one subpopulation involved in cancer; (d) CD169+ macrophages; a subpopulation found in lymphoid organs and implicated in immune tolerance and antigen presentation; and (e) one macrophage subpopulation, recently described as T cell receptor positive (TCR+) macrophages. Despite the limited information about CD169+ and TCR+ macrophages, we include them because there are accumulating data showing their role under specific conditions. We describe experimental evidence relative to phenotypic heterogeneity and effector functions and add a short description of the relationship between macrophage subsets and major pathologies where they have been involved.
Precursor Cell Origin of Macrophages
Monocytes, defined as circulating blood cells, are a population of mononuclear leukocytes, morphologically and phenotypically heterogeneous that constitute approximately 10% of peripheral blood cells in humans. In murine model, it was that blood monocytes are produced by bone marrow (BM) from macrophage/dendritic cell progenitor (MDP) cells. MDP lacks the ability to differentiate into granulocytic, megakaryocytic, lymphoid cells, and are considered as the direct precursor of monocytes from BM and consequently of blood monocytes (7, 8). It has been established that CCR2 and the chemokine chemoattractant protein 3 (MCP-3) are critical for BM monocyte mobilization and homeostasis maintenance (9). Under specific circumstances, the egress of monocytes from blood to inflamed tissue is dependent on both CCR2 and CX3 chemokine receptor 1 (CX3CR1) (10). However, it remains to be determined if these molecules are necessary for the migration of monocytes from BM to blood and finally to tissue, and, if they are also responsible for the recirculation of monocytes through the BM.
In 2003, Geissman et al. identified two populations of murine monocytes: a short-lived subset phenotypically described as Ly6C+CX3CR1loCCR2+CD62L+ and found in inflamed tissue, and a second subset with a longer half-life, phenotypically shown as Ly6CloCX3CR1hiCCR2−CD62L− and observed in non-inflamed tissues (11). These subsets of murine monocytes can be compared with the two subpopulations of human monocytes already described nearly 30 years ago (12). Their main characteristics were related to different surface expression markers (CD14, CD16) and their distinct ability to deliver pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α or TNF). Currently, these populations are called ‘classical’ (CD14++CD16−) and non-classical (CD14+CD16++) monocyte subsets and these are synonymous of CD62L+ and CD62L− murine subsets, respectively (13, 14).
Hence, once monocytes are recruited from the BM to circulating blood, they can migrate to various tissues of the body and differentiate into macrophages and subsequently become macrophages with their own phenotypic and functional characteristics in a tissue-dependent manner. According to the specific anatomical site where they are recruited, they are differently named as alveolar macrophages in the lungs, microglia in the central nervous system (CNS), and Kupffer cells in the liver (Figure 1). Recent literature has reviewed macrophage populations and considered the large diversity in phenotype and functions according to their anatomic localization. Italiani and Boraschi have recently reviewed human and murine monocyte subsets and the fate of monocyte/macrophage populations and functions in the main body tissues during steady state and inflammatory conditions (15). Dey et al. provided us an analysis about polarization, ontogeny, and plasticity of tissue macrophages inside a context of treatment of acute and chronic inflammatory diseases (15, 16). Finally, Gordon et al. have summarized the heterogeneity, functionality, and interactions of macrophage populations within body tissues (17).
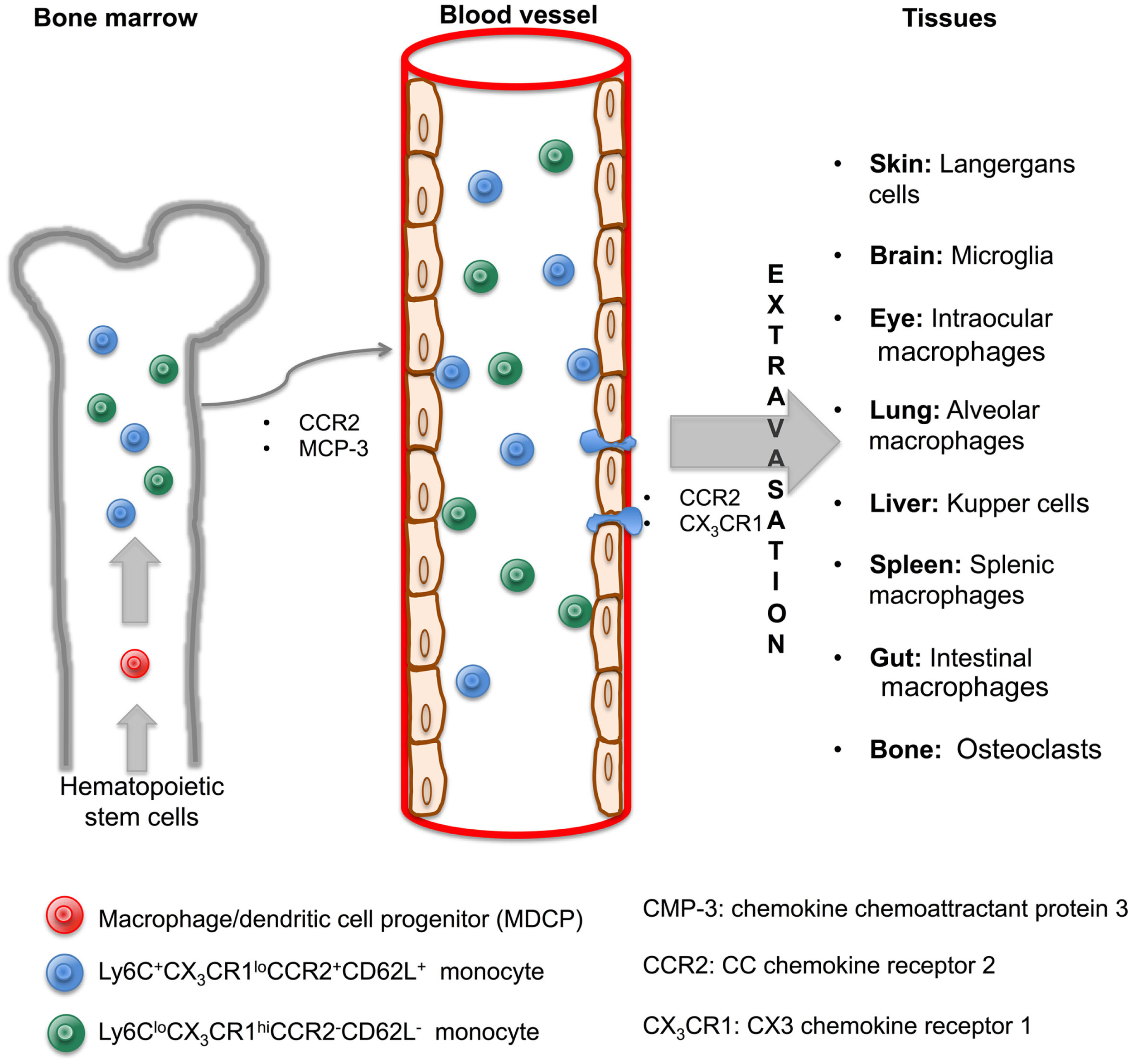
Monocytes are generated in the bone marrow and mobilized to blood and tissue through cytokine signaling to originate macrophage subsets. In the bone marrow, hematopoietic stem cells give rise to MDCP, which divides into two subsets of monocytes, Ly6C+CX3CR1loCCR2+CD62L+ and Ly6CloCX3CR1hiCCR2−CD62L−. Both are mobilized to blood through CCR2 and MCP-3 signaling. Once inside the blood vessels, monocytes are able to leave by extravasation and arrive to tissues via CCR2 and CX3CR1 signaling. A macrophage will originate in a tissue-dependent manner and with its own characteristic and name.
In this review, we consider blood monocytes as tissue-macrophage progenitors, because the major fraction of macrophages originates from blood-borne monocytes. However, it is important to note that there are tissue-resident macrophages that are not derived from monocytes, and their self-renewal, proliferation, origin, and mechanisms of replacement have not been totally elucidated. In 1989, the presence of macrophages in the mouse yolk sac at day 9 of gestation was reported, suggesting that part of the macrophages may exist before promonocyte and monocyte development (18, 19). In the CNS, a murine model of microgliosis has illustrated that microglia can be functionally maintained independently of BM-derived progenitors (20). By contrast, there is also experimental evidence proving that microglia is derived from hematopoietic cells. Inhibition of monocyte recruitment to the CNS prevented experimental autoimmune encephalitis, suggesting that within the CNS, macrophages can originate from both dependent and independent blood monocytes (21, 22). Recently, Davies et al. reported an extensive analysis confirming the co-existence of macrophages of dependent and independent blood monocyte-derived origin in several tissues including brain, spleen, and lung, and defined cellular phenotypes and functions of these tissue-resident macrophages (23). How the resident macrophage population is self-renewing is still an open question. Colony-stimulating factor 1 (CSF-1) was proposed as a protein necessary to regulate the number of tissue-resident macrophages (24); nevertheless, it has been shown that interleukin-4 (IL-4) plays a major role in favoring the local proliferation of macrophages in parasitic infections. Nevertheless, IL-4-mediated proliferation seems to be independent of CSF-1 (25, 26).
Origin of M1 and M2 Macrophage Terminology
The concepts M1 and M2 oversimplify the pattern of macrophages because, as we will discuss, the combinatorial spectrum of these cellular populations is extremely large. The first classification of macrophages was adopted from the work on T helper cell polarization. Two main lymphocytic subpopulations were characterized with diametrically contrasting functions and categorized according to patters of cytokines they deliver: T helper cells type 1 (TH1) and type 2 (TH2) (27).
From early 1980s, Nathan et al. identified that interferon-gamma (IFN-γ) stimulating the peroxide-releasing capacity of macrophages, contributes to macrophage ability to kill intracellular pathogens (28). Posteriorly, IL-4, an anti-inflammatory cytokine, was identified and shown to induce a different macrophage gene expression compared with IFN-γ dependent (29). However, it was only in 2000 when Mills et al. proposed the M1–M2 terminology. These authors used T lymphocytes from two mouse strains, one with a background producing mainly IFN-γ, which activates macrophages to generate nitric oxide (NO) from arginine versus mice which T lymphocytes producing IL-4 and TGF-β1, and generating ornithine from arginine. This elegant study showed that, in a strain-dependent manner, macrophages express distinct metabolic programs and their responses influence inflammatory reactions in opposite way (30).
Recently, Murray et al. proposed a nomenclature and experimental guideline for generating in vitro M1 and M2 subpopulations, with the objective to obtain data reproducibility across laboratories (31). In fact, the existence of this guideline notes the relevance to study M1/M2 paradigm as a useful network, which plays different roles inside immune responses.
Classically Activated Macrophages (M1 Macrophages)
M1 macrophages are defined as macrophages that produce pro-inflammatory cytokines, mediate resistance to pathogens, and exhibit strong microbicidal properties, but these also contribute to tissue destruction. Classical activation of macrophages occurs when the cell receive stimuli such as: (1) IFN-γ, mainly secreted by other cell types (TH1 cells, cytotoxic T cells, and NK cells); (2) lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria; and (3) granulocyte-macrophage colony-stimulating factor (GM-CSF) that stimulates the production of pro-inflammatory cytokines (32–34).
M1 macrophages are characterized by an elevated ability to secrete cytokines such as IL-1β, TNF, IL-12, and IL-18; phenotypically, they express high levels of main histocompatibility complex class II (MHC-II), CD68 marker, and CD80 and CD86 costimulatory molecules. Recently, it has been shown that M1 macrophages up-regulate the expression of intracellular protein called suppressor of cytokine signaling 3 (SOCS3), activate the inducible nitric oxide synthase (NOS2 or iNOS) generating NO. Hence, M1 macrophages, under specific conditions, exacerbate inflammatory processes that can be detrimental to health (35–37). However, these macrophages also have the ability to phagocyte large numbers of pathogens and can kill intracellular bacteria. When macrophages are under classical activation conditions, they initiate microbicidal mechanisms by the synthesis of NO, the restriction of iron or nutrients for microorganisms and acidification of the phagosome (38–40).
At present, the pathway that regulates the macrophage polarization is not fully understood, but there are several molecules implicated in this process. For instance, members of the family of interferon regulatory factor (IRF), signal transducers and activators of transcription (STAT), and SOCSs. In 1990s, STAT1, a 91-kDa cytoplasmic protein, was shown to be crucial for M1 macrophage polarization (41, 42). STAT1 can form homodimers or heterodimers (STAT1–STAT2) that bind to interferon-stimulated response elements (ISREs) and members of the IRF can also bind to ISRE sequences. In 2011, Krausgruber et al. showed that IRF5 is a critical protein for M1 macrophage polarization. Both GM-CSF and IFN-γ stimuli induce IRF5 expression that directly activate 20 M1-specific genes and inhibit 19 M2-specific genes encoding cytokines (43).
Lipopolysaccharide stimulus generates M1 macrophages through interaction with its receptor, TLR-4, by inducing phosphorylation of both STAT1α and STAT1β. This pathway is MyD88-independent but is toll/IL-1R motif-dependent (44). A contribution from Bruton’s tyrosine kinase (Btk) is possible at this level since Btk is required downstream of TLR-4 for optimal phosphorylation of STAT1, and its absence exacerbates M2 recruitment under allergic inflammation conditions (45). Recently, Eun et al. showed that the P2Y(2) receptor (P2Y(2)R), a G-protein-coupled receptor, is up-regulated in response to LPS and facilitates the release of ATP, thus, P2Y(2)R increases NOS2–NO levels, which is a signature of M1 polarization (46). Arnold et al. reported experimental evidence supporting the hypothesis that up-regulation of SOCS3 is essential for an effective M1 macrophage activation. Indeed, SOCS3 controls activation and translocation of nuclear factor-κB (NF-κB) and activity of phosphatidylinositol 3-kinase (PI3K), favoring NO production (37).
Finally, it has been shown that in an autocrine or paracrine manner, Activin A, a growth and differentiation factor of the TGF-β superfamily, promotes the expression of M1 markers and down-regulates the production of IL-10 probably leading to M1 polarization (47, 48).
Autoimmune Diseases-Related M1 Macrophages
Autoimmune diseases are frequently associated with inflammatory processes. Here, we briefly describe experimental evidence showing a relationship between molecules previously described that support M1 polarization and the pathophysiology of autoimmune diseases.
The inflammatory bowel disease (IBD) is characterized by a chronic recurrent inflammation of the gastrointestinal tract. In both, murine model and biopsies of IBD patients, increase of SOCS3 expression has been observed, which was correlated with the severity of inflammation. Furthermore, SOCS3 expression has been proposed as a useful marker for cells undergoing acute or chronic inflammation (49–52). In systemic lupus erythematosus (SLE), an autoimmune illness characterized by chronic inflammation, patients displayed elevated levels of IFN-γ and IRF-5 (53). In addition, a risk haplotype of IRF5 has been described in SLE patients associated with the presence of anti-double-stranded DNA antibodies, which preceded clinical symptoms in many individuals (54). In addition, there is evidence that the SLE risk haplotype also influences systemic sclerosis predisposition, which is a fibrotic autoimmune diseases (55).
Multiple sclerosis (MS) is a demyelinating disease of the CNS. The role of macrophages in the CNS appears dual. Initially, it was shown that activated monocytes/macrophages could play a role in the acute exacerbation of MS in patients (56). However, experimental evidence suggested that GM-CSF (M1 inductor) was necessary to induce axonal regeneration (57). Using the experimental autoimmune encephalomyelitis (EAE) murine model, it has been reported that macrophages producing NO played an active role in the inflammation pathogenesis (58, 59). Experimental approaches attempt to reduce CNS inflammation using clodronate liposomes to eliminate infiltrating macrophages as well as G-protein-coupled receptor (GPBAR1), agonist to reduce pro-inflammatory cytokines; mice treated with clodronate liposomes or GPBAR1 agonist exhibited a significant decrease in EAE clinical score (60, 61).
Rheumatoid arthritis (RA) is an autoimmune disease characterized by a chronic inflammation of the synovial membrane that leads to joint destruction. Macrophage number has been correlated with disease activity in RA patients (62). Specifically, it has been shown that STAT1 is a signature in RA synovial fluid macrophages and dependent on autocrine TNF (63). At date, an effective treatment is proposed to block members of Janus kinase (JAK) family, JAK1 and JAK3, since these molecules are necessary to phosphorylated STATs modulating gene expression toward M1 profile (64).
Type 1 diabetes mellitus is an autoimmune disease characterized by depletion of insulin-producing pancreatic beta cells (β-cells) and consequently, high levels of glucose. Under hyperglycemic condition, the generation of reactive oxygen intermediates and apoptosis by IFN-γ/STAT1-dependent pathway is increased (65, 66). In murine model, it has been shown that β-cells increase the expression of CCL2 and allow pro-inflammatory monocyte recruitment in the pancreas and spontaneous development of diabetes (67). It has also been shown that macrophages from diabetic mice have elevated expression of anti-apoptotic proteins as a potential mechanism to promote an attack toward β-cells (68).
Obesity-Related M1 Macrophages
Obesity has been implicated as the major risk factor in developing diverse diseases such as type 2 diabetes mellitus (69). Below, we discuss diverse evidence showing that obesity has a pro-inflammatory background. Although on a cellular level still remains largely unknown; however, it has been described, both in vitro and in vivo, that human adipocytes up-regulate of NF-κB-regulated genes, such as CCL2, E-selectin, IL-6, and IL-8, favoring the recruitment of macrophages (70, 71).
Weisberg et al. showed that macrophage numbers were increased in adipose tissue in both mice and obese humans; their percentage correlated positively with their level of obesity, and these cells were responsible for almost all of the TNF and iNOS present in adipose tissue (72). Macrophages isolated from white adipose tissue from lean animals showed hallmarks of polarization toward an alternative activation state. By contrast, obesity induced increasing gene expression of molecules characteristic of M1 macrophages, such as those encoding TNF and NOS2, suggesting that diet-induced obesity leads to a change from M2 to M1 polarization (72, 73). Animal models have shown that a high-fat diet increases systemic and tissue pro-inflammatory cytokines such as bioactive TNF, IL-6, and IL-12, reinforcing the hypothesis that obesity favors a pro-inflammatory microenvironment (74). In agreement with these data, Kawanishi et al. reported that physical activity markedly inhibited TNF and increased CD163 (a M2 marker) mRNA expression in adipose tissue. Thus, it appears likely that exercise might induce the phenotypic switching from M1 macrophages to M2 macrophages in high-fat-diet animals (75).
Infectious Diseases-Related M1 Macrophages
Pro-inflammatory macrophages are necessary in controlling infectious processes, principally intracellular pathogens such as Mycobacterium tuberculosis (M. tuberculosis) or Listeria monocytogenes (L. monocytogenes), causal agents of tuberculosis and listeriosis, respectively. The array of pro-inflammatory and chemoattractant cytokines induced by M. tuberculosis infection play a significant role in cell recruitment, granuloma formation and progression, and severity of the disease (76). However, the inductions of pro-inflammatory cytokines and microbicidal macrophage functions are necessary to activate protective host immune responses against mycobacterial infections (77, 78). It has also been proposed in murine model that during the later stages of infection the presence of alternatively active macrophages are increased, impairing NO production, and consequently favoring the intracellular persistence of mycobacteria (79). Leemans et al. reported that macrophages play a dual role during tuberculosis since depletion of non-selective macrophage populations improved the clinical outcome, while depletion of activated macrophages enhanced mycobacterial outgrowth and decreased granuloma number, which is associated with a deficiency of TNF production (80). Mycobacterial proteins suppress functions of M1 macrophages as a mechanism of pathogen evasion. For instance, macrophages infected with a mycobacterial deficient strain of SecA2 produced high levels of TNF, IL-6, and reactive nitrogen intermediate (81), but the PE-PGRS62 protein supported virulence through down-regulating IL-1β and iNOS mRNA levels (82, 83). These data, therefore, indicate that for the adequate control of M. tuberculosis infection, the development of a bactericidal mechanism of M1 macrophages is required.
Listeria monocytogenes is a foodborne bacteria infecting a majority of organs including liver in which induces recruitment of pro-inflammatory monocytes and formation of micro-abscesses containing among other cells M1 monocyte-derived macrophages. Several chemokines have been shown to play a critical role in monocyte recruitment and infection control such as MCP-1 (also referred as CCL2, the ligand of CCR2), and MCP-3 (84). Mice deficient in CCR2 were unable to clear L. monocytogenes infection (85–87). The Notch signaling regulates diverse cellular process and after ligation with its ligand, it is cleaved, translocated to the nucleus, and binds to the DNA-binding protein RBP-J (88). Notch-RBP-J and TLR-4 pathways synergistically induce expression of IRF8 protein that plays a role in activation of M1 macrophage polarization, autophagy, and clearance of L. monocytogenes (89, 90). An interesting study by Bleriot et al. has shown that during of L. monocytogenes infection, necroptosis of Kupffer cells delivers IL-1β activating hepatocytes to produce the alarmin IL-33 triggering the basophil to produce IL-4 and resulting in a shift of M1 to M2 macrophage phenotype. These M2 macrophages can replace dead Kupffer cells allowing attenuation of the inflammatory process and return to liver homeostasis. This study illustrates that sequential type 1 and type 2 immune responses after L. monocytogenes infection enable first host defense and then homeostasis (91).
Alternatively Activated Macrophages (M2 Macrophages)
Macrophages activated through a pathway opposite to the classical pathway are referred to as M2 or alternative pathway. It has been demonstrated that stimuli such as CSF-1, IL-4, IL-10, TGF-β, and IL-13, fungi and helminth infections, favor M2 subpopulation polarization, delivering IL-10 in high concentrations, and IL-12 in low amounts. M2 macrophages play a central role in responses to parasites, tissue remodeling, angiogenesis, and allergic diseases (25, 92).
Phenotypically, this population is characterized by the expression of the macrophage mannose receptor (MMR), also called CD206. However, in 2013 Jaguin et al. observed no difference in CD206 expression between M1 and M2 macrophages and proposed that specific characteristic of M2 macrophages is the up-regulation of the CD200R membrane glycoprotein (93). CD163 has been suggested as M2 marker, but more recently was shown, in human tissue, that CD163 is M2 macrophage marker only in combination with the transcription factor CMAF, thus CD163 cannot be considered a M2 marker when used as unique marker (94). MGL1 and MGL2, two members of the macrophage galactose-type C-type lectin family, are also expressed in macrophages stimulated upon conditions of alternative activation (95). Response gene to complement 32 (RGC-32) is a cell cycle regulator expressed in many cells including macrophages but not monocytes (96). Recently, it has been shown that absence of RGC-32 does not affect monocyte-macrophage differentiation, however, under M-CSF or IL-4 stimuli, RGC-32 has a relevant role to promote M2 polarization and its level of expression still increases M2 macrophages, proposing this protein to be included as a marker for M2 polarization (97).
Using a murine model, a genetic profile for M2 macrophages has been reported, and among other genes, arginase 1 (Arg1), MMR (Mrc1), resistin-like molecule α (FIZZ1), and chitinase-like protein Ym1 were shown to be up-regulated (98). In conclusion, to make an adequate phenotypic characterization of macrophage subpopulations, it is important to consider all described markers rather than individual markers, considering that some molecules can be shared by distinct cellular subsets.
The molecular network activated to favor M2 polarization involves distinct members of families already discussed to participate in M1 polarization. On the one hand, Ohmori et al. showed that IL-4 is antagonistic to IFN-γ because it can suppress IFN-γ-stimulated gene transcription and increase the activation of STAT6 (99). Arginase 1 production, a hallmark of M2 macrophages, depends on IL-4 and IL-13, and is a direct consequence of STAT6 activation (100). It has been shown that the NFκB p50 subunit, in the form of homodimers, has a regulatory activity essential for M2 polarization, both in vitro and in vivo (101). IRF4, a member of IRF family, is involved in M2 polarization and functions as a negative regulator of TLR signaling by association with the MyD88 adaptor molecule (102). Peroxisome proliferator-activated receptor γ (PPARγ), a nuclear receptor with anti-inflammatory properties, has been proposed to enhance the M2 phenotype; however, only native monocytes can be primed by PPARγ, but not M2 resting or M1 macrophages (103). The treatment of human monocytes with bone morphogenetic protein-7 (BMP-7) induces M2 polarization and appears to be mediated by the p-PI3K–Akt–mTOR complex (104). On the other hand, it has been proposed that IL-21 can affect M2 polarization by inhibiting ERK phosphorylation and decreasing iNOS, thus consequently increasing STAT3 phosphorylation (105). The detailed molecular network controlling M2 polarization still appears to be complex and is not fully understood.
M2 Macrophages in Allergy and Asthma
Allergic asthma is an inflammatory disease of the airway characterized by an increased reactivity to different allergens, and by TH2 immune responses expressing IL-4, IL-5, and IL-13, as recently revised by Lambrecht and Hammad (106). Some clinical hallmarks of asthma include increased IgE, eosinophilia, and airway hyperresponsiveness (AHR) (107). At the protein level, asthma pathology is known to depend on IL-4/IL-13 acting through IL-4 receptor-alpha chain (IL-4Rα) and activating the STAT6 pathway (108). At present, exactly how M2 macrophages contribute to this pathology is not completely clear. Evidence using ovalbumin and house dust mites to induce an allergic airway disease in IL-4Rα KO mice, showed a decrease in macrophage Arginase1+ and Ym-1+ but the clinical pathological features were not reduced. By contrast, the presence of YM1-1+ macrophages responding to IL-4/IL-13 increased the severity of allergic lug inflammation as a consequence of M2 macrophage production of CCL11 and CCL24, two important mediators of eosinophil chemotaxis (109, 110). Thus, it is possible that the discrepancy regarding the role of M2 macrophages in asthma physiopathology depends on different factors including the nature of the stimulus and the time of exposure.
M2 Macrophages in Helminth Parasite Infection
Helminths have subversive immune strategies that allow them to evade the host’s immune response and to establish persistent infections. In 1998, Barner et al. showed that IL-4Rα-deficient mice were extremely susceptible to helminth infections suggesting that TH2 responses regulate this type of infection (111). M2 macrophages are necessary for an adequate immune response against parasites; however, the exact mechanism through which M2 macrophages act has not been fully elucidated. It has been proposed that M2 macrophages are an effector cell population impairing parasite health and mobility through the arginase 1 pathway and contributing to expulsion of adult worms (112). Using a model of the Strongyloides stercoralis infection, it has been reported that M2 macrophages can kill the parasites but this function depends on the collaboration between neutrophils and the complement system (113). Finally, the role of M2 macrophages during nematode infection has been considered to be not only limited to the elimination of the parasite but rather the TH2-type response is essential for controlling acute tissue damage and repair, which is their traditional role (114).
Tumor-Associated Macrophages
In a strict sense, TAM are not always considered as an additional subset of macrophages because these cells do not exist at steady-state condition but are observed in many tumors. TAM are macrophages associated with a specific pathological context and their specific polarization status has been the object of extensive studies supporting TAM as M2-like macrophages. However, there are also experimental evidence not only proposing TAM as one unique and distinct M2 myeloid population but also sharing M1 and M2 signature polarization (115–117). Switch of M2 macrophages toward M1 phenotype has been proposed as a therapeutic approach (118).
The microenvironment of solid tumors, releasing many chemoattractants, has the ability to induce recruitment of circulating monocytes, which may differentiate into TAM and are frequently found in tumors forming the main inflammatory infiltrate. It has been proposed that TAM can induce angiogenesis, lymphogenesis, stroma remodeling, immune suppression, and metastasis. TAM release different enzymes comprising plasmin, uPA, MMP, and cathepsin B contributing to tumor cell invasiveness and metastasis (119, 120). These cells accumulate in tumors mainly in necrotic regions, and are associated with a poor prognosis (121, 122).
Even if some features of TAM resemble M2 polarization, such as high production of IL-10, the same cells co-express IFN-inducible chemokines; this duality was recently described in freshly isolated TAM from pancreatic ductal adenocarcinoma. Cells exhibited anti- as well as pro-inflammatory properties supporting the idea that macrophages are main players of the epithelial–mesenchymal-transition favoring tumor formation and metastasis (123).
Although TAM show a pattern of M1 and M2 macrophages, it is known that these cells have a transcriptional profile distinct from M1 or M2 macrophages. Considering that there is an “overlap” of molecules between M1/M2/TAM, Table 1 summarizes some of the discrete differences that have been reported on molecules considered as specify marker for every one of these three types of macrophages.
| M1 | M2 | TAM | |
|---|---|---|---|
| iNOS production | ↑ | − | + |
| MHC-II expression | ↑ | ↓ | ↓ |
| CD163 expression | − | + | + |
| CD200R | − | + | − |
| Macrophage galactase-type C-type lectins | ↓ | ↑ | ↑ |
| Response to stimulus | IFN-y, LPS, GM-CSF | IL-4, IL-13, M-CSF, helminths | Tumor microenviroment |
| Arginase 1 | − | ↑ | + |
| Cytokine production | IL-18, IL-12, IL-1, TNF | IL-10, IL-12(low) | IL-10, TGF-β, CCL2, CCL5 |
| STAT molecules | STAT1, STAT2 | STAT3, STAT6 | STAT1 |
| IRF molecules | IRF5 | IRF4 | ↑ IRF3 |
| NFκB participation | p65 | p50 | p50 |
| PI3K participation | + | + | + |
| Genes | Nos2, Ciita, ll12 | Arg1, Ym1, ll10, Mcr1, Fizz1 | Ccl2, Ccl5, ll10, CD81, H2Eb |
Molecules involved in the polarization of M1, M2, and TAM macrophages.
‘+’ Present. ‘−’ Ausent. ↑ High expression or production. ↓ Low expression or production.
As yet, it is not completely clear how the cascade of events generating TAM is orchestrated. Resting TAM are characterized by high expression of CCL2, CCL5, and IL-10 and surface molecular markers such as MGL1, Dectin-1, CD81, MHC-II, and scavenger receptor A (121, 124). It has been described that TAM activation pathway enhances IRF3, STAT1, and the release of CCL2, CCL3, CCL5, IL-10, IL-12 (low), as well as other molecules such as PGE2 and epidermal growth factor (124, 125). In a model of TAM derived from murine and human tumors, it has been shown that LPS activation of TAM resulted in defective NF-κB activation as well as inhibition of IL-12p40 promoter transcriptional activity. However, when TAM were cultured in standard conditions (outside the tumor microenvironment), they recovered the capacity to express IL-12 and TNF (126). These data suggest that tumor environment induces TAM to maintain a status of tolerance and consequently this may attenuate macrophage cytokine responses.
There is no precise information on how a monocyte precursor can generate the coexistance of both M1 and M2 markers in the same TAM. Regarding this question, Movahedi et al. reported that the tumor-infiltrating monocyte pool were predominantly Ly6C+CX3CR1lo and suggested that Ly6Chigh monocytes were direct precursors of TAM. Then, they subdivided TAM in two groups according to intensity of MHC-II expression and suppression of T cell proliferation: (1) MHC-IIhi TAM suppressing proliferation using an iNOS-pathway and (2) MHC-IIlow TAM suppressing proliferation in an iNOS-independent pathway (127). Recently, Franklin et al. reported experimental evidence supporting the idea that inflammatory monocytes could differentiate into TAM; as a late differentiation event, the integrin receptor VCAM1 was up-regulated, but terminal differentiation was dependent on the transcriptional regulator of Notch signaling, RBPJ (128). Laoui et al. have reviewed TAM subsets in breast cancer defining markers and functions (129).
It is important to note that, as previously discussed, STAT3 is involved in M2 macrophage differentiation; however, in addition, STAT3 also participates in TAM functions. STAT3 is required for expression of DC-SIGN on macrophages that might help tumor progression because these cells releasing IL-10 favor the maintenance of an activated STAT3 in a tumor context (130). In a hepatocellular carcinoma (HCC) model, it was shown that TAM secrete IL-6 activating STAT3 in HCC cells and promoting expansion of cancer stem cells. This group proposed that targeting IL6/STAT3 to inhibit cancer stem cell function has important therapy implications for the treatment of HCC (131). More recently, using the HCC model, a study identified that TAM produced high levels of IL-8, chemokine that induced epithelial–mesenchymal transition and promoted cellular migration by JAK2/STAT3 signaling pathway (132). Thus, these data indicate that STAT3 has a relevant participation in tumor progression, and could be considered as target molecule for anticancer therapy.
Mycobacterium bovis bacillus Calmette–Guerin (BCG) has been used for the immunotherapy of bladder carcinoma for more than 30 years. It is clearly established that experimental BCG infections in murine model and in human vaccination induce a Th1 type immune response with activation of IFN-γ and TNF (77). Two strains of BCG, which are currently used for bladder carcinoma immunotherapy, were recently compared and the results showed that BCG Connaught strain provided better clinical outcome and better patient survival. In the same study, comparison of the two BCG strains in a murine model of bladder carcinoma demonstrated that BCG Connaught strain-induced stronger Th1 immune responses suggesting that better BCG efficacy is associated to the activation of Th1 type immunity (133). Attempts to associate BCG treatment with TAM and M1 and M2 are in progress. The efficacy of BCG instillation in non-muscle invasive bladder carcinoma was associated with decrease M2, suggesting that M2 tumor infiltration can be considered as a marker for recurrence of tumors (134). Clinical trial using sequential combination of BCG and mitomycin with the objective to improve bladder cancer immunotherapy and to increase M1 TAM are in development (135).
CD169+ Macrophages
The antigen CD169, or Siglec-1, was originally reported as a marker of one macrophage subpopulation isolated from BM, lymph node, liver, and spleen (136). These cells had the ability to bind red cells. The CD169 molecule is highly expressed by macrophages found within the subcapsular sinus (SCS) and the medulla (M) of lymph nodes (LN) and marginal-zone (MZ) in spleen. So far, there is not sufficient information regarding the signaling pathway and activation of CD169+ macrophages as defined for M1 and M2 macrophages (136, 137).
The spleen has crucial roles in our body as filtering the blood, erythropoiesis, and is the largest secondary lymphoid organ. This organ is structured in compartments, comprising the white pulp that consists of a central arteriole and the T and B cell areas (splenic nodule), the red pulp surrounds the white pulp, and both pulps interact at the MZ (Figure 2A). From 1986, it was described that the murine MZ contains marginal metallophilic cells that are CD169+ and also promote adaptive immune response (138, 139).
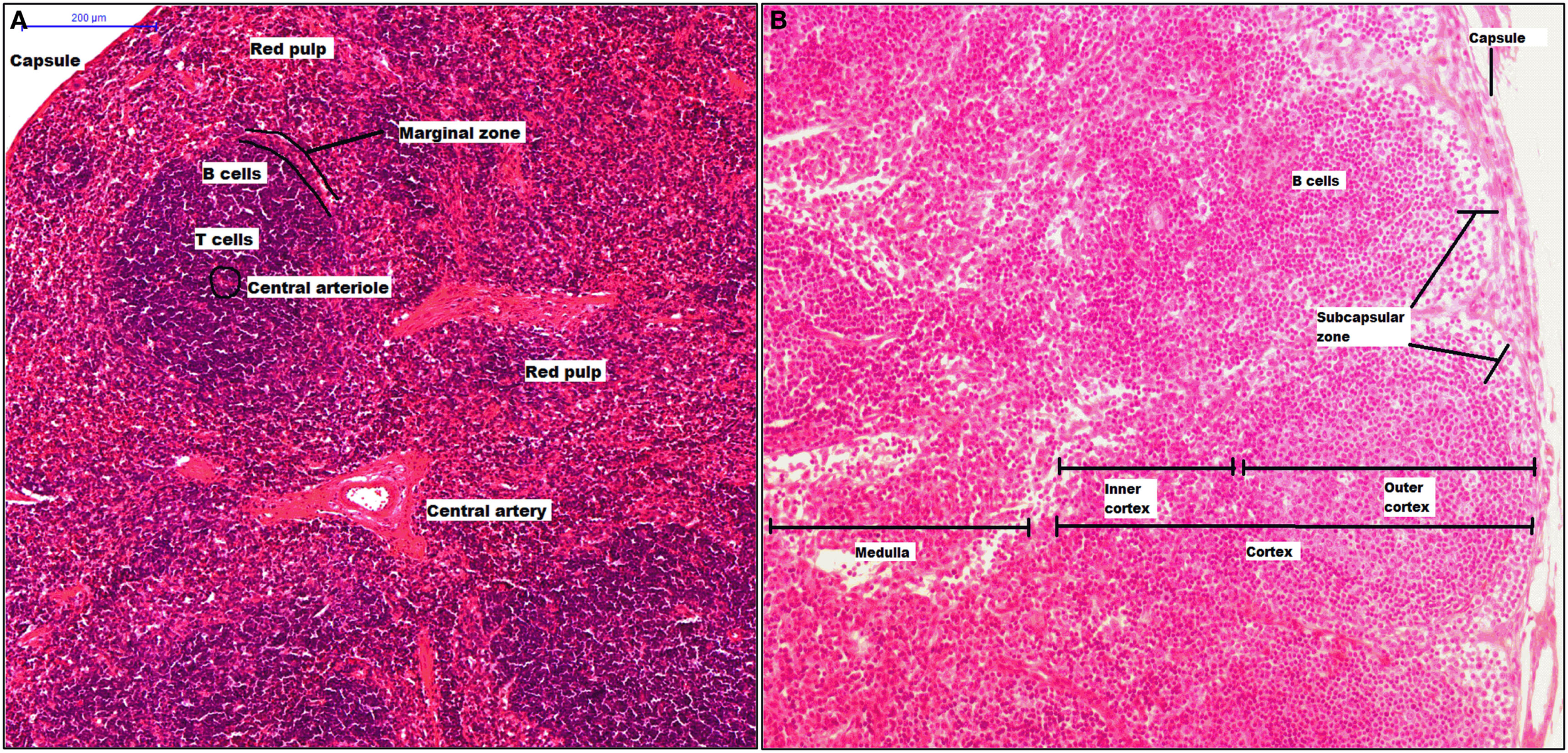
Structure of spleen and lymph node. The spleen has two mayor components, white pulp that includes a central arteriole, T and B cells, and red pulp. Between red and white zone, there is a marginal zone where there are CD169+ macrophages (A). A lymph node is surrounded by a capsule, and the parenchyma is divided into cortex and medulla. The cortex has two zones: outer and inner zone. The subcapsular sinus and medulla zone contain CD169+ macrophages (B).
Lymph nodes are secondary lymphoid organs integrated into the lymphatic system, whose main activities include filtering the lymph, maintaining and producing B cells, and detection of lymph-borne antigens. A LN is surrounded by a capsule, which is underlined with lymphatic endothelial cells forming the SCS. The parenchyma is divided into cortex, which has two zones: the outer cortex (B cell-rich) and the inner cortex (CD4+ T cell-rich), and the medulla (Figure 2B) (140). Thus, CD169+ macrophages are localized inside the SCS and M and their phenotype characterization remains ambiguous. In murine model, it has been clearly described some molecules as part of CD169+ macrophages characterization, the molecules included are CD11b, MHC-II, CD68, CD11c, and F4/80. However, F4/80 is expressed only on some CD169+ macrophage subpopulations. It has been proposed that inside of SCS there are CD169+ macrophages that are F4/80− while macrophages from the M are F4/80+. Finally, SCS macrophages, as splenic CD169+ macrophages, require lymphotoxin alpha (LT-α) signaling and can be depleted using clodronate liposomes (141, 142).
Recently, it was reported that CD169+ macrophages could be detected in the colon, interestingly, these macrophages are vitamin A dependent for their proper development in contrast to splenic CD169+ macrophages, which are LT-α-dependent and express low levels of F4/80 and CD11c (143, 144).
The biological functions of CD169+ macrophages are still imprecise. CD169+ macrophages do not mediate phagocytosis and are mainly involved in the regulation of the immune system rather than in steady-state hematopoiesis (145). However, as discussed below, their best described functions may be conditioned primarily upon their anatomic localization, and it has been proposed some activity in kidney pathologies and under viral infection.
CD169+ Macrophages and Erythropoiesis
The BM is the main site for adult hematopoiesis where erythroid progenitors develop into red blood cells, a process orchestrated mainly by erythropoietin. Chow et al. demonstrated a new role for the mononuclear phagocytes as erythropoietin-complementary regulators. They found that CD169+ macrophages from the BM promoted retention of the hematopoietic stem cells (HSC) by acting on the Nestin+ HCS, suggesting that G-CSF signaling in macrophages is sufficient to promote HSC mobilization (146). Posteriorly, it was reported that G-CSF and depletion of CD169+ macrophages blocked erythropoiesis in the BM but not in the spleen (147). Supporting the relevant role of CD169+ macrophages in erythropoiesis, Falchi et al., using an in vitro erythroid stress model, recently reported that dexamethasone (Dex) amplified the number of proerytroblasts (proEry) and maintained proliferation in an indirect form. They showed that Dex promoted the CD169+ macrophage maturation and instructed to exert their erythroid function, thus acting as a regulator of stress erythropoiesis (148).
Polycythemia vera (PV) and β-thalasemia (β-T) are diseases associated with an elevated erythropoietic activity. PV is a clonal stem cell disorder with the somatic JAK2V617F mutation, whereas β-T is an expansion of the pool of erythroid progenitors. Consistent with previous reports, Ramos et al. have provided experimental evidence suggesting that macrophage depletion in mice with the JAK2V617F+ mutation delayed the appearance of PV and proposed the JAK2V617F mutation as responsible for initiating the pathology, but also a stress erythropoiesis macrophage-supporting activity (SEMA) is required for a full manifestation of the erythroid phenotype in vivo (149). Together, these results are crucial for the identification of new therapies for hematopoiesis disorders where the strategies may use macrophages from BM as a new target cell.
CD169+ Macrophages and Their Immunological Role
Invariant natural killer T cells (iNKT cells) are a subset of T lymphocytes that express a specific αβ T cell receptor (TCR), Vα14-Jα18 in mice and Vα24-Jα18 in human, co-express molecules of NK cells (CD16, CD56), and recognize lipid antigens presented by CD1d molecules. CD169+ macrophages can mediate rapid and long-lasting interactions with iNKT cells after administration of lipid antigens (150). CD169+ macrophages can induce a similar robust activation of iNKT cells in vivo, using liposomes decorated with glycan (151).
Immunological tolerance is the ability the body has to distinguish between self and foreign, allowing homeostasis maintenance and prevention of autoimmune diseases. Two models propose that CD169+ macrophages are necessary to maintain immunological tolerance. The first model suggested that in the splenic MZ, apoptotic cells induce the expression of CCL22 in CD169+ macrophages, resulting in rapid follicular accumulation of Tregs and 103neg DC subset. Thus, recruited Tregs could be activated by apoptotic cell antigens when presented by professional antigen presenting cells or constitutively self-antigen presented to maintain tolerogenic stimulation (152). The second model proposed that CD169+ macrophages from the lymph node or spleen are responsible for capturing exosomes, cell-derived vesicles that are a potential source of self-antigens, thus suggesting that CD169+ macrophages control the access of exosomes to lymphoid organs as a mechanism decreasing the probability of self-antigen responses (153). Although at date, mechanisms favoring generation and maintenance of immunological tolerance have not been totally elucidated, CD169+ macrophages can be considered important players.
CD169+ Macrophages in Kidney Diseases
Some kidney diseases have been associated with macrophage accumulation. In a model of anti-glomerular basement membrane (anti-GBM), adoptive transfer of macrophages showed their contribution to renal injury-induced proteinuria and glomerular cell proliferation (154). Posteriorly, CD169+ macrophages were found in glomerulonephritis and there was a correlation with proteinuria and histologic damage, however, as CD169 marker is absent from blood monocytes, this work did not identify if a specific factor, within the glomerular microenvironment, induced the expression of CD169 on macrophages or if CD169+ macrophages were recruited (155).
More recently, using a model of renal ischemia–reperfusion injury (IRI), Karasawa et al. identified a subset of peripheral blood monocytes (inside CX3CR1+ subset) and kidney-resident macrophages, which were CD169+. However, in contrast to anti-GBM model, the depletion of CD169+ cells resulted in progressive renal injury by IRI, suggesting that CD69+ cells contributed to the suppression or resolution of IRI (156).
CD169+ Macrophages in Their Antiviral Role
NK cells play a pivotal role in viral infection. Garcia et al. have shown that during experimental infection with recombinant modified vaccinia virus Ankara (MVA), viral vector used for vaccine purposes, NK cells accumulated in LN and became activated in an IFN-dependent manner. Indeed, CD169+ macrophages from the SCS are the main type I IFN producers and therefore SCS macrophages appear essential for NK cell recruitment (154). A second infectious viral model using vesicular stomatitis virus (VSV) also supported that SCS macrophage can contribute to antiviral immune surveillance (157, 158). With the same model of VSV, other group reported that CD169+ macrophages from the MZ were able to capture virus but allowed viral replication even in the presence of type I IFN and also overexpressed Usp18, a potent inhibitor of IFN signaling pathway. The lack of either CD169+ cells or Usp18 led to impaired adaptive immunity against VSV suggesting that enforced viral replication in CD169+ macrophages is essential for the induction of an efficient adaptive immune response (159).
It has been shown that increased Siglec-1 expression on human CD14+ monocytes in response to human immunodeficient virus (HIV-1) infection, correlated with viral loads that range from undetectable (<50 copies/ml) to unsuppressed (>800,000 RNA copies/ml), proposing that Siglec-1 avidly binds HIV-1 and facilitates virus dissemination. It is possible that sialic acids on the viral envelope facilitated HIV-1 infection of macrophages through interacting with Siglec-1 (160, 161).
TCR+ Macrophages
T cell receptor (TCR), a molecule necessary for antigen recognition with broad antigen specificity, forms a complex with CD3. This complex TCR-CD3 consists of eight chains: two from TCR that are mainly αβ, but occasionally γδ, plus six chains from CD3: δϵ, γϵ, and ζζ. For many years, it has been accepted that TCR expression is exclusive to T cells; however, there are no reports providing explicit or systematic experimental evidence that others leukocytes, different from the T cell lineage are unable to express TCR. Puellmann et al. reported experimental data changing this dogma (162).
In 2006, it was reported that 5–8% of neutrophils in the circulation express TCR-αβ complex, comprising CD3 and individual-specific TCR Vαβ repertoires, the engagement of the CD3-dependent TCR signal in neutrophils inhibited apoptosis and increased IL-8 expression (162, 163). In addition, other cellular subsets from polymorphonuclear group were also shown to express TCR. Interestingly, there are eosinophil subsets that are TCRγδ+ and TCRαβ−, when these cells were activated by CD3 (unspecific stimulus) led to ROS production, eosinophil peroxidase (EPO), eosinophil-derived neurotoxin (EDN), and cytokine release. However, when eosinophils were activated by mycobacterial ligands (specific stimulus), which are efficient to activate T cells TCRγδ+, they produced ROS and EPO but released cytokines were not documented. Finally, this subpopulation also had an anti-tumor cytotoxicity activity, thus eosinophils TCRγδ+ are relevant to the immune defense (164).
Regarding macrophage cellular subpopulations, several publications have reported the presence of both humans and murine TCR+ macrophages. The TCR-αβ was described to be expressed by peripheral blood monocytes and in vitro by activated monocyte-derived macrophages. Using an in vitro model, Beham et al. showed that TCRβ locus rearrangement and expression of Vβ repertoires in the myeloid lineage occurred during the early phase of macrophage differentiation. TCR+ macrophages have the ability to release CCL2 and possess high-phagocytosis capacity. These cells express molecules such as ZAP70, LAT, Fyn, and Lck, necessary for TCR signaling on lymphocytes; however, their concentration was different when macrophages received IL-4 or IFN-γ stimuli that determine different macrophages’ polarization. Interestingly, during M. tuberculosis infection, which derived pathology involves granuloma formation, macrophages at the inner epithelioid cell zone of caseous granulomas are TCR+ macrophages. Neutralization of TNF (cytokine necessary for host resistance to M. tuberculosis) in patients with lung tuberculosis, suppressed the expression of the CD3 ζ-subunit on TCR+ macrophages, becoming unstable to form the complex TCR-CD3, and the number of TCR+ macrophages decreased. Anti-TNF treatment is also associated with granuloma disorganization and reduced CCL2 expression, thus suggesting that TNF is a regulator of TCRαβ expression on macrophages (165).
Recently, Fuchs et al. reported that in both murine and human lesions of atherosclerosis, there is accumulation of TCRαβ+ macrophages. Using an in vitro cholesterol import and export model, this group proposed that high-density lipoprotein (HDL)-mediated cholesterol efflux induced downregulation of TCRβ within 24 h, but after 72 h, low-density lipoprotein (LDL) ingestion by macrophages-induced changes of TCRβ chains in human carotid artery lesion. Thus, cholesterol import/export was identified as a potent in vitro modulator of macrophages-TCRβ repertoire expression and the presence of TCRαβ+ macrophages was suggested as a new signature of atherosclerosis representing a novel molecular target for diagnostic and treatment of diseases where cholesterol plays a main role in physiopathology (166).
A recent report has shown that monocytes/macrophages from both humans and mice, constitutively express a second type of combinatorial receptor based on γδ variable chains, however, after a bacterial exposure, a distinct TCR Vδ repertoire is induced suggesting that TCRγδ represents a flexible host defense system that responds to bacterial challenge (167).
In conclusion, although the numbers of reports are still limited, there are evidences suggesting that the expression of TCR on cellular surface of non-lymphoid cells, including neutrophils, eosinophils, and monocytes/macrophages, specially, both TCRαβ+ and TCRγδ+ macrophages are implicated in inflammatory and infectious diseases.
Perspectives
For several decades, great progress has been accomplished in the definition of lymphocyte populations by refining their phenotypic profile and characterizing their distinct functions. At present, the knowledge of macrophage subpopulations is still growing. Initially divided according to their ability to release pro-inflammatory or anti-inflammatory cytokines as a paradigm to mimic Th1 and Th2 subsets, these macrophages were designed as M1 and M2 macrophages. Nowadays, besides M1 and M2 macrophages, other different subpopulations, which have been described, such as TAM, CD169+, or TCR+, that are differently located and possess their own characteristics and functionalities. The importance of macrophages on the immune response is indubitable; they are necessary in controlling infectious processes, but their presence is also necessary to maintain homeostasis under sterile injury. In the Figure 3, we summarize some of the main features of macrophage subpopulations discussed in this review.
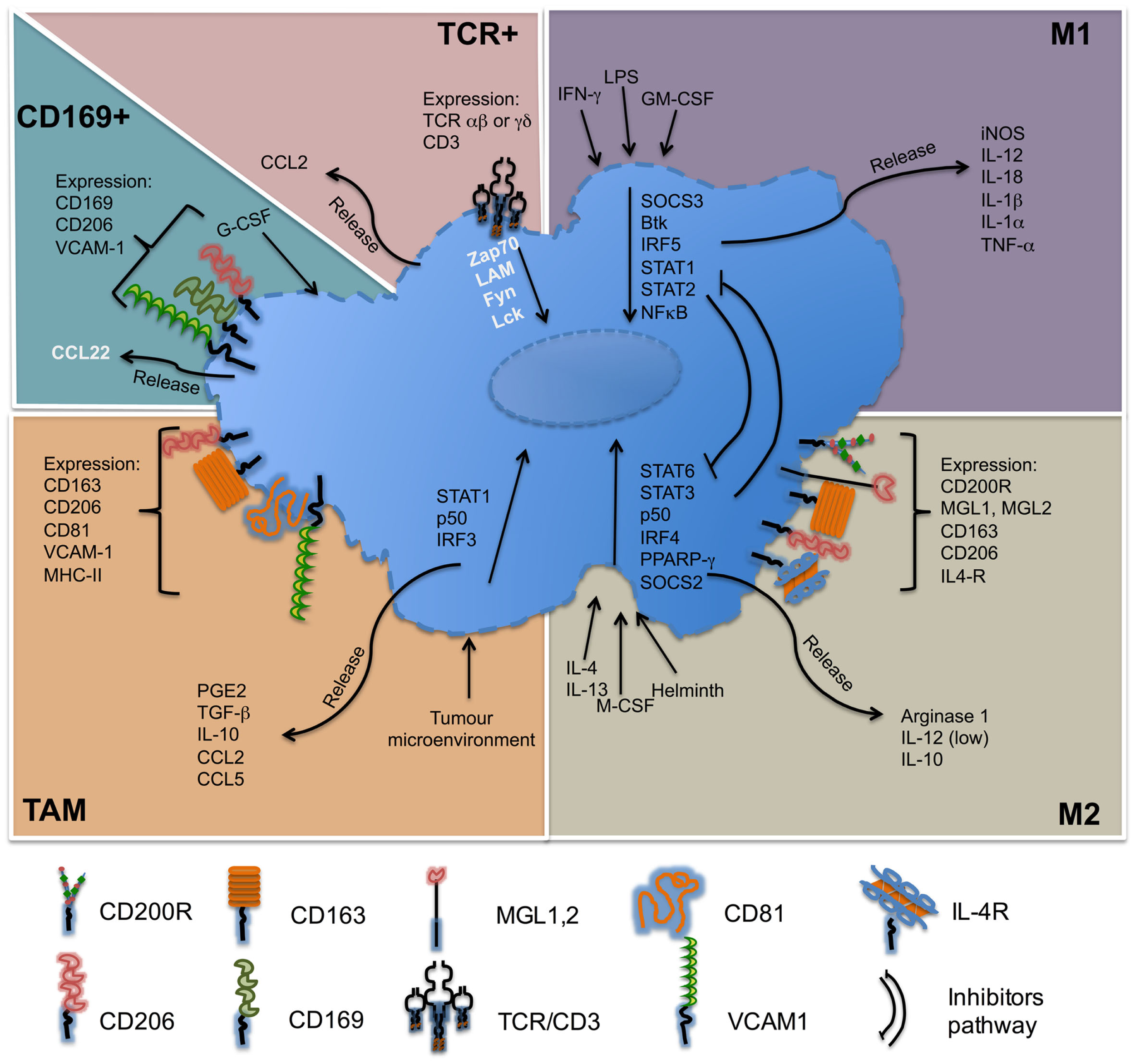
Molecules associated with macrophage subsets. M1 macrophages, also called classically activated, respond to stimuli such as LPS, IFN-γ, and are important producers of pro-inflammatory cytokines. M2 macrophages, also called alternatively active respond to stimuli such as IL-4 or IL-13, are producer of anti-inflammatory cytokines. Tumor-associated macrophages (TAM) respond to self-tumor microenvironment and secrete cytokines such as TGF-β or IL-10. CD169+ macrophages are involved in immune tolerance and erythropoiesis. TCR+ macrophages are a new subpopulation of macrophages that release chemokine CCL2 and play a role in inflammatory and infectious diseases. Names in black = usually described. Names in white = under specific circumstances can be present in different concentrations.
Many questions remain unsolved in this field, and more efforts are needed to establish origin, specific markers, conditions of appropriate stimuli for macrophage polarization, and steps of the differentiation process leading to different subpopulation of macrophages. It is still not clear the relationship between human macrophages versus animal model system and this knowledge is important to advance in the design of new therapeutic strategies supplementing those established in different pathological conditions such as cancer, infectious disease, obesity, and asthma. Moreover, preclinical and clinical observations demonstrate an association between macrophage number/type and prognosis in variety malignancies.
Statements
Acknowledgments
We thank the fellowship for LCG (207760), provided by the Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico, and the FNS (31-46833) and the Ligue Pulmonaire Genevoise.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1
Collin M McGovern N Haniffa M . Human dendritic cell subsets. Immunology (2013) 140(1):22–30.10.1111/imm.12117 - 2
Springall R Amezcua-Guerra LM Gonzalez-Pacheco H Furuzawa-Carballeda J Gomez-Garcia L Marquez-Velasco R et al Interferon-gamma increases the ratio of matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 in peripheral monocytes from patients with coronary artery disease. PLoS One (2013) 8(8):e72291.10.1371/journal.pone.0072291 - 3
Schaale K Brandenburg J Kispert A Leitges M Ehlers S Reiling N . Wnt6 is expressed in granulomatous lesions of _Mycobacterium tuberculosis_-infected mice and is involved in macrophage differentiation and proliferation. J Immunol (2013) 191(10):5182–95.10.4049/jimmunol.1201819 - 4
Crane MJ Daley JM van Houtte O Brancato SK Henry WL Jr Albina JE . The monocyte to macrophage transition in the murine sterile wound. PLoS One (2014) 9(1):e86660.10.1371/journal.pone.0086660 - 5
Fantin A Vieira JM Gestri G Denti L Schwarz Q Prykhozhij S et al Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood (2010) 116(5):829–40.10.1182/blood-2009-12-257832 - 6
Simon MC . PU.1 and hematopoiesis: lessons learned from gene targeting experiments. Semin Immunol (1998) 10(2):111–8.10.1006/smim.1998.0112 - 7
Fogg DK Sibon C Miled C Jung S Aucouturier P Littman DR et al A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science (2006) 311(5757):83–7.10.1126/science.1117729 - 8
Varol C Landsman L Fogg DK Greenshtein L Gildor B Margalit R et al Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med (2007) 204(1):171–80.10.1084/jem.20061011 - 9
Tsou CL Peters W Si Y Slaymaker S Aslanian AM Weisberg SP et al Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest (2007) 117(4):902–9.10.1172/JCI29919 - 10
Li L Huang L Sung SS Vergis AL Rosin DL Rose CE Jr et al The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int (2008) 74(12):1526–37.10.1038/ki.2008.500 - 11
Geissmann F Jung S Littman DR . Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity (2003) 19(1):71–82.10.1016/S1074-7613(03)00174-2 - 12
Passlick B Flieger D Ziegler-Heitbrock HW . Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood (1989) 74(7):2527–34. - 13
Zimmermann HW Seidler S Nattermann J Gassler N Hellerbrand C Zernecke A et al Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One (2010) 5(6):e11049.10.1371/journal.pone.0011049 - 14
Tacke F Randolph GJ . Migratory fate and differentiation of blood monocyte subsets. Immunobiology (2006) 211(6–8):609–18.10.1016/j.imbio.2006.05.025 - 15
Italiani P Boraschi D . From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol (2014) 5:514.10.3389/fimmu.2014.00514 - 16
Dey A Allen J Hankey-Giblin PA . Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol (2014) 5:683.10.3389/fimmu.2014.00683 - 17
Gordon S Pluddemann A Martinez Estrada F . Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev (2014) 262(1):36–55.10.1111/imr.12223 - 18
Takahashi K Yamamura F Naito M . Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol (1989) 45(2):87–96. - 19
Naito M Yamamura F Nishikawa S Takahashi K . Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol (1989) 46(1):1–10. - 20
Ajami B Bennett JL Krieger C Tetzlaff W Rossi FM . Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci (2007) 10(12):1538–43.10.1038/nn2014 - 21
Hess DC Abe T Hill WD Studdard AM Carothers J Masuya M et al Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol (2004) 186(2):134–44.10.1016/j.expneurol.2003.11.005 - 22
Ajami B Bennett JL Krieger C McNagny KM Rossi FM . Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci (2011) 14(9):1142–9.10.1038/nn.2887 - 23
Davies LC Jenkins SJ Allen JE Taylor PR . Tissue-resident macrophages. Nat Immunol (2013) 14(10):986–95.10.1038/ni.2705 - 24
Lee AW Mao Y Penninger JM Yu S . Gab2 promotes colony-stimulating factor 1-regulated macrophage expansion via alternate effectors at different stages of development. Mol Cell Biol (2011) 31(22):4563–81.10.1128/MCB.05706-11 - 25
Jenkins SJ Ruckerl D Thomas GD Hewitson JP Duncan S Brombacher F et al IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med (2013) 210(11):2477–91.10.1084/jem.20121999 - 26
Jenkins SJ Ruckerl D Cook PC Jones LH Finkelman FD van Rooijen N et al Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science (2011) 332(6035):1284–8.10.1126/science.1204351 - 27
Mosmann TR Cherwinski H Bond MW Giedlin MA Coffman RL . Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol (1986) 136(7):2348–57. - 28
Nathan CF Murray HW Wiebe ME Rubin BY . Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med (1983) 158(3):670–89.10.1084/jem.158.3.670 - 29
Stein M Keshav S Harris N Gordon S . Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med (1992) 176(1):287–92.10.1084/jem.176.1.287 - 30
Mills CD Kincaid K Alt JM Heilman MJ Hill AM . M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol (2000) 164(12):6166–73.10.4049/jimmunol.164.12.6166 - 31
Murray PJ Allen JE Biswas SK Fisher EA Gilroy DW Goerdt S et al Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity (2014) 41(1):14–20.10.1016/j.immuni.2014.06.008 - 32
Billiau A Matthys P . Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev (2009) 20(2):97–113.10.1016/j.cytogfr.2009.02.004 - 33
Guha M Mackman N . LPS induction of gene expression in human monocytes. Cell Signal (2001) 13(2):85–94.10.1016/S0898-6568(00)00149-2 - 34
Fleetwood AJ Lawrence T Hamilton JA Cook AD . Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol (2007) 178(8):5245–52.10.4049/jimmunol.178.8.5245 - 35
Okamoto T Gohil K Finkelstein EI Bove P Akaike T van der Vliet A . Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol (2004) 286(1):L198–209.10.1152/ajplung.00136.2003 - 36
Zeidler PC Millecchia LM Castranova V . Role of inducible nitric oxide synthase-derived nitric oxide in lipopolysaccharide plus interferon-gamma-induced pulmonary inflammation. Toxicol Appl Pharmacol (2004) 195(1):45–54.10.1016/j.taap.2003.10.005 - 37
Arnold CE Whyte CS Gordon P Barker RN Rees AJ Wilson HM . A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology (2014) 141(1):96–110.10.1111/imm.12173 - 38
Andrade MR Amaral EP Ribeiro SC Almeida FM Peres TV Lanes V et al Pathogenic Mycobacterium bovis strains differ in their ability to modulate the proinflammatory activation phenotype of macrophages. BMC Microbiol (2012) 12:166.10.1186/1471-2180-12-166 - 39
Nairz M Schleicher U Schroll A Sonnweber T Theurl I Ludwiczek S et al Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med (2013) 210(5):855–73.10.1084/jem.20121946 - 40
Podinovskaia M Lee W Caldwell S Russell DG . Infection of macrophages with Mycobacterium tuberculosis induces global modifications to phagosomal function. Cell Microbiol (2013) 15(6):843–59.10.1111/cmi.12092 - 41
Shuai K Stark GR Kerr IM Darnell JE Jr . A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science (1993) 261(5129):1744–6.10.1126/science.7690989 - 42
Zhong Z Wen Z Darnell JE Jr . Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science (1994) 264(5155):95–8.10.1126/science.8140422 - 43
Krausgruber T Blazek K Smallie T Alzabin S Lockstone H Sahgal N et al IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol (2011) 12(3):231–8.10.1038/ni.1990 - 44
Toshchakov V Jones BW Perera PY Thomas K Cody MJ Zhang S et al TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol (2002) 3(4):392–8.10.1038/ni774 - 45
Ni Gabhann J Hams E Smith S Wynne C Byrne JC Brennan K et al Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS One (2014) 9(1):e85834.10.1371/journal.pone.0085834 - 46
Eun SY Seo J Park SW Lee JH Chang KC Kim HJ . LPS potentiates nucleotide-induced inflammatory gene expression in macrophages via the upregulation of P2Y2 receptor. Int Immunopharmacol (2014) 18(2):270–6.10.1016/j.intimp.2013.11.026 - 47
Sierra-Filardi E Puig-Kroger A Blanco FJ Nieto C Bragado R Palomero MI et al Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood (2011) 117(19):5092–101.10.1182/blood-2010-09-306993 - 48
Escribese MM Sierra-Filardi E Nieto C Samaniego R Sanchez-Torres C Matsuyama T et al The prolyl hydroxylase PHD3 identifies proinflammatory macrophages and its expression is regulated by activin A. J Immunol (2012) 189(4):1946–54.10.4049/jimmunol.1201064 - 49
Suzuki A Hanada T Mitsuyama K Yoshida T Kamizono S Hoshino T et al CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med (2001) 193(4):471–81.10.1084/jem.193.4.471 - 50
Miyanaka Y Ueno Y Tanaka S Yoshioka K Hatakeyama T Shimamoto M et al Clinical significance of mucosal suppressors of cytokine signaling 3 expression in ulcerative colitis. World J Gastroenterol (2007) 13(21):2939–44.10.3748/wjg.v13.i21.2939 - 51
Koeberlein B zur Hausen A Bektas N Zentgraf H Chin R Nguyen LT et al Hepatitis B virus overexpresses suppressor of cytokine signaling-3 (SOCS3) thereby contributing to severity of inflammation in the liver. Virus Res (2010) 148(1–2):51–9.10.1016/j.virusres.2009.12.003 - 52
White GE Cotterill A Addley MR Soilleux EJ Greaves DR . Suppressor of cytokine signalling protein SOCS3 expression is increased at sites of acute and chronic inflammation. J Mol Histol (2011) 42(2):137–51.10.1007/s10735-011-9317-7 - 53
Feng D Stone RC Eloranta ML Sangster-Guity N Nordmark G Sigurdsson S et al Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus. Arthritis Rheum (2010) 62(2):562–73.10.1002/art.27223 - 54
Niewold TB Kelly JA Kariuki SN Franek BS Kumar AA Kaufman KM et al IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann Rheum Dis (2012) 71(3):463–8.10.1136/annrheumdis-2011-200463 - 55
Carmona FD Martin JE Beretta L Simeon CP Carreira PE Callejas JL et al The systemic lupus erythematosus IRF5 risk haplotype is associated with systemic sclerosis. PLoS One (2013) 8(1):e54419.10.1371/journal.pone.0054419 - 56
Imamura K Suzumura A Hayashi F Marunouchi T . Cytokine production by peripheral blood monocytes/macrophages in multiple sclerosis patients. Acta Neurol Scand (1993) 87(4):281–5.10.1111/j.1600-0404.1993.tb05508.x - 57
Bouhy D Malgrange B Multon S Poirrier AL Scholtes F Schoenen J et al Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J (2006) 20(8):1239–41.10.1096/fj.05-4382fje - 58
Ponomarev ED Shriver LP Maresz K Dittel BN . Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res (2005) 81(3):374–89.10.1002/jnr.20488 - 59
Smith KJ Lassmann H . The role of nitric oxide in multiple sclerosis. Lancet Neurol (2002) 1(4):232–41.10.1016/S1474-4422(02)00102-3 - 60
Van Rooijen N Sanders A . Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods (1994) 174(1–2):83–93.10.1016/0022-1759(94)90012-4 - 61
Lewis ND Patnaude LA Pelletier J Souza DJ Lukas SM King FJ et al A GPBAR1 (TGR5) small molecule agonist shows specific inhibitory effects on myeloid cell activation in vitro and reduces experimental autoimmune encephalitis (EAE) in vivo. PLoS One (2014) 9(6):e100883.10.1371/journal.pone.0100883 - 62
Mulherin D Fitzgerald O Bresnihan B . Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum (1996) 39(1):115–24.10.1002/art.1780390116 - 63
Gordon RA Grigoriev G Lee A Kalliolias GD Ivashkiv LB . The interferon signature and STAT1 expression in rheumatoid arthritis synovial fluid macrophages are induced by tumor necrosis factor alpha and counter-regulated by the synovial fluid microenvironment. Arthritis Rheum (2012) 64(10):3119–28.10.1002/art.34544 - 64
Boyle DL Soma K Hodge J Kavanaugh A Mandel D Mease P et al The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis (2015) 74(6):1311–6.10.1136/annrheumdis-2014-206028 - 65
Du X Stocklauser-Farber K Rosen P . Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase?Free Radic Biol Med (1999) 27(7–8):752–63.10.1016/S0891-5849(99)00079-9 - 66
Kim JY Song EH Lee S Lim JH Choi JS Koh IU et al The induction of STAT1 gene by activating transcription factor 3 contributes to pancreatic beta-cell apoptosis and its dysfunction in streptozotocin-treated mice. Cell Signal (2010) 22(11):1669–80.10.1016/j.cellsig.2010.06.007 - 67
Martin AP Rankin S Pitchford S Charo IF Furtado GC Lira SA . Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes (2008) 57(11):3025–33.10.2337/db08-0625 - 68
Kim HS Park JM Lee MS . A defect in cell death of macrophages is a conserved feature of nonobese diabetic mouse. Biochem Biophys Res Commun (2012) 421(1):145–51.10.1016/j.bbrc.2012.04.017 - 69
Surani SR . Diabetes, sleep apnea, obesity and cardiovascular disease: why not address them together?World J Diabetes (2014) 5(3):381–4.10.4239/wjd.v5.i3.381 - 70
Vitseva OI Tanriverdi K Tchkonia TT Kirkland JL McDonnell ME Apovian CM et al Inducible toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (2008) 16(5):932–7.10.1038/oby.2008.25 - 71
Ekstrom M Halle M Bjessmo S Liska J Kolak M Fisher R et al Systemic inflammation activates the nuclear factor-kappaB regulatory pathway in adipose tissue. Am J Physiol Endocrinol Metab (2010) 299(2):E234–40.10.1152/ajpendo.00115.2010 - 72
Weisberg SP McCann D Desai M Rosenbaum M Leibel RL Ferrante AW Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest (2003) 112(12):1796–808.10.1172/JCI19246 - 73
Lumeng CN Bodzin JL Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest (2007) 117(1):175–84.10.1172/JCI29881 - 74
Olleros ML Martin ML Vesin D Fotio AL Santiago-Raber ML Rubbia-Brandt L et al Fat diet and alcohol-induced steatohepatitis after LPS challenge in mice: role of bioactive TNF and Th1 type cytokines. Cytokine (2008) 44(1):118–25.10.1016/j.cyto.2008.07.001 - 75
Kawanishi N Yano H Yokogawa Y Suzuki K . Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev (2010) 16:105–18. - 76
Shaler CR Horvath CN Jeyanathan M Xing Z . Within the enemy’s camp: contribution of the granuloma to the dissemination, persistence and transmission of Mycobacterium tuberculosis. Front Immunol (2013) 4:30.10.3389/fimmu.2013.00030 - 77
Olleros ML Vesin D Bisig R Santiago-Raber ML Schuepbach-Mallepell S Kollias G et al Membrane-bound TNF induces protective immune responses to M. bovis BCG infection: regulation of memTNF and TNF receptors comparing two memTNF molecules. PLoS One (2012) 7(5):e31469.10.1371/journal.pone.0031469 - 78
Garcia I Olleros ML Quesniaux VF Jacobs M Allie N Nedospasov SA et al Roles of soluble and membrane TNF and related ligands in mycobacterial infections: effects of selective and non-selective TNF inhibitors during infection. Adv Exp Med Biol (2011) 691:187–201.10.1007/978-1-4419-6612-4_20 - 79
Kahnert A Seiler P Stein M Bandermann S Hahnke K Mollenkopf H et al Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol (2006) 36(3):631–47.10.1002/eji.200535496 - 80
Leemans JC Thepen T Weijer S Florquin S van Rooijen N van de Winkel JG et al Macrophages play a dual role during pulmonary tuberculosis in mice. J Infect Dis (2005) 191(1):65–74.10.1086/426395 - 81
Kurtz S McKinnon KP Runge MS Ting JP Braunstein M . The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect Immun (2006) 74(12):6855–64.10.1128/IAI.01022-06 - 82
Thi EP Hong CJ Sanghera G Reiner NE . Identification of the Mycobacterium tuberculosis protein PE-PGRS62 as a novel effector that functions to block phagosome maturation and inhibit iNOS expression. Cell Microbiol (2013) 15(5):795–808.10.1111/cmi.12073 - 83
Huang Y Wang Y Bai Y Wang ZG Yang L Zhao D . Expression of PE_PGRS 62 protein in Mycobacterium smegmatis decrease mRNA expression of proinflammatory cytokines IL-1beta, IL-6 in macrophages. Mol Cell Biochem (2010) 340(1–2):223–9.10.1007/s11010-010-0421-x - 84
Ebe Y Hasegawa G Takatsuka H Umezu H Mitsuyama M Arakawa M et al The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int (1999) 49(6):519–32.10.1046/j.1440-1827.1999.00910.x - 85
Kurihara T Warr G Loy J Bravo R . Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med (1997) 186(10):1757–62.10.1084/jem.186.10.1757 - 86
Jia T Serbina NV Brandl K Zhong MX Leiner IM Charo IF et al Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol (2008) 180(10):6846–53.10.4049/jimmunol.180.10.6846 - 87
Serbina NV Pamer EG . Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol (2006) 7(3):311–7.10.1038/ni1309 - 88
Kopan R Ilagan MX . The canonical Notch signaling pathway: unfolding the activation mechanism. Cell (2009) 137(2):216–33.10.1016/j.cell.2009.03.045 - 89
Xu H Zhu J Smith S Foldi J Zhao B Chung AY et al Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol (2012) 13(7):642–50.10.1038/ni.2304 - 90
Gupta M Shin DM Ramakrishna L Goussetis DJ Platanias LC Xiong H et al IRF8 directs stress-induced autophagy in macrophages and promotes clearance of Listeria monocytogenes. Nat Commun (2015) 6:6379.10.1038/ncomms7379 - 91
Bleriot C Dupuis T Jouvion G Eberl G Disson O Lecuit M . Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity (2015) 42(1):145–58.10.1016/j.immuni.2014.12.020 - 92
Martinez FO Sica A Mantovani A Locati M . Macrophage activation and polarization. Front Biosci (2008) 13:453–61.10.2741/2692 - 93
Jaguin M Houlbert N Fardel O Lecureur V . Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol (2013) 281(1):51–61.10.1016/j.cellimm.2013.01.010 - 94
Barros MH Hauck F Dreyer JH Kempkes B Niedobitek G . Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One (2013) 8(11):e80908.10.1371/journal.pone.0080908 - 95
Raes G Brys L Dahal BK Brandt J Grooten J Brombacher F et al Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol (2005) 77(3):321–7.10.1189/jlb.0304212 - 96
Tang R Zhang G Chen SY . Response gene to complement 32 protein promotes macrophage phagocytosis via activation of protein kinase C pathway. J Biol Chem (2014) 289(33):22715–22.10.1074/jbc.M114.566653 - 97
Zhao P Gao D Wang Q Song B Shao Q Sun J et al Response gene to complement 32 (RGC-32) expression on M2-polarized and tumor-associated macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4. Cell Mol Immunol (2014).10.1038/cmi.2014.108 - 98
Raes G De Baetselier P Noel W Beschin A Brombacher F Hassanzadeh Gh G . Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol (2002) 71(4):597–602. - 99
Ohmori Y Hamilton TA . IL-4-induced STAT6 suppresses IFN-gamma-stimulated STAT1-dependent transcription in mouse macrophages. J Immunol (1997) 159(11):5474–82. - 100
Pauleau AL Rutschman R Lang R Pernis A Watowich SS Murray PJ . Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol (2004) 172(12):7565–73.10.4049/jimmunol.172.12.7565 - 101
Porta C Rimoldi M Raes G Brys L Ghezzi P Di Liberto D et al Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A (2009) 106(35):14978–83.10.1073/pnas.0809784106 - 102
Satoh T Takeuchi O Vandenbon A Yasuda K Tanaka Y Kumagai Y et al The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol (2010) 11(10):936–44.10.1038/ni.1920 - 103
Bouhlel MA Derudas B Rigamonti E Dievart R Brozek J Haulon S et al PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab (2007) 6(2):137–43.10.1016/j.cmet.2007.06.010 - 104
Rocher C Singla DK . SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One (2013) 8(12):e84009.10.1371/journal.pone.0084009 - 105
Li SN Wang W Fu SP Wang JF Liu HM Xie SS et al IL-21 modulates release of proinflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Mediators Inflamm (2013) 2013:548073.10.1155/2013/548073 - 106
Lambrecht BN Hammad H . Asthma: the importance of dysregulated barrier immunity. Eur J Immunol (2013) 43(12):3125–37.10.1002/eji.201343730 - 107
Zhu Z Homer RJ Wang Z Chen Q Geba GP Wang J et al Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest (1999) 103(6):779–88.10.1172/JCI5909 - 108
Yang M Hogan SP Henry PJ Matthaei KI McKenzie AN Young IG et al Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol (2001) 25(4):522–30.10.1165/ajrcmb.25.4.4620 - 109
Nieuwenhuizen NE Kirstein F Jayakumar J Emedi B Hurdayal R Horsnell WG et al Allergic airway disease is unaffected by the absence of IL-4Ralpha-dependent alternatively activated macrophages. J Allergy Clin Immunol (2012) 130(3):743–50 e8.10.1016/j.jaci.2012.03.011 - 110
Ford AQ Dasgupta P Mikhailenko I Smith EM Noben-Trauth N Keegan AD . Adoptive transfer of IL-4Ralpha+ macrophages is sufficient to enhance eosinophilic inflammation in a mouse model of allergic lung inflammation. BMC Immunol (2012) 13:6.10.1186/1471-2172-13-6 - 111
Barner M Mohrs M Brombacher F Kopf M . Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol (1998) 8(11):669–72.10.1016/S0960-9822(98)70256-8 - 112
Anthony RM Urban JF Jr Alem F Hamed HA Rozo CT Boucher JL et al Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med (2006) 12(8):955–60.10.1038/nm1451 - 113
Bonne-Annee S Kerepesi LA Hess JA O’Connell AE Lok JB Nolan TJ et al Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun (2013) 81(9):3346–55.10.1128/IAI.00625-13 - 114
Chen F Liu Z Wu W Rozo C Bowdridge S Millman A et al An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med (2012) 18(2):260–6.10.1038/nm.2628 - 115
Mantovani A Schioppa T Porta C Allavena P Sica A . Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev (2006) 25(3):315–22.10.1007/s10555-006-9001-7 - 116
Sica A Schioppa T Mantovani A Allavena P . Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer (2006) 42(6):717–27.10.1016/j.ejca.2006.01.003 - 117
Allavena P Sica A Garlanda C Mantovani A . The yin-yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev (2008) 222:155–61.10.1111/j.1600-065X.2008.00607.x - 118
Mantovani A Germano G Marchesi F Locatelli M Biswas SK . Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol (2011) 41(9):2522–5.10.1002/eji.201141894 - 119
Gocheva V Wang HW Gadea BB Shree T Hunter KE Garfall AL et al IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev (2010) 24(3):241–55.10.1101/gad.1874010 - 120
Wang R Zhang J Chen S Lu M Luo X Yao S et al Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer (2011) 74(2):188–96.10.1016/j.lungcan.2011.04.009 - 121
Bailey C Negus R Morris A Ziprin P Goldin R Allavena P et al Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis (2007) 24(2):121–30.10.1007/s10585-007-9060-3 - 122
Fujimoto H Sangai T Ishii G Ikehara A Nagashima T Miyazaki M et al Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer (2009) 125(6):1276–84.10.1002/ijc.24378 - 123
Helm O Held-Feindt J Grage-Griebenow E Reiling N Ungefroren H Vogel I et al Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer (2014) 135(4):843–61.10.1002/ijc.28736 - 124
Biswas SK Gangi L Paul S Schioppa T Saccani A Sironi M et al A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood (2006) 107(5):2112–22.10.1182/blood-2005-01-0428 - 125
DeNardo DG Barreto JB Andreu P Vasquez L Tawfik D Kolhatkar N et al CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell (2009) 16(2):91–102.10.1016/j.ccr.2009.06.018 - 126
Saccani A Schioppa T Porta C Biswas SK Nebuloni M Vago L et al p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res (2006) 66(23):11432–40.10.1158/0008-5472.CAN-06-1867 - 127
Movahedi K Laoui D Gysemans C Baeten M Stange G Van den Bossche J et al Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res (2010) 70(14):5728–39.10.1158/0008-5472.CAN-09-4672 - 128
Franklin RA Liao W Sarkar A Kim MV Bivona MR Liu K et al The cellular and molecular origin of tumor-associated macrophages. Science (2014) 344(6186):921–5.10.1126/science.1252510 - 129
Laoui D Movahedi K Van Overmeire E Van den Bossche J Schouppe E Mommer C et al Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol (2011) 55(7–9):861–7.10.1387/ijdb.113371dl - 130
Dominguez-Soto A Sierra-Filardi E Puig-Kroger A Perez-Maceda B Gomez-Aguado F Corcuera MT et al Dendritic cell-specific ICAM-3-grabbing nonintegrin expression on M2-polarized and tumor-associated macrophages is macrophage-CSF dependent and enhanced by tumor-derived IL-6 and IL-10. J Immunol (2011) 186(4):2192–200.10.4049/jimmunol.1000475 - 131
Wan S Zhao E Kryczek I Vatan L Sadovskaya A Ludema G et al Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology (2014) 147(6):1393–404.10.1053/j.gastro.2014.08.039 - 132
Fu XT Dai Z Song K Zhang ZJ Zhou ZJ Zhou SL et al Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol (2015) 46(2):587–96.10.3892/ijo.2014.2761 - 133
Rentsch CA Birkhauser FD Biot C Gsponer JR Bisiaux A Wetterauer C et al bacillus Calmette-Guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol (2014) 66(4):677–88.10.1016/j.eururo.2014.02.061 - 134
Suriano F Santini D Perrone G Amato M Vincenzi B Tonini G et al Tumor associated macrophages polarization dictates the efficacy of BCG instillation in non-muscle invasive urothelial bladder cancer. J Exp Clin Cancer Res (2013) 32:87.10.1186/1756-9966-32-87 - 135
Svatek RS Zhao XR Morales EE Jha MK Tseng TY Hugen CM et al Sequential intravesical mitomycin plus bacillus Calmette-Guerin for non-muscle-invasive urothelial bladder carcinoma: translational and phase I clinical trial. Clin Cancer Res (2015) 21(2):303–11.10.1158/1078-0432.CCR-14-1781 - 136
Crocker PR Gordon S . Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med (1986) 164(6):1862–75.10.1084/jem.164.6.1862 - 137
Martinez-Pomares L Kosco-Vilbois M Darley E Tree P Herren S Bonnefoy JY et al Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med (1996) 184(5):1927–37.10.1084/jem.184.5.1927 - 138
Kraal G Janse M . Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology (1986) 58(4):665–9. - 139
Mebius RE Kraal G . Structure and function of the spleen. Nat Rev Immunol (2005) 5(8):606–16.10.1038/nri1669 - 140
Liao S von der Weid PY . Lymphatic system: an active pathway for immune protection. Nature (2007) 450(7166):110–4.10.1016/j.semcdb.2014.11.012 - 141
Junt T Moseman EA Iannacone M Massberg S Lang PA Boes M et al Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature (2007) 450(7166):110–4.10.1038/nature06287 - 142
Phan TG Green JA Gray EE Xu Y Cyster JG . Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol (2009) 10(7):786–93.10.1038/ni.1745 - 143
Matsumoto M Mariathasan S Nahm MH Baranyay F Peschon JJ Chaplin DD . Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science (1996) 271(5253):1289–91.10.1126/science.271.5253.1289 - 144
Hiemstra IH Beijer MR Veninga H Vrijland K Borg EG Olivier BJ et al The identification and developmental requirements of colonic CD169(+) macrophages. Immunology (2014) 142(2):269–78.10.1111/imm.12251 - 145
Oetke C Vinson MC Jones C Crocker PR . Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin M levels. Mol Cell Biol (2006) 26(4):1549–57.10.1128/MCB.26.4.1549-1557.2006 - 146
Chow A Lucas D Hidalgo A Mendez-Ferrer S Hashimoto D Scheiermann C et al Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med (2011) 208(2):261–71.10.1084/jem.20101688 - 147
Jacobsen RN Forristal CE Raggatt LJ Nowlan B Barbier V Kaur S et al Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80(+)VCAM1(+)CD169(+)ER-HR3(+)Ly6G(+) erythroid island macrophages in the mouse. Exp Hematol (2014) 42(7):547–61 e4.10.1016/j.exphem.2014.03.009 - 148
Falchi M Varricchio L Martelli F Masiello F Federici G Zingariello M et al Dexamethasone targeted directly to macrophages induces macrophage niches that promote erythroid expansion. Haematologica (2015) 100(2):178–87.10.3324/haematol.2014.114405 - 149
Ramos P Casu C Gardenghi S Breda L Crielaard BJ Guy E et al Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med (2013) 19(4):437–45.10.1038/nm.3126 - 150
Barral P Polzella P Bruckbauer A van Rooijen N Besra GS Cerundolo V et al CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol (2010) 11(4):303–12.10.1038/ni.1853 - 151
Kawasaki N Vela JL Nycholat CM Rademacher C Khurana A van Rooijen N et al Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proc Natl Acad Sci U S A (2013) 110(19):7826–31.10.1073/pnas.1219888110 - 152
Ravishankar B Shinde R Liu H Chaudhary K Bradley J Lemos HP et al Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci U S A (2014) 111(11):4215–20.10.1073/pnas.1320924111 - 153
Saunderson SC Dunn AC Crocker PR McLellan AD . CD169 mediates the capture of exosomes in spleen and lymph node. Blood (2014) 123(2):208–16.10.1182/blood-2013-03-489732 - 154
Ikezumi Y Hurst LA Masaki T Atkins RC Nikolic-Paterson DJ . Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int (2003) 63(1):83–95.10.1046/j.1523-1755.2003.00717.x - 155
Ikezumi Y Suzuki T Hayafuji S Okubo S Nikolic-Paterson DJ Kawachi H et al The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrol Dial Transplant (2005) 20(12):2704–13.10.1093/ndt/gfi105 - 156
Karasawa K Asano K Moriyama S Ushiki M Monya M Iida M et al Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol (2015) 26(4):896–906.10.1681/ASN.2014020195 - 157
Garcia Z Lemaitre F van Rooijen N Albert ML Levy Y Schwartz O et al Subcapsular sinus macrophages promote NK cell accumulation and activation in response to lymph-borne viral particles. Blood (2012) 120(24):4744–50.10.1182/blood-2012-02-408179 - 158
Moseman EA Iannacone M Bosurgi L Tonti E Chevrier N Tumanov A et al B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity (2012) 36(3):415–26.10.1016/j.immuni.2012.01.013 - 159
Honke N Shaabani N Cadeddu G Sorg UR Zhang DE Trilling M et al Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol (2012) 13(1):51–7.10.1038/ni.2169 - 160
Rempel H Calosing C Sun B Pulliam L . Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One (2008) 3(4):e1967.10.1371/journal.pone.0001967 - 161
Zou Z Chastain A Moir S Ford J Trandem K Martinelli E et al Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS One (2011) 6(9):e24559.10.1371/journal.pone.0024559 - 162
Puellmann K Kaminski WE Vogel M Nebe CT Schroeder J Wolf H et al A variable immunoreceptor in a subpopulation of human neutrophils. Proc Natl Acad Sci U S A (2006) 103(39):14441–6.10.1073/pnas.0603406103 - 163
Kaminski WE Beham AW Kzhyshkowska J Gratchev A Puellmann K . On the horizon: flexible immune recognition outside lymphocytes. Immunobiology (2013) 218(3):418–26.10.1016/j.imbio.2012.05.024 - 164
Legrand F Driss V Woerly G Loiseau S Hermann E Fournie JJ et al A functional gammadeltaTCR/CD3 complex distinct from gammadeltaT cells is expressed by human eosinophils. PLoS One (2009) 4(6):e5926.10.1371/journal.pone.0005926 - 165
Beham AW Puellmann K Laird R Fuchs T Streich R Breysach C et al A TNF-regulated recombinatorial macrophage immune receptor implicated in granuloma formation in tuberculosis. PLoS Pathog (2011) 7(11):e1002375.10.1371/journal.ppat.1002375 - 166
Fuchs T Puellmann K Emmert A Fleig J Oniga S Laird R et al The macrophage-TCRalphabeta is a cholesterol-responsive combinatorial immune receptor and implicated in atherosclerosis. Biochem Biophys Res Commun (2015) 456(1):59–65.10.1016/j.bbrc.2014.11.034 - 167
Fuchs T Puellmann K Hahn M Dollt C Pechlivanidou I Ovsiy I et al A second combinatorial immune receptor in monocytes/macrophages is based on the TCRgammadelta. Immunobiology (2013) 218(7):960–8.10.1016/j.imbio.2012.11.005
Keywords
monocytes, macrophages, M1/M2 polarization, TAM generation, pathologies
Citation
Chávez-Galán L, Olleros ML, Vesin D and Garcia I (2015) Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front. Immunol. 6:263. doi: 10.3389/fimmu.2015.00263
Received
26 January 2015
Accepted
12 May 2015
Published
26 May 2015
Volume
6 - 2015
Edited by
Uday Kishore, Brunel University London, UK
Reviewed by
Robert Adam Harris, Karolinska Institutet, Sweden; Geanncarlo Lugo-Villarino, Centre National de la Recherche Scientifique (CNRS), France
Copyright
© 2015 Chávez-Galán, Olleros, Vesin and Garcia.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leslie Chávez-Galán, Department of Pathology and Immunology, Centre Medical Universitaire (CMU), University of Geneva, 1, Rue Michel-Servet, Geneva 1211, Switzerland, leslie.chavezgalan@unige.ch, lchavez_galan@iner.gob.mx
Specialty section: This article was submitted to Molecular Innate Immunity, a section of the journal Frontiers in Immunology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.