Dynamin, a membrane remodelling GTPase (original) (raw)
. Author manuscript; available in PMC: 2012 Dec 12.
Published in final edited form as: Nat Rev Mol Cell Biol. 2012 Jan 11;13(2):75–88. doi: 10.1038/nrm3266
Abstract
Dynamin, the founding member of a family of dynamin-like GTPases (DLPs) implicated in membrane remodelling, has a critical role in endocytic membrane fission events. The use of complementary approaches, including live cell imaging, cell free-studies, X-ray crystallography and genetic studies in mice has greatly advanced our understanding of the mechanisms by which dynamin acts, its essential roles in cell physiology and the specific function of different dynamin isoforms. In addition, several connections between dynamin and human disease have also emerged that highlight specific contributions of this GTPase to the physiology of different tissues.
Introduction
Endocytosis, the process through which cells internalize portions of their plasma membrane along with extracellular material, is fundamentally important in cell physiology. Endocytosis counterbalances the continuous delivery of membrane to the cell surface by exocytosis, regulates the plasma membrane abundance of proteins, controls the signalling output of receptors, mediates cellular uptake of nutrients and is also exploited by pathogens to enter cells. To support these various functions, multiple molecularly and morphologically distinct forms of endocytosis, both constitutive and regulated, operate in all cells 1-4.
Generation of an endocytic vesicle requires the recruitment of various proteins from the cytosol that orchestrate inward plasma membrane bending to form a deeply invaginated bud and subsequently promote its fission. One such protein that directly participates in the fission reaction is the GTPase dynamin, the founding member of a family of GTPases that have diverse roles in membrane remodelling events throughout the cell (Figure 1). The role of dynamin in endocytosis has now been investigated for more than 20 years 5-8. Considerable evidence indicates that dynamin assembles into helical polymers at the necks of budding vesicles and that its GTP-hydrolysis dependent conformational change promotes fission of the underlying tubular membrane to generate a free endocytic vesicle (Figure 2). However, several aspects of its function, its precise mechanisms of action and specific roles of multiple dynamin isoforms in mammalian cells have remained elusive. Furthermore, although multiple dynamin-dependent and dynamin-independent endocytic pathways have been described, we only have a good understanding of how dynamin is recruited and then acts at fission sites for clathrin-mediated endocytosis. In this review, we discuss how complementary experimental approaches have greatly increased our understanding of dynamin's action. We also highlight how this information has been further enhanced by new insights into the function and mechanitic action of dynamin-like proteins.
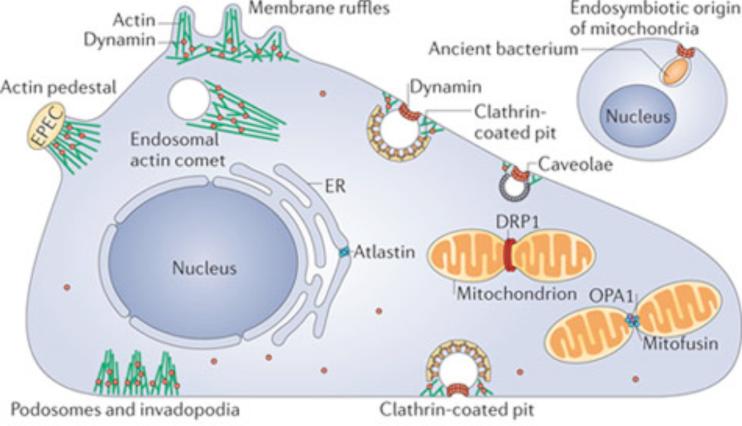
Figure 1. Sites of action of dynamin and dynamin related proteins (DLPs) in a mammalian cell.
(a) Dynamin is localized at sites of endocytosis. It is also found at actin meshworks nucleated by the Arp2/3 complex such as membrane ruffles, podosomes and invadopodia, and at actin pedestals induced by pathogenic bacteria. Other DLPs, including atlastin, Drp1, OPA1 and mitofusin, localize to sites of intracellular membrane fission and fusion in the endoplasmic reticulum and mitochondria. The location of dynamin and DLPs is shown in red while F-actin is coloured green. The endosymbiotic origin of mitochondria (i.e. their formation by the endocytosis of an ancient prokaryote) may explain the role of a DLP in the fission of their outer mitochondrial membrane
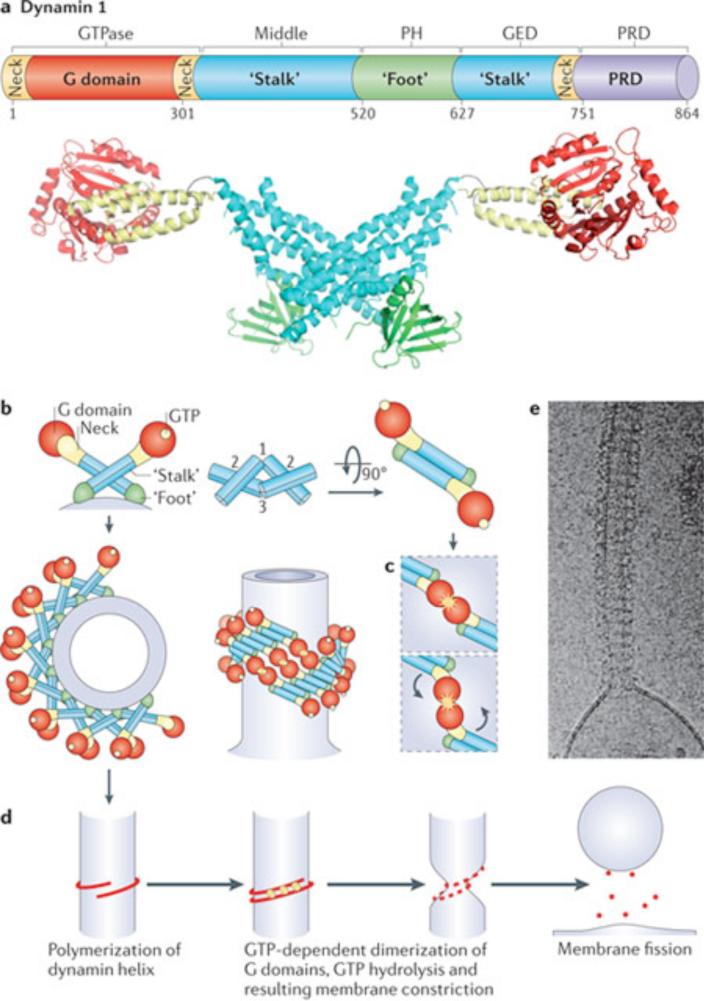
Figure 2. Structure of dynamin and putative mechanism of dynamin-mediated membrane fission.
(a) Top: Linear representation of the domain organization of dynamin based on its 3D structure as revealed by crystallographic studies (numbers indicate amino acid position within the primary sequence of human dynamin 1, xa splice variant). Regions that belong to the same folded module are shown in the same color. Bottom: crystal structure of a dynamin dimer (color coded to match the linear representation). Molecular graphics were created with Pymol, PDB code 3SNH 42). (b) Schematic representation of dynamin dimers and of helical dynamin polymers around a tubular template in two different orientations (90 degrees rotation). The colour-coding of the domains matches the colors of panel (a). The approximate location of the bound nucleotide is highlighted in yellow. Dynamin polymerization occurs as a result of interactions between the stalks of dynamin monomers (interface 2) and between stalk dimers (interfaces 1 and 3). The GTP-dependent dimerization of G domains between adjacent rungs of the dynamin helix (highlighted in yellow stars, longitudinal view of the helix), is thought to promote assembly-stimulated GTPase activity, resulting in membrane constriction and ultimately fission. (d) Proposed GTP hydrolysis-dependent lever-like movement of dynamin's neck (BSE) relative to the G domain. (e) Schematic view of the key steps leading to dynamin-mediated membrane fission. (e) Cryo-EM image showing a helical polymer of purified dynamin that has driven the formation of a tubule from a liposome. Image kindly provided by Adam Frost and Vincenz Unger (University of Utah and Northwestern University respectively).
The discovery of dynamin
Dynamin was originally identified as a GTPase that co-purified with brain microtubules 6, 9. Its role in endocytosis was revealed only subsequently, when the mutations responsible for the temperature-sensitive paralytic phenotype of Drosophila melanogaster shibire mutants were mapped to the dynamin gene 7, 8. In these mutants, paralysis results from the neuronal activity-dependent depletion of synaptic vesicles that is accompanied by the accumulation of arrested ‘collared’ endocytic pits at the presynaptic plasma membrane 5 Subsequent studies revealed that similar collared pits formed at mammalian presynaptic plasma membranes upon exposure to GTPγS, a slowly hydrolyzable GTP analog, and that they contain oligomeric assemblies of dynamin 10. The assembly of helical dynamin polymers either in solution 11 or on membrane templates was additionally reconstituted with the purified protein 11 (Figure 2c). Further investigations in non-neuronal cells showed that dynamin is a general component of clathrin-coated endocytic pits and that its GTPase activity is important for endocytosis: expression of dynamin mutants with an impaired GTP binding (such as the commonly used K44A mutant) and/or hydrolysis cycle have dominant-negative effects on endocytosis 12-15.
Three dynamin isoforms in mammals
Mammalian genomes contain 3 dynamin genes 16. The proteins encoded by these genes share the same domain organization and an overall 80% homology, but have distinct expression patterns. Dynamin 1 is selectively expressed at high levels in neurons and is generally not present in non-neuronal tissues 17, 18, although it can be detected in many cultured cell lines 19, 20. Dynamin 2 is expressed ubiquitously 17, 21. Dynamin 3 is found predominantly in the brain (at much lower levels than dynamin 1) and testis, and at lower levels in some tissues such as the lung 16, 17, 22. Dynamin diversity is compounded by the existence of multiple splice variants for each of the three dynamins 16.
Invertebrates such as C. elegans and D. melanogaster possess only a single dynamin gene 7, 23. So, the existence of three dynamin genes in mammals could in principle reflect differences between the housekeeping role of dynamin in clathrin-mediated endocytosis, mediated by dynamin 2, and specialized forms of endocytosis in cells that additionally express dynamin 1 and/or 3. In addition, this triplication of the dynamin gene during evolution may be partly explained by a need to fine tune overall dynamin levels in specific tissues. Different dynamin isoforms have some unique protein-protein interactions24-27. However, most differences between isoforms are quantitative rather than qualitative; these include affinities for SH3-domain-containing proteins 22, rates of GTPase activity, oligomerization efficiency and lipid binding properties28. Nonetheless, a recent report that differences in the lipid binding characteristics of dynamin 1 and 2 cause robust differences in their membrane fission activity 28 indicates that this remains an area of active research and additional studies are required to fully address the issue of redundancy between dynamin isoforms.
A family of dynamin-like proteins
Dynamins are the founding members of a family of GTPases known as dynamin-like proteins (DLPs), which participate in membrane remodelling (Figure 1 and Table 1) and share basic features of domain organization (Box 1). They also have similar catalytic mechanisms and modes of action that differ from those of the Ras superfamily of regulatory GTPases. The GTPase domains (G domains) of DLPs undergo nucleotide-dependent dimerization and dimer-dependent GTP hydrolysis 29-32. G domain dimerization reciprocally induces structural rearrangements in the catalytic centre of the partner DLP which stimulate GTP hydrolysis 33. In contrast to small regulatory GTPases of the Ras family, DLP family GTPases have low affinity for nucleotides and do not require separate guanine nucleotide-exchange factors (GEFs) or guanine nucleotide-activating proteins (GAPs), as they load spontaneously with GTP and have intrinsic mechanisms for stabilizing the transition state during GTP hydrolysis 29. In the case of dynamin, this is thought to be supported by a sodium or potassium ion within the active site 30. In other DLPs, divergent mechanisms are used to stabilize the transition state. However, the lack of requirement for additional GAP proteins and the importance of G domain dimerization is conserved within the DLP family.
Table 1.
Diverse roles for DLPs at membrane interfaces
| DLP | Organism | Subcellular localization/Site of action | Function | References |
|---|---|---|---|---|
| Classical Dynamins | Animals | Endocytic sites in the plasma membraneArp2/3 containing actin meshworkMidbody | Endocytic membrane fissionRegulation of Arp2/3-dependent actin polymerizationCytokinesis | Discussed extensively in this review. |
| Drpl | Animals | Mitochondria Outer MembranePeroxisomes | Mitochondria fissionPeroxisome division | 161-164 |
| Mitofusin | Animals | Mitochondria Outer Membrane | Mitochondria fusion | 161-164 |
| OPA1 | Animals | Mitochondria Inner Membrane | Mitochondria Fusion | 161-164, 191 |
| Atlastin | Animals | Endoplasmic reticulum | Fusion of ER tubules | 170, 171, 192 |
| MX proteins | Animals | Endoplasmic reticulum | Antiviral | 172, 193 |
| Guanylate Binding Proteins | Animals | Intracellular vesicles | Defense against viral and bacterial pathogens | 173, 194, 195 |
| Phragmoplastin | Plants | Cell Plate | Cell Division | 174 |
| ARC5, ADL | Plants | Chloroplast | chloroplast fission | 196, 197 |
| Vpsl | Fungi | EndosomesPlasma Membrane? | Membrane fission | 100, 198 |
| BDLP | Cyanobacteria | Integral membrane protein | Membrane fusion? | 40 |
Furthermore, whereas the GTP binding and hydrolysis cycle of regulatory GTPases mediates recruitment and release of effector proteins, the cellular function of DLPs is tightly coupled to the dimerization of their G-domains and, at least for some of them, to their polymerization29, 31, 32, 34, 35. The conformational changes produced by GTP binding and hydrolysis in the G domain of DLPs is transduced into a movement relative to adjacent domains 36-38 that when propagated along the polymer is expected to produce a force on membranes 31, 32. Thus, for dynamin, the cycle of GTP loading and hydrolysis is intimately coupled to its membrane fission activity (Figure 2b). This explains why mutations in dynamin designed to lock it into a GTP-bound state do not result in constitutive activation of endocytosis 15, in contrast to the persistent activity exhibited by regulatory GTPases that harbour equivalent mutations.
Domain organization of dynamin
On the basis of its primary sequence, dynamin, a cytosolic protein, has been typically described as comprising: an N-terminal GTPase or G domain; a ‘middle’ or ‘stalk’ region; a pleckstrin homology (PH) domain; a GED domain, so called because its interactions with the GTPase domain had suggested a function as a GTPase effector domain 39; and a proline-rich C-terminal region, typically referred to as the proline-rich domain (PRD, Figure 2a)16. More recently, information about the structure of dynamin and DLPs 30, 34, 36, 37, including the crystal structure of dimers of nearly full length dynamin (lacking only the PRD)31, 32 have allowed for a modified definition of dynamin domains that better reflects the predicted three-dimensional hairpin-like folding of the full-length protein (Figure 2a and b).
The G domain and bundle signalling element
The G domain sits upon a helical bundle, also known as the bundle signalling element (BSE)30 or neck 40, which is formed by 3 helices derived from sequences at the N and C terminal sides of the G domain and from the C-terminal region of the GED domain, respectively (Figure 2). Consistent with the idea that this region of the GED domain is in close physical proximity with, and functionally linked to, the GTPase domain, a screen for suppressor mutations of a mutation in the G domain of Drosophila dynamin identified a mutation within the GED C-terminus 41. The BSE is followed by a stalk that is composed of helices from the middle domain and the N-terminal region of the GED 34, 42, 43; and a PH domain 44 that forms the vertex or ‘foot’ of the stalk hairpin and binds membranes. The PRD, expected to be unfolded, emerges at the boundary between the BSE and the G-domain, most likely projecting away from the membrane, where it might interact with other proteins.
Dimerization through the stalk
The stalk of dynamin dimerizes in a cross-like fashion (Figure 2b) to yield a dynamin dimer in which the two G-domains are oriented in opposite directions 31, 32, 34. This dimer represents the basic dynamin unit, and is different from the additional dimerization interface that is generated by the interaction of two G domains, which will be discussed below.
Phospholipid association through the PH Domain
The PH domain binds acidic phospholipids in the cytosolic leaflet of the plasma membrane and PI(4,5)P2 in particular via a positively charged surface at the foot of the dynamin hairpin 44, 45. PH domain mutants that impair phosphoinositide binding exert dominant-negative effects on clathrin-mediated endocytosis 46, 47. The binding between the isolated dynamin PH domain and phosphoinositides is of very modest affinity (>1mM) but this membrane interaction is strengthened by charge-dependent association with other negatively charged phospholipids and by avidity afforded by dynamin polymerization 45-48. A hydrophobic loop emerging from the PH domain may promote membrane interaction and may also have curvature generating or sensing properties 28, 49.
Coordinating dynamin function through the PRD
The PRD contains an array of PxxP amino acid motifs that interact with many SH3 domain containing proteins (Table 2) to localize dynamin at endocytic sites and coordinate dynamin's function with these other factors during endocytosis50-53. Accordingly, dynamin lacking the PRD cannot rescue endocytic defects in dynamin KO fibroblasts 19. The PRD-SH3 interactions are typically of moderate affinity but the presence of multiple SH3-binding motifs in the PRD and multiple SH3-domain-containing proteins at endocytic sites, and the polymeric state of these proteins results in a significant avidity effect that enhances the ability of such interactions to concentrate dynamin. At least some interactions of the dynamin PRD are regulated by phosphorylation 50.
Coordinating polymerization and activity
Purified dynamin spontaneously polymerizes into rings and helices when incubated in low ionic strength solutions 11 or in the presence of narrow negatively charged tubular templates (such as membrane tubules, microtubules or actin bundles) 6, 54-56. It can also tubulate membrane bilayers under appropriate conditions by forming a continuous membrane coat around them 10, 57-59. Dynamin polymerization resulting from the side-by-side apposition of dimers via stalk tip interactions (Figure 2b, interfaces 1 and 3) occurs an angle that determines the diameter of the ring 31, 32. The tetrameric form of dynamin, which can be abundant in solution 60, may represent an intermediate in higher order assembly 34, 38. Thus, the stalks form the core of the ring, whereas the BSE and G-domains of each dimer project towards the adjacent rungs of the dynamin helix 31, 32 (Figure 2b). It follows that G-domain dimerization, which is critical for GTP hydrolysis and for dynamin function, can only occur across adjacent rungs (Figure 2b), which explains the robust stimulation of dynamin's GTPase activity upon polymerization 56. A similar overall domain organization and mode of polymerization is predicted to be shared by all DLPs implicated in membrane fission 61. However, the module that binds the membrane in these other proteins is divergent from dynamin and the PRD is replaced by sequences with different binding properties. Such divergence likely reflects the different mechanisms that recruit different DLPs to membranes and may speak against a key role of the PRD and the PH domain in the mechanics of fission.
Membrane fission by the dynamin helix
The mechanisms by which dynamin drives membrane fission have been the subject of intense debate, and have been analysed in living cells 12, 15, 46, 56, broken cell preparations 10, 62 and minimal systems based on purified dynamin and artificial lipid bilayers 57, 58, 63-65. Models derived from these experiments have suggested that once dynamin has polymerized around the neck of an endocytic bud (or a tubular membrane template in vitro), its GTP-hydrolysis-dependent structural reorganization triggers constriction (twistase and/or constrictase models) or stretching (poppase model) to promote membrane fission (Fig. 2b). Importantly, cell-free studies have shown that purified dynamin alone can cut synthetic lipid tubules in the presence of GTP 57, 58, 63, 65. Thus, while other factors may assist the action of dynamin in vivo, dynamin is sufficient to mediate membrane fission.
Insights obtained from recent crystallographic and cryo-EM studies of dynamin and DLPs have offered important new clues about how dynamin works. First, the demonstration that the G domain of dynamin belongs to the superfamily of GTPases that undergo GTP-dependent homodimerization (GADs) 29 strongly argues against the possibility that GTP-bound dynamin may function by recruiting another effector 39. Second, as G domain dimerization is critical for GTPase activity 29, 30, 61, 63, 66 and requires interactions between adjacent rungs of the dynamin helix (Figure 2b) 30, dynamin's action requires at minimum a polymer that wraps around a membrane template to connect to the next rung. Third, studies of dynamin and of the DLP atlastin suggest that GTP hydrolysis by a G domain dimer leads to a prominent lever-like movement of the adjacent domain, the BSE in the case of dynamin 36, 37,38. Such a movement could constrict the dynamin helix when propagated along its subunits. As this movement is triggered by the interaction of G domains on adjacent rungs of a helix, it could produce a coordinated rotational sliding of one rung on the next, which is consistent with the reported twisting of dynamin-coated tubules during GTP hydrolysis 57. However, in principle, such movement could also simply constrict the tubule by producing a structural change in the ring without any rotation43.
Surprisingly, however, if a dynamin helix has been assembled in the absence of hydrolysable GTP, subsequent GTP loading and hydrolysis does not necessarily trigger fission, possibly because a long dynamin coat can function as a stabilizing scaffold 57, 63, 64. In cell-free systems, efficient fission of lipid tubules occurs optimally under conditions where dynamin polymerization occurs in the presence of GTP, allowing rapid coupling between dynamin assembly, GTP hydrolysis and polymer disassembly 58, 63, 65. Under these conditions, fission is proposed to result from the membrane destabilization that is predicted to occur when the constricted ring disassembles (Figure 2b). A long continuous dynamin helix may be less efficient in promoting fission because it lacks a constriction focal point or has less efficient subunit dissociation. In fact, taking into consideration bulk cytoplasmic GTP concentrations of ~2 mM versus the binding affinity of dynamin for GTP (Kd=~1μM) 67, dynamin oligomerizing into helices should be in a predominantly GTP-bound state and be poised to hydrolyse GTP and disassemble quickly once G domains of one turn can dimerize with the G domains of the next turn. Under some physiological conditions, local regulation of GTP levels probably contributes to proper dynamin function as mutations in the nucleoside diphosphokinase gene, which helps maintain such levels, make synapses more sensitive to dynamin mutations 68.
Although there have been important advances in understanding dynamin structure, we have much to learn about how conformational changes produced by the GTP hydrolysis cycle of dynamin relate to dynamin-mediated membrane fission. For example, the relative contributions of G domain dimerization versus subsequent GTP hydrolysis in the constriction and fission of membranes remain undefined. Furthermore, GTP hydrolysis may not only produce a powerstoke but also contribute to membrane destabilization by inducing helix disassembly..
Additional factors cooperating in fission
Although dynamin alone can constrict and cut lipid tubules, the constriction of the dynamin ring in vivo is likely to be assisted or regulated by other factors to achieve fission (Figure 3). These include: membrane tension generated by actin, either via its polymerization or via myosin motors 57, 63, 69, 70; a heterogeneous distribution of lipids on the two sides of the constriction contributing to line tension 71; enzymatic degradation of PI(4,5)P2 leading to catastrophic dissociation of dynamin and other endocytic factors after GTP hydrolysis 72; or the partial destabilization of the bilayer by neighbouring lipid-binding proteins, such as proteins containing BAR domains 73. The potential role of BAR domain proteins has acquired interest with the realization that dynamin GTPase activity involves interactions between dynamin rungs, because the intercalation of BAR domains between rungs could hinder such an interaction 73-75, and thus potentially inhibit dynamin's action. Cryo-EM studies aimed at elucidating the precise organizational relationship between dynamin rings and BAR domain proteins should help to address this question.
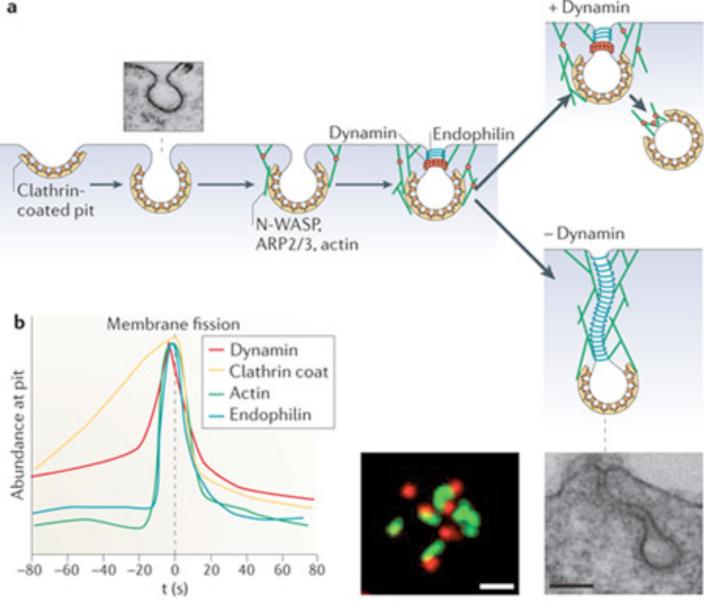
Figure 3. Dynamin and clathrin-mediated endocytosis.
(a) Putative sequence of action of actin, BAR proteins and dynamin at clathrin coated endocytic pits as revealed by studies of cells that lack dynamin (dynamin 1,2 double KO fibroblasts) 19. Lack of dynamin results in an arrest of the endocytic reaction at the stage of deeply invaginated pits and in the actin-dependent elongation of their BAR protein coated tubular necks (EM micrograph at lower right, reproduced with permission from 19). When actin is depolymerized (latrunculin B treatment), clathrin coated buds collapse to omega shaped pits with short wide necks (upper left EM micrograph, reproduced with permission from 19). The fluorescence micrograph shows tubulated endocytic clathrin coated pits in dynamin 1,2 double KO fibroblasts as revealed by live cell imaging of the BAR protein endophilin 2-GFP (tubular neck) and of RFP-clathrin light chain (reproduced with permission from 19) (b) Schematic timeline showing the accumulation of endocytic proteins at endocytic pits. The zero time represents the fission reaction, as determined by the loss of accessibility of the bud lumen to the extracellular medium (sketched based on data from 69). Note that the role of actin may be non-essential under some conditions 80, 102.
A key component of clathrin-coated pits
Dynamin may contribute to multiple forms of endocytosis, but its action is better understood in the context of clathrin-mediated endocytosis 12-14, 17, 19, 20, 22, 69, 76 (Figure 3). During formation of endocytic buds (Figure 3), a subset of scaffold proteins (such as FCHO1, Eps15 and intersectin)69, 77 and clathrin adaptors (for example the AP-2 complex, AP180/CALM and epsin) are first recruited to the PI(4,5)P2-rich plasma membrane, coincident with the binding of some of these proteins to endocytic sorting motifs of integral membrane proteins1, 78. Such components cluster cargo, induce membrane curvature and also have actin nucleating properties 1, 78. The coat subsequently grows through the assembly of the clathrin lattice, which, through positive feedback, recruits additional cargo adaptors and endocytic factors 1, 78, 79. Dynamin also slowly accumulates around the growing pit69. Deep invagination of the bud and formation of a narrow neck, which is often assisted by a burst of actin polymerization19, 80, involves the recruitment of BAR-domain-containing proteins, several of which bind dynamin as well as the PI(4,5)P2 phosphatase synaptojanin (Box 2, Figure 2 b, c, d, f and g) 19, 74, 75, 81. It is at this point that dynamin rapidly accumulates at bud necks to mediate fission (Figure 3a and c)69, 82.
The enrichment of dynamin at clathrin-coated endocytic pits has been extensively demonstrated by fluorescence microscopy12, 53, 69, 82 including super-resolution methods 62 and electron microscopy 10, 83. Furthermore, live imaging of cells expressing fluorescent dynamin shows that dynamin levels at the pits are low during early stages of clathrin-coated pit maturation (Figure 2 a and c), most likely reflecting low affinity interactions with other endocytic factors andrise markedly during later stages - reflecting polymerization at the bud neck - to reach a peak that coincides with membrane fission (Figure 3c) 69, 82, 84. Factors that cooperatively, but redundantly, regulate dynamin assembly here include: PI(4,5)P2 and other acidic phospholipids present in the plasma membrane 85, 86; the tubular membrane template at the bud neck55; proteins containing dynamin binding SH3 domains (primarily BAR domain containing proteins 74, 75 whose polymerization forms a powerful high avidity affinity matrix 73, see Table 2); and actin, which can directly bind dynamin 87 (Figure 3). The coinciding accumulation of BAR proteins, actin and dynamin at late stages of endocytosis 69, 82 is particularly striking (Figure 3c), consistent with feed-forward mechanisms.
The sequence of events underlying clathrin-mediated endocytosis is evolutionarily conserved, as this process shows mechanistic similarity to endocytosis at actin patches in yeast 88, 89. Surprisingly, however, there are mixed reports as to whether the closest yeast homologue of dynamin, Vps1, does 90, 91 or does not 88, 92 participate in actin patch endocytosis, questioning how essential dynamin is for clathrin-mediated endocytosis. It is possible that unique properties of the yeast plasma membrane, for example tension and/or the presence of a cell wall, explains this difference93.
Dynamin in other endocytic pathways
Endocytosis can also occur without clathrin, and dynamin has been implicated in some clathrin-independent endocytic pathways (Figure 1). For example, dynamin has been implicated in caveolin-dependent internalization and both dynamin and actin were transiently detected at caveolae, coincident with their fission from the plasma membrane 94, 95. However, the dynamics and function of dynamin at caveolae remains largely undefined. Surprisingly, the absence of dynamin is accompanied by a loss of caveolae and of their core component caveolin1 rather than by the increase in caveolae abundance that might be expected if dynamin were required for their fission19. Thus, it is not yet clear whether dynamin has a key role during endocytic fission of caveolae.
With respect to clathrin- and caveolin-independent endocytic pathways, evidence for a role of dynamin is in many cases primarily dependent on dominant-negative mutant approaches (which may exert indirect effects) or pharmacological inhibition with drugs (which may have off-target or non-physiological effects; Box 3). Thus, definitive answers about how dynamin affects such pathways will require a combination of methods and should include loss of function studies. As robust bulk endocytosis still occurs in fibroblasts lacking dynamin 19 and in the presence of dominant-negative dynamin mutants 12, dynamin cannot have a universal role in endocytic vesicle fission.
Roles in intracellular budding
Vesicle budding, and thus membrane fission, occurs at multiple points throughout the secretory and endocytic pathways. Although dynamin does not affect most of these events, several experimental approaches that it might contribute to the fission of clathrin-coated vesicles that bud from the trans-Golgi network (TGN) 96 as well as of other vesicles that bud from either the Golgi complex or endosomes 20, 97-99. The yeast dynamin homologue Vps1 has also been implicated in retrograde traffic between endosomes and the Golgi complex100. However, there was no obvious accumulation of clathrin-coated pits at the trans-Golgi network (TGN) of fibroblasts lacking dynamin19, suggesting that dynamin is not universally required for clathrin-mediated budding. It is also important to consider that some defects observed at non-endocytic sites when dynamin is inhibited may arise indirectly through clathrin sequestration at the cell surface, perturbation of membrane traffic, effects on signalling pathways and/or cytoskeletal dynamics.
Dynamin and the cytoskeleton
Dynamin interacts both directly and indirectly with the cytoskeleton. The relation between these interactions and the endocytic function of dynamin represents one of the most interesting and poorly understood aspects of dynamin biology.
Intimate links with actin
A major property of dynamin, and one likely to be fundamentally important to its action, is its link to the actin cytoskeleton. A link between dynamin and actin at endocytic sites is not surprising, consistent with the importance of actin for many endocytic events, including at least a subset of clathrin-mediated endocytosis19, 69, 70, 101, 102. However, immunofluorescence studies and live cell analysis show that dynamin also colocalizes prominently with actin meshworks that are nucleated by the actin regulators N-WASP/WAVE and Arp2/3 at several other sites, including lamellipodia 103, membrane ruffles (including circular dorsal ruffles 104 and ruffles that mediate phagocytosis 105), invadopodia 106, podosomes 107, actin comets 108, 109 and the actin pedestals that can be associated with bacterial cell entry110 (Figure 1 and 3). At these sites, dynamin is not restricted to the membrane interface, but is present in the actin meshwork itself (Figure 1). This colocalization is consistent with the PRD-mediated binding of dynamin to numerous actin regulatory proteins, including proteins that contain Cdc42 interacting or regulatory domains (summarized in Table 2). Indeed, dynamin can bundle around actin filaments assembled in the presence of its partner cortactin, which can simultaneously bind F-actin54, 104. The stalk region of dynamin itself can also bind F-actin directly87. This raises the question of whether dynamin can directly influence actin dynamics and may have a role in actin function independent of endocytosis. However, as membrane remodelling is the shared property of dynamin and other DLPs, and is thus likely to be the most critical function of dynamin, links between dynamin and actin may predominantly reflect its role in membrane remodelling during endocytosis. Studies in yeast emphasize the critical role of actin in endocytosis, further supporting this possibility 88, 92, 111.
The interaction of dynamin with actin and its regulatory proteins may help position dynamin at endocytic sites (Figures 1 and 3a,b). For example, a role of actin in directing the localization of dynamin at endocytic sites is supported by studies of mammalian cells that lack dynamin, which have placed F-actin upstream of dynamin at clathrin coated pits (Figure 2d and g)19.. Actin may also generate membrane tension at the neck of endocytic buds, thus synergizing with dynamin during fission. Conversely, perturbation of dynamin function in living cells (by the expression of mutant dynamin or via pharmacological inhibition) can perturn actin dynamics87, 103, 108, 109, 112, 113. However, in cells that lack dynamin, stress fibers do not seem to be affected19. The most obvious change observed in these cells is the accumulation of Arp2/3-dependent actin meshworks around the necks of arrested endocytic clathrin-coated pits (Figure 3) 19. There is also evidence of alterations to other Arp2/3-dependent actin-based structures in these cells, such as circular membrane ruffles and invadopodia/podosomes (Shawn M. Ferguson, Olivier Destaing, Roland Baron and Pietro De Camilli, unpublished results) 106, 107, 113. However, it is difficult to discriminate between direct and indirect effects in these experiments, as disrupted endocytosis may alter signalling pathways (see below).
The recent crystallographic studies of dynamin 30-32 have raised new questions about potential non-endocytic effects of dynamin on actin dynamics. The helical organization of dynamin polymers at the bud neck appears to be optimally suited to allow an interaction in trans between dynamin G domains to promote GTP-hydrolysis (Figure 2B. However, purified dynamin can also polymerize into helices around actin bundles and microtubules. It will be important to determine whether such a transient helical arrangement occurs physiologically at non-endocytic sites; these structures would be expected to produce detectable bursts of fluorescence in dynamin-GFP-expressing cells ) but have not yet been observed.
Possible ties to microtubule dynamics?
Although dynamin interacts with microtubules in vitro 6, dynamin only shows strong colocalization with microtubules in cells when its PH domain is mutated 114. Moreover, disruption of dynamin function or lack of dynamin both result in a robust increase in the level of acetylated tubulin 19, 114, a modification associated with stable microtubules. This effect may be directly related to the ability of dynamin to bind microtubules, or to altered microtubule dynamics that result from alterations in the structure and functional state of the cell cortex (for example the great abundance of endocytic clathrin coated pits and of a robust accumulation of actin around them), where the stability of the “plus” end of microtubules is regulated.
Control of cytokinesis
Dynamin 2 concentrates at sites of abscission and was implicated in the completion of cytokinesis 115-117. Given the role of both microtubules as well as membrane traffic during abscission 118-120 and the connections of dynamin to each of these pathways, the precise role of dynamin in cytokinesis remains unclear. Interestingly, dynamin 2 is phosphorylated during mitosis by Cyclin dependent kinase 1 (Cdk1), and its dephosphorylation by calcineurin at these sites is required during cytokinesis 116. This phosphorylation cycle of dynamin 2 is reminiscent of the phosphorylation cycle of dynamin 1 in nerve terminals, which is mediated by cyclin-dependent kinase (Cdk5) and calcineurin (Figure 4d) 121. The broad use of this signaling pathway may be indicative of its fundamental importance. Indeed, this extends to Cdk1 phosphorylation of DRP1 that promotes mitochondrial fission during mitosis122.
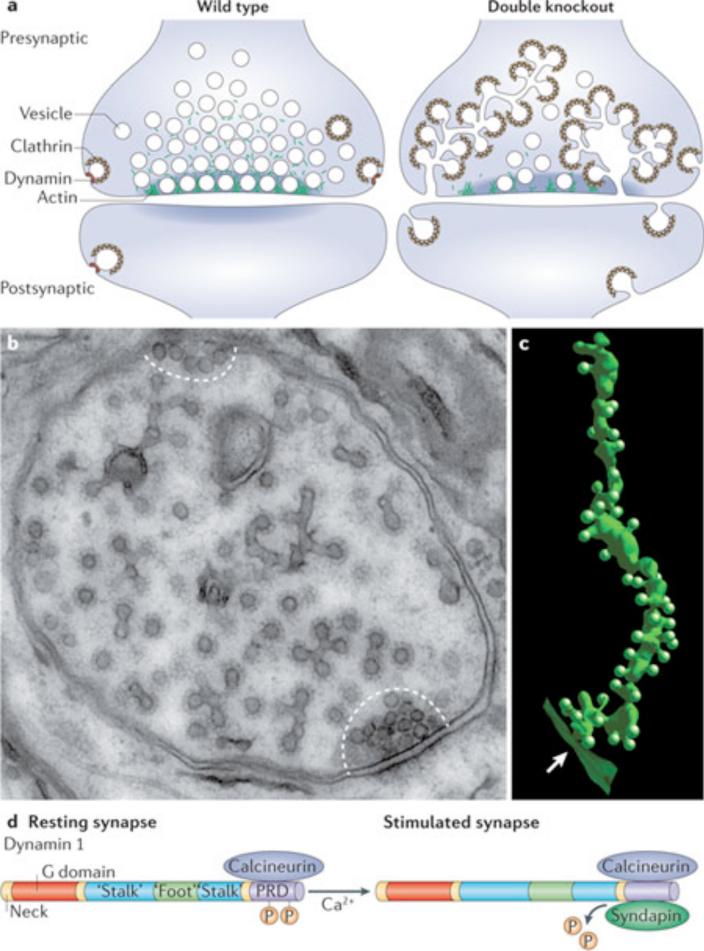
Figure 4. Dynamin participates in synaptic vesicle recycling at neuronal synapses.
(a) From electron microscopy analysis, wild type synapses (left) are characterized by an abundance of synaptic vesicles and very few clathrin-coated pits or clathrin-coated vesicles, consistent with the transient nature of clathrin-coated structures even during intense synaptic activity. At synapses that lack the great majority of their normal dynamin content (for example, from dynamin 1 knockout neurons or even more strikingly from dynamin 1,3 double KO neurons) (right) there is a depletion of synaptic vesicles and a striking accumulation of uniformly sized clathrin coated pits which bud from long invaginations of the plasma membrane 17, 22, 147. A small increase in the abundance of clathrin coated pits is also observed post-synaptically, where pits are heterogeneous in size. (b) Representative electron micrograph of a presynaptic terminal from a dynamin 1 knockout neuron. Presynaptic vesicle clusters at two synaptic junctions are very small (regions defined by dashed lines) while clathrin-coated vesicular structures are highly abundant and occupy the bulk of the terminal. As shown by electron tomography (see field c), all such structures are coated pits, although in this thin section (~60nm) their tubular stalks can only seen in few cases and their connection with the plasma membrane cannot be appreciated 17, 147. (c) Partial electron tomography reconstruction from a dynamin 1,3 double KO nerve terminals showing a long tubular plasma membrane invagination studded with numerous individual clathrin coated pits. The arrow indicates the opening of this tubular structure at the peripheral presynaptic plasma membrane. (d) In resting synapses, the PRD region of dynamin 1 is phosphorylated, preventing association with syndapin. Upon stimulation and increased cytosolic [Ca2+], the protein phosphatase calcineurin, which directly binds a dynamin 1 splice variant (xb), dephosphorylates dynamin 1, allowing its interaction with syndapin. This regulated interaction is thought to promote synaptic vesicle endocytosis at times of elevated neuronal activity.
Endocytosis and signalling
As clathrin-mediated endocytosis is the major pathway of receptor internalization, dynamin regulates the plasma membrane abundance, and thus signalling output, of diverse receptors including growth factor receptors, GPCRs, ionotropic neurotransmitter receptors, ion channels, cell adhesion proteins and signalling proteins such as Notch 123-126. This was first emphasized by the major neurogenic developmental effects of the Drosophila shibire mutation 127 that arise from inhibition of Notch signaling (a phenotype also observed when dynamin is lost126). In addition to these indirect effects of dynamin on signalling, dynamin also interacts directly with a large collection of signalling proteins, (Table 2). These interactions may help coordinate signalling from receptors internalized by clathrin-coated pits with the dynamics of the pits and resulting vesicles and probably reflects an inseparable partnership between endocytosis and signalling. The broader effects of endocytosis on signalling have been extensively studied and reviewed elsewhere 3, 123, 128.
A full and systematic assessment of how dynamin affects signalling has yet to be performed. However, dynamin loss significantly increases phosphorylation and activation of the tyrosine kinase Ack, that interacts with clathrin and other factors implicated in clathrin-mediated endocytosis 129. These findings suggest that dynamin may limit Ack signalling by promoting clathrin coated pit turnover.
Unique roles for dynamin isoforms
Gene knockout studies in mice 17, 19, 22 and the cells derived from them have provided numerous insights into dynamin function and the specific roles of the three dynamin isoforms. Dynamin 2 KO mice die early in embryonic development, consistent with the ubiquitous expression and housekeeping functions of this isoform19. Conditional dynamin 2 KO cells showed transient defects in clathrin-mediated endocytosis. However, the severity of this phenotype was reduced by dynamin 1 upregulation, demonstrating that these two isoforms have at least some overlapping functions 19, 20.
A double conditional KO of dynamin 1 and 2 in mice19 and analysis of derived fibroblasts confirmed that dynamin has an essential role in clathrin-mediated endocytosis but also yielded unexpected results. Surprisingly, dynamin 1, 2 double knockout fibroblasts survived for several weeks in culture without proliferation 19, which precluded analysis of the proposed role for dynamin in cytokinesis130. Most strikingly, dynamin 1 and 2 double KO cells accumulated endocytic clathrin-coated pits connected to the plasma membrane by long tubular necks (Figure 3a) 19, similarly to the phenotype produced by some dynamin mutants83 or to the phenotype observed in cells recovering from a temperature-induced block in endocytosis 131. These tubular necks, which were highly dynamic had an outer diameter of approximately 36 nm 19, consistent with that of tubules coated by BAR-domain-containing proteins wich narrow curvature73. Accordingly, fluorescent imaging analysis shows that a variety of BAR domain proteins such as endophilin, SNX9, amphiphysin and tuba are present in these tubules 19, 129, as were N-WASP, components of the Arp2/3 complex, other actin regulatory proteins and F-actin (Figure 3a and d and ref. 19).
Thus, although dynamin may modulate early stages of clathrin-coated pit formation, it is essential only for a late step of clathrin-mediated endocytosis, when membrane fission occurs. Moreover, the key function of dynamin is to terminate the formation of a deeply invaginated pit that is orchestrated by other factors. These studies have also helped elucidate the sequence of events that occur upstream of dynamin, by allowing otherwise transient intermediates to accumulate (Figure 3a). They showed that the actin cytoskeleton has a primary role in elongating the tubular necks of the coated pits and that the recruitment of BAR proteins on tubules, which is mediated to some extent by their curvature-sensing properties may help shape, stabilize and elongate the tubules in tight coordination with actin.
Dynamin and the nervous system
Endocytosis has a housekeeping role in neurons as in other cells. However, endocytosis, and clathrin-mediated endocytosis in particular, also has a specialized role in nerve terminals, mediating the local recycling of synaptic vesicle membranes after exocytosis (Figure 4a) 132. Consistent with this, disruption of synaptic transmission is the most dramatic and rapid effect produced by the temperature-sensitive inactivation of dynamin in Drosophila 5, 7, 8. Furthermore, perturbing dynamin impairs synaptic vesicle recycling, and thus neuronal communication in several experimental models 5, 17, 22, 23, 133-138.
The fact that all three dynamins are expressed in neurons, with both dynamin 1 and 3 being expressed primarily in the nervous system, raises questions about the specific roles that each of the three dynamins has here 17, 21. On the basis of KO studies, only dynamin 2 is essential for the development of the nervous system 19. Its conditional KO using the Cre/Lox system in early neuronal progenitors139, prior to the onset of expression of dynamins 1 and 3, drastically impairs brain development (S.M.F. and P.D.C., unpublished observations). However, conditional deletion of dynamin 2 in neurons at an early postnatal stage140 does not affect mouse viability or cause any discernible neurological phenotype (S.M.F. and P.De C., unpublished observations). This indicates that, once dynamins 1 and/or 3 are expressed, they can replace the function of dynamin 2.
Dynamin 1 and nervous system function
Dynamin 1 is present at massive concentrations in the nervous system, is concentrated at synapses, and shows markedly increased levels during synaptogenesis 17, 18. Additionally, dynamin 1 and several other endocytic proteins collectively called ‘dephosphins’ 141-143 are constitutively phosphorylated in resting synapses. This dynamin phosphorylation depends at least in part on Cdk5 121 and, upon nerve stimulation, dynamin is rapidly dephosphorylated by the Ca2+ and calmodulin-dependent phosphatase calcineurin (Figure 4d)141; this is predicted to facilitate dynamin interactions with endocytic proteins to promote endocytosis50. The direct interaction of a dynamin 1 splice variant (the ‘b’ C-terminal variant) with calcineurin, via a PxIxIT-like calcineurin binding motif (PRITIS), further emphasizes the potential physiological importance of this Ca2+-dependent dephosphorylation reaction for synaptic function (24, 25 and Silvia Giovedi’, S. M. F. and P. De C., unpublished observations). On the basis on these considerations, dynamin 1 was expected to be essential for synaptic vesicle endocytosis.
Surprisingly, dynamin 1 is dispensable for basic functions of the nervous system 17. However, dynamin 1 KO mice rapidly develop a severe neurological phenotype, fail to thrive and die within two weeks of birth 17. Thus, dynamin 1 is not unique among the three dynamins in its ability to support synaptic vesicle recycling. However, the massive increase in dynamin 1 expression during the first post-natal weeks is critical to sustain the increase in synaptic activity that occurs as the nervous system matures. Consistent with this, direct measurements of endocytic rates at the calyx of Held synapse in the mouse brain stem show that the endocytic rate in the dynamin 1 KO is normal after small stimuli but rapidly saturates and fails to scale with exocytosis as stimulus strength increases 138.
Dynamin 3 at the synapse
Dynamin 3 was proposed to have a specific postsynaptic role, , forming specialized endocytic sites that locally recycle AMPA receptors in dendritic spines144. Consistent with this, dynamin 3 binds the EVH1 domain of the post-synaptic protein Homer via a PxxF motif that is not present in dynamin 1 and 2 and this property appeared consistent with the reported strong localization of dynamin 3 in dendritic spines27. However, this dendritic enrichment of dynamin 3 was not confirmed by another study that instead emphasized an overlapping role with dynamin 1 presynaptically 22. Thus, it is unclear whether any one dynamin isoform shows preferential enrichment or function within the postsynaptic compartment, although strong evidence indicates that the dynamins plays an important role in the endocytosis of neurotransmitter receptors 145, 146.
Dynamin 3 KO mice do not show any obvious pathological phenotype 22. By contrast, dynamin 1, 3 double KO mice have a more severe phenotype than the dynamin 1 single KO 22. They develop normally in utero but, although they maintain a limited degree of synaptic transmission, they die within several hours after birth. This result supports a partially overlapping function of the two neuronally enriched dynamins in synaptic transmission.
Synaptic transmission in dynamin 1,3 double KO mice
Electrophysiological recordings from cultured dynamin 1 single and dynamin 1, 3 double KO neurons confirmed that synaptic transmission can still occur in the absence of these ‘neuronal’ dynamins, although the ability to release neurotransmitter and to sustain release upon sustained stimulation is strongly reduced 17, 22. Likewise, these mutants show a delay in compensatory endocytosis in response to stimulus17, 22. The main ultrastructural defect observed is reduced synaptic vesicle number and a massive accumulation of endocytic clathrin coated pits located along deep invaginations of the plasma membrane (Figure 4a and b)17, 22, 147. Dynamin 1 has been implicated in bulk endocytosis that helps to recover synaptic vesicle membrane in response to strong stimuli 148. However, a form of bulk endocytosis still occurs in response to massive stimulation in both dynamin 1 KO 138, 147 or dynamin 1, 3 double KO neurons (Yumei Wu, S. M.F. and P. De C., unpublished observations).
Overall, the results of these mouse KO studies demonstrate that even the combined absence of dynamin 1 and 3 does not abolish synaptic vesicle recycling. Most likely, dynamin 2 is sufficient to support synaptic vesicle endocytosis, albeit at a slower rate. This conclusion is supported by the ability of dynamin 2 overexpression to at least partially rescue synaptic vesicle recycling defects in dynamin 1 KO neurons 17. However, the additional occurrence of dynamin-independent synaptic vesicle recycling cannot be excluded. These findings support a model wherein the occurrence of 2 neuronally enriched dynamin isoforms (dynamin 1 and 3) and the extremely high levels of one of them (dynamin 1), are primarily needed not to support a specific form of endocytosis, either pre- or postsynaptically, but to allow clathrin-mediated endocytosis to operate over a very broad range of neuronal activities. Nonetheless, unique properties of dynamin 1 and 3 (and their splice variants) may fine tune their functions in neurons.
Dynamin links to human disease
Roles of abnormal dynamin function in genetic disease have begun to emerge. Whereas dynamin 2 mutations show links to tissue-specific diseases, dynamin 1 mutations affect selectively the nervous system..
Dynamin 2 and tissue-specific diseases
Multiple unique missense mutations, or short deletions, within the middle, PH and stalk domains of dynamin 2 have been identified in patients with two autosomal dominant genetic conditions, intermediate Charcot-Marie-Tooth disease 149 and centronuclear myopathy 150. Charcot-Marie-Tooth disease is a peripheral neuropathy characterized by muscular weakness of the extremities and defects in neuronal axon conduction. Centronuclear myopathy is a congenital disease characterized by muscle weakness. The dominant mode in which these disorders are inherited supports the idea that the mutant dynamin acquires a toxic gain of function (Box 3).
How perturbed dynamin 2 function results in these two conditions is unclear. There is a lack of clear correlation between either of the diseases and the mutation sites in the dynamin structure, making mechanistic interpretations difficult. It is interesting to note, however, that PH domain mutations in Charcot-Marie-Tooth disease are more frequently located in the N-terminal portion of the domain whereas mutations in centronuclear myopathy are more frequently located in its C-terminal portion 151. Structural analysis shows that, although disease-linked mutations in the PH and stalk domains are distant within the primary sequence of dynamin, they actually cluster at an interface between these two domains and so can potentially influence dynamin oligomerization 31. It additionally remains unknown why altering the function of dynamin 2 so selectively affects certain tissues. Mutations in amphiphysin 2, a major binding partner of dynamin in brain and muscle, can also give rise to an autosomal recessive form of centronuclear myopathy 152. The muscle-specific isoform of amphiphysin 2 is expressed at very high levels, and affects muscle function153, raising the possibility that muscle pathology in these centronuclear myopathies r involves a dynamin interaction with amphiphysin 2.
A role for dynamin 1 in epilepsy?
So far, no human disease has been mapped to the dynamin 1 or dynamin 3 genes. Nonetheless, given the prominent expression and functions of these proteins in neurons, mutations that produce neurological phenotypes are likely. Supporting this possibility, a spontaneous mouse mutation - the fitful mutant mouse – that causes seizures and hearing impairment maps to the dynamin 1 gene 154. This missense mutation (A408T) occurs in an alternatively spliced region within the middle domain of dynamin 1 and may affect its oligomerization. Electrophysiological recordings from neurons of these mice demonstrated defects in GABAergic neurotransmission in response to prolonged stimulation 154, a defect similar to that of dynamin 1 KO mice 17, 147. Thus, subtle perturbations of dynamin 1 function that are otherwise compatible with life can enhance seizure susceptibility and dynamin 1 should be considered as a candidate gene in idiopathic cases of human epilepsy. It is of further interest that KO mice for three dynamin-binding proteins; amphiphysin 155, syndapin 156 and endophilin 157 also have seizures. Additionally, mutations in human synapsin 1, another protein implicated in synaptic vesicle recycling, have been identified in patients with epilepsy 158. Most likely, impairing synaptic vesicle recycling lowers seizure threshold through greater effects on inhibitory neurons that have a high rate of synaptic vesicle turnover and thus a high endocytic demand.
Another spontaneous animal model with impaired dynamin 1 function is Exercise Induced Collapse, a recessive condition that has been observed in dogs 159. These otherwise healthy animals display acute and severe muscle weakness resulting in a life threatening collapse in response to intense exercise or excitement. The underlying missense mutation, R256L, is located within the dynamin 1 G domain. The functional consequence of this mutation on GTPase activity remains uncharacterized.
Conclusions and future questions
The function of dynamin has been extensively investigated both in vitro and in vivo, and this has provided powerful insights into its cellular roles. Studies of dynamin have also helped identify factors that deform membranes and/or sense membrane curvature and that participate in the control membrane fission. Cryo-EM and crystallographic studies of dynamin and other members of the dynamin superfamily have provided insight into how it mediates membrane fission. However, major questions remain open.
The precise mechanism through which dynamin achieves fission remains to be elucidated. The similarities and evolutionary relationship with DLPs that mediate membrane fission (such as dynamin and Drp1) and membrane fusion (such as atlastin, OPA1 and mitofusin) are intriguing and unexpected because it suggests analogies between the mechanisms that mediate these seemingly opposite membrane-remodelling processes. Elucidating the basis of this similarity will be important.
Likewise, it will be interesting to determine why DLPs are needed for only a subset of membrane fission reactions. Other GTPases have been implicated in some membrane budding reactions that do not involve dynamin or other DLPs: for example, the Arf1 GTPase affects COP1-dependent budding from the Golgi complex and the Sar1 GTPase regulates COPII-dependent budding from the endoplasmic reticulum 160. However, both Arf1 and Sar1 are classic regulatory GTPases that function by recruiting downstream effectors, indicating that they use a fundamentally different mechanisms to that of dynamin. The two intracellular fission reactions besides endocytosis that clearly require DLPs are the fission of mitochondria 161-164 and the fission of chloroplast (in plants). The endosymbiotic origin of these organelles implies that their outer membrane is ancestrally derived form the plasma membrane, possibly explaining why fission of their outer membrane requires a dynamin.
Other exciting areas that need to be explored include possible non-endocytic functions of dynamin, particularly those involving the cytoskeleton, and the actin cytoskeleton in particular. The possibility that dynamin could be a target for cancer therapy because of its role in either cytokinesis 130, 165 or cell migration and tissue invasion 166, deserves consideration. Interestingly, the invasive activity of pancreatic ductal carcinoma was associated with elevated dynamin 2 expression 166. Are the potential non-endocytic actions of dynamin mediated by its polymerization in a manner that is analogous to that at the neck of clathrin coated pits? Structural studies indicate that a helical polymerization of dynamin around tubular templates 30-32 is optimally suited to promote G domain dimerization and thus GTP hydrolysis. However, perhaps other structural configurations are possible.
It can be anticipated that the spectrum of genetic diseases resulting directly from mutations in dynamin genes will expand. Indeed, several genes implicated in endocytosis, including PICALM and genes that encode the dynamin-binding proteins CD2AP and amphiphysin 2 have been linked to Alzheimer's disease by genome-wide association studies 167, 168. Dynamin 2 also mediates internalization and infectivity of bacterial 169 and viral pathogens including HIV 2, 26. In addition to these possibilities, as dynamin-dependent endocytosis is implicated in multiple processes that are relevant to disease, polymorphisms within dynamin genes may contribute to susceptibility to many other specific diseases.
Twenty years after the discovery that dynamin has a critical role in endocytosis, the function and mechanisms by which this protein acts remains a most important and timely field of investigation.
Supplementary Material
Supplemental Data
Box 1. A family of dynamin-like proteins.
Dynamins are the founding members of a family of GTPases known as dynamin-like proteins (DLPs), which share basic features of domain organization and roles in membrane remodelling (Figure 1). Within this family, some proteins act similarly to dynamin in mediating membrane fission. For example, Drp1 (Dynamin related protein 1) is important for fission of mitochondria and peroxisomes 161-164. Others are more distant members and mediate homotypic membrane fusion 161-164. These include, for example, mitofusin which controls fusion of mitochondrial outer membranes, OPA1 (optic atrophy 1 gene) which is important for inner mitochondrial membrane fusion, and atlastin which mediates fusion of endoplasmic reticulum membranes 161-164, 170, 171. Also included in this family on the basis of their structure and assembly properties, are the anti-viral, interferon-inducible myxovirus resistance (Mx) proteins 9, 172 and guanylate-binding proteins (GBPs) 173 whose precise functions remain unknown. Plant DLPs control membrane traffic during cell plate formation in cytokinesis 174 and mediate chloroplast division 175, 176. A bacterial DLP implicated in membrane remodeling has also been identified and structurally characterized 40, 175. Additionally, although differing from the DLPs in that they are ATPases rather than GTPases, the EHD proteins share structural similarities and are also implicated in membrane remodelling 177.
Mutations in DLPs other than dynamin, and more specifically in DLPs that affect mitochondrial and endoplasmic reticulum dynamics, have been identified as causes of inherited diseases. These include mutations in OPA1 (optic atrophy type 1) 178, MFN2 (Charcot-Marie-Tooth Type 2A) 179, atlastin (hereditary spastic paraplegia) 180 and a DRP1 (lethal perinatal defects in mitochondria and peroxisome fission 181.
Box 2: Sensing and generating membrane curvature.
Some proteins that function at the membrane interface bind preferentially to curved membranes. This can result from: the intrinsic curvature of the membrane binding surface of these proteins; their propensity to oligomerize into curved scaffolds; or the presence of amphipathic helices whose partial insertion into the bilayer is facilitated by the loose packing of phospholipids at sites of high positive curvature. The same proteins can also force the membrane to curve, and thus induce, propagate or stabilize bilayer curvature 73, 182-185. The ability of these proteins to function primarily as curvature sensors or curvature inducers depends on various factors, such as regulated affinity for the membrane (through changes in phosphorylation, folding state or surface charge of the membrane) and local concentration 182. Dynamin itself has important curvature sensing and generating properties 55, owing to its polymerization into rings 11 and to the presence of a hydrophobic loop emerging from its PH domain 49.
Bar domains. Many proteins that bind dynamin's PRD via an SH3 domain also contain BAR domains, protein modules with curvature generating and sensing properties (Table 2) 73, 81, 186. BAR domains comprise amino-acid sequences that fold into coiled-coils and dimerize into elongated curved structures optimally suited to bind the negatively charged cytosolic leaflet of the plasma membrane 187, 188. The presence of amphipathic helices or lipid-binding modules that flank the BAR domains and their propensity to polymerize further enhance the bilayer-molding properties of these proteins 73. In BAR domain proteins that bind dynamin, the bilayer binding surface is typically concave (with shallow curvature in F-BAR domains such as those of syndapin/pacsin and of FBP17/Toca1/CIP4 73, 188, 189, and narrow curvature in classic BAR domains such as those of endophilin, amphiphysin and Snx9/18 73) and thus optimally suited to bind endocytic intermediates 73. BAR domain proteins are proposed to coordinate the progressive acquisition of bilayer curvature at the necks of coated pits with the recruitment of factors that drive constriction, fission and immediate post-fission events (Figure 3). Dynamin binding to the SH3 domain of BAR or F-BAR domain proteins also relieves autoinhibitory intramolecular interactions that limit the membrane-binding activity of the BAR or F-BAR domain 189, 190.
Acknowledgments
This work was supported in part by the Howard Hughes Medical Institute, the G. Harold and Leila Y. Mathers Charitable Foundation, National Institutes of Health grants (R37NS036251, DK45735 and DA018343), the W.M. Keck Foundation and a NARSAD distinguished Investigator Award to P.D.C.. We wish to thank Hongying Shen, Michelle Pirucello, Andrea Raimondi and Oliver Daumke for their helpful suggestions and insights and Michelle Pirucello and Andrea Raimondi for assistance with preparation of figures.
Glossary
GAPs and GEFs
GTPase-activating proteins (GAPs) promote GTP hydrolysis by GTPases while guanine nucleotide exchange factors (GEFs) displace the GDP generated by the reaction , thus allowing the next cycle of GTP binding and hydrolysis to proceed.
Pleckstrin homology domains (PH domains)
These domains often contain a binding sites for phosphoinositides (most typically PI4P and PI(4,5)P2) and thus help targeting proteins to specific membranes. However they can also function in protein-protein interactions
Src homology 3 (SH3) domains
These domains mediate protein-protein interactions and bind proline containing short amino acid motifs. They are frequently found in proteins involved in signalling, endocytosis and actin regulation.
Line tension
A force that acts to minimize the length of the energetically unfavourable interface between adjacent membrane domains of different composition.
Caveolae
Small flask shaped invaginations of the plasma membrane that are involved in the endocytic uptake of various cell surface molecules and some viruses, in signaling and in the regulation of plasma membrane tension.
Lamellipodia and membrane ruffles
Broad, thin, plasma membrane protrusions that are driven by WASP/WAVE and Arp2/3 complex dependent actin polymerization and are critical for cell motility and for some forms of bulk endocytosis.
Invadopodia/Podosomes
Focal sites of dynamic, Arp2/3 dependent, actin polymerization at the plasma membrane which are found in motile cells at sites of cell-matrix interaction, where they promote local degradation of the matrix. These structures are critical for supporting cell migration through the extracellular matrix and for bone resorption by osteoclasts.
Actin comets
This term describes a “tail” of F-actin nucleated by endosomes or intracellular pathogens that propels the endosome/pathogen through the cytoplasm via the force produced by actin polymerization.
Synaptic vesicle
The small (~40 nm diameter) vesicles within neuronal presynaptic terminals that store and release neurotransmitter.
Calyx of Held
A large synapse within the mammalian auditory brainstem that is suitable (in mice and rats) for presynaptic measurement of membrane capacitance and thus for the detection of synaptic vesicle exo- and endocytosis on a millisecond timescale.
Dendritic spine
Small (~1μm) (spine-like) actin rich protrusions of neuronal dendrites that function as post-synaptic sites for excitatory neurotransmission. They are closely opposed to presynaptic sites of neurotransmitter release and are enriched in neurotransmitter receptors.
Charcot-Marie-Tooth Disease
Peripheral nervous system dysfunction characterized by slow action potential conduction due to defects in either the myelin sheath that insulates neuronal axons or in the axons themselves.
Centronuclear Myopathies
Skeletal muscle diseases that result in muscle weakness and which are characterized by the abnormal central location of nuclei within muscle fibres.
Biography
Shawn M. Ferguson is an assistant professor in the Department of Cell Biology at Yale University. He received his PhD from Vanderbilt University with Randy Blakely and pursued postdoctoral research with Pietro De Camilli at Yale. His laboratory investigates mechanisms controlling lysosome homeostasis and the impacts of this process on cancer and neurodegenerative diseases.
Pietro De Camilli is a Professor in the Department of Cell Biology at Yale University, an Investigator in the Howard Hughes Medical Institute and a founding Director of the Yale Program in Cellular Neuroscience, Neurodegeneration and Repair. He received his MD from the University of Milano and was a postdoc with Paul Greengard at Yale. His research focus on mechanisms in membrane traffic, with emphasis on membrane traffic at synapses.
References
- 1.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews. Molecular cell biology. 2011;12:517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 2.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annual review of biochemistry. 2010;79:803–33. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 3.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–73. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 4.Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Current opinion in cell biology. 2010;22:519–27. doi: 10.1016/j.ceb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–60. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–32. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 7.van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–4. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen MS, et al. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–6. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 9.Obar RA, Collins CA, Hammarback JA, Shpetner HS, Vallee RB. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990;347:256–61. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- 10.Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature. 1995;374:186–90. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 11.Hinshaw JE, Schmid SL. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–2. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 12.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–34. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–78. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Bliek AM, et al. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–63. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks B, et al. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–5. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 16.Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson SM, et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–4. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 18.Nakata T, et al. Predominant and developmentally regulated expression of dynamin in neurons. Neuron. 1991;7:461–9. doi: 10.1016/0896-6273(91)90298-e. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson SM, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Developmental cell. 2009;17:811–22. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YW, Surka MC, Schroeter T, Lukiyanchuk V, Schmid SL. Isoform and splice-variant specific functions of dynamin-2 revealed by analysis of conditional knock-out cells. Mol Biol Cell. 2008;19:5347–59. doi: 10.1091/mbc.E08-08-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook TA, Urrutia R, McNiven MA. Identification of dynamin 2, an isoform ubiquitously expressed in rat tissues. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:644–8. doi: 10.1073/pnas.91.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raimondi A, et al. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70:1100–14. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark SG, Shurland DL, Meyerowitz EM, Bargmann CI, van der Bliek AM. A dynamin GTPase mutation causes a rapid and reversible temperatureinducible locomotion defect in C. elegans. Proc Natl Acad Sci U S A. 1997;94:10438–43. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue J, et al. Calcineurin Selectively Docks with the Dynamin Ixb Splice Variant to Regulate Activity-dependent Bulk Endocytosis. The Journal of biological chemistry. 2011;286:30295–303. doi: 10.1074/jbc.M111.273110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodmer D, Ascano M, Kuruvilla R. Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth. Neuron. 2011;70:1085–99. doi: 10.1016/j.neuron.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzato M, et al. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6812–7. doi: 10.1073/pnas.0607622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray NW, et al. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Current biology : CB. 2003;13:510–5. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 28.Liu YW, et al. Differential curvature sensing and generating activities of dynamin isoforms provide opportunities for tissue-specific regulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E234–42. doi: 10.1073/pnas.1102710108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango: regulation of G proteins by dimerization. Nature reviews. Molecular cell biology. 2009;10:423–9. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- 30.Chappie JS, Acharya S, Leonard M, Schmid SL, Dyda F. G domain dimerization controls dynamin's assembly-stimulated GTPase activity. Nature. 2010;465:435–40. doi: 10.1038/nature09032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faelber K, et al. Crystal structure of nucleotide-free dynamin. Nature. 2011 doi: 10.1038/nature10369. In press. [DOI] [PubMed] [Google Scholar]
- 32.Ford MGJ, Jenni S, Nunnari J. The crystal structure of dynamin. Nature. 2011 doi: 10.1038/nature10441. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chappie JS, et al. An intramolecular signaling element that modulates dynamin function in vitro and in vivo. Molecular biology of the cell. 2009;20:3561–71. doi: 10.1091/mbc.E09-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao S, et al. Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature. 2010;465:502–6. doi: 10.1038/nature08972. [DOI] [PubMed] [Google Scholar]
- 35.Ingerman E, et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. The Journal of cell biology. 2005;170:1021–7. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bian X, et al. Structures of the atlastin GTPase provide insight into homotypic fusion of endoplasmic reticulum membranes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3976–81. doi: 10.1073/pnas.1101643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrnes LJ, Sondermann H. Structural basis for the nucleotide-dependent dimerization of the large G protein atlastin-1/SPG3A. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2216–21. doi: 10.1073/pnas.1012792108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chappie JS, et al. A pseudoatomic model of the dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell. 2011;147:209–22. doi: 10.1016/j.cell.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sever S, Muhlberg AB, Schmid SL. Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–6. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]
- 40.Low HH, Lowe J. A bacterial dynamin-like protein. Nature. 2006;444:766–9. doi: 10.1038/nature05312. [DOI] [PubMed] [Google Scholar]
- 41.Narayanan R, Leonard M, Song BD, Schmid SL, Ramaswami M. An internal GAP domain negatively regulates presynaptic dynamin in vivo: a two-step model for dynamin function. The Journal of cell biology. 2005;169:117–26. doi: 10.1083/jcb.200502042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faelber K, et al. Crystal structure of nucleotide-free dynamin. Nature. 2011;477:556–60. doi: 10.1038/nature10369. [DOI] [PubMed] [Google Scholar]
- 43.Ford MG, Jenni S, Nunnari J. The crystal structure of dynamin. Nature. 2011;477:561–6. doi: 10.1038/nature10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Crystal structure at 2.2 A resolution of the pleckstrin homology domain from human dynamin. Cell. 1994;79:199–209. doi: 10.1016/0092-8674(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 45.Zheng J, et al. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: implications for stimulation of GTPase activity. Journal of molecular biology. 1996;255:14–21. doi: 10.1006/jmbi.1996.0002. [DOI] [PubMed] [Google Scholar]
- 46.Lee A, Frank DW, Marks MS, Lemmon MA. Dominant-negative inhibition of receptor-mediated endocytosis by a dynamin-1 mutant with a defective pleckstrin homology domain. Current biology : CB. 1999;9:261–4. doi: 10.1016/s0960-9822(99)80115-8. [DOI] [PubMed] [Google Scholar]
- 47.Vallis Y, Wigge P, Marks B, Evans PR, McMahon HT. Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Current biology : CB. 1999;9:257–60. doi: 10.1016/s0960-9822(99)80114-6. [DOI] [PubMed] [Google Scholar]
- 48.Bethoney KA, King MC, Hinshaw JE, Ostap EM, Lemmon MA. A possible effector role for the pleckstrin homology (PH) domain of dynamin. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13359–64. doi: 10.1073/pnas.0906945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramachandran R, et al. Membrane Insertion of the Pleckstrin Homology Domain Variable Loop 1 Is Critical for Dynamin-catalyzed Vesicle Scission. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anggono V, et al. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nature neuroscience. 2006;9:752–60. doi: 10.1038/nn1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grabs D, et al. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. The Journal of biological chemistry. 1997;272:13419–25. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- 52.Lundmark R, Carlsson SR. Regulated membrane recruitment of dynamin-2 mediated by sorting nexin 9. The Journal of biological chemistry. 2004;279:42694–702. doi: 10.1074/jbc.M407430200. [DOI] [PubMed] [Google Scholar]
- 53.Shpetner HS, Herskovits JS, Vallee RB. A binding site for SH3 domains targets dynamin to coated pits. The Journal of biological chemistry. 1996;271:13–6. doi: 10.1074/jbc.271.1.13. [DOI] [PubMed] [Google Scholar]
- 54.Mooren OL, Kotova TI, Moore AJ, Schafer DA. Dynamin2 GTPase and cortactin remodel actin filaments. J Biol Chem. 2009 doi: 10.1074/jbc.M109.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roux A, et al. Membrane curvature controls dynamin polymerization. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4141–6. doi: 10.1073/pnas.0913734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stowell MH, Marks B, Wigge P, McMahon HT. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nature cell biology. 1999;1:27–32. doi: 10.1038/8997. [DOI] [PubMed] [Google Scholar]
- 57.Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–31. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 58.Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–9. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 59.Takei K, et al. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–41. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran R, et al. The dynamin middle domain is critical for tetramerization and higher-order self-assembly. The EMBO journal. 2007;26:559–66. doi: 10.1038/sj.emboj.7601491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low HH, Lowe J. Dynamin architecture--from monomer to polymer. Current opinion in structural biology. 2010;20:791–8. doi: 10.1016/j.sbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Wu M, et al. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nature cell biology. 2010;12:902–8. doi: 10.1038/ncb2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bashkirov PV, et al. GTPase Cycle of Dynamin Is Coupled to Membrane Squeeze and Release, Leading to Spontaneous Fission. Cell. 2008 doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danino D, Moon KH, Hinshaw JE. Rapid constriction of lipid bilayers by the mechanochemical enzyme dynamin. Journal of structural biology. 2004;147:259–67. doi: 10.1016/j.jsb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Pucadyil TJ, Schmid SL. Real-Time Visualization of Dynamin-Catalyzed Membrane Fission and Vesicle Release. Cell. 2008 doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghosh A, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature. 2006;440:101–4. doi: 10.1038/nature04510. [DOI] [PubMed] [Google Scholar]
- 67.Binns DD, et al. The mechanism of GTP hydrolysis by dynamin II: a transient kinetic study. Biochemistry. 2000;39:7188–96. doi: 10.1021/bi000033r. [DOI] [PubMed] [Google Scholar]
- 68.Krishnan KS, et al. Nucleoside diphosphate kinase, a source of GTP, is required for dynamin-dependent synaptic vesicle recycling. Neuron. 2001;30:197–210. doi: 10.1016/s0896-6273(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 69.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS biology. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collins A, Warrington A, Taylor KA, Svitkina T. Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Current biology : CB. 2011;21:1167–75. doi: 10.1016/j.cub.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Sun Y, Drubin DG, Oster GF. The mechanochemistry of endocytosis. PLoS Biol. 2009;7:e1000204. doi: 10.1371/journal.pbio.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang-Ileto B, et al. Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Developmental cell. 2011;20:206–18. doi: 10.1016/j.devcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–6. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farsad K, et al. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–9. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 76.Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–61. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 77.Henne WM, et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–4. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–96. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 79.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annual review of cell and developmental biology. 1999;15:705–32. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 80.Saffarian S, Cocucci E, Kirchhausen T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 2009;7:e1000191. doi: 10.1371/journal.pbio.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Itoh T, et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci U S A. 2006;103:19332–7. doi: 10.1073/pnas.0609795104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Damke H, Binns DD, Ueda H, Schmid SL, Baba T. Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Molecular biology of the cell. 2001;12:2578–89. doi: 10.1091/mbc.12.9.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Massol R, Boll W, Griffin AM, Kirchhausen T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0603369103. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barylko B, et al. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. The Journal of biological chemistry. 1998;273:3791–7. doi: 10.1074/jbc.273.6.3791. [DOI] [PubMed] [Google Scholar]
- 86.Zoncu R, et al. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2007;104:3793–8. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu C, et al. Direct dynamin-actin interactions regulate the actin cytoskeleton. The EMBO journal. 2010;29:3593–606. doi: 10.1038/emboj.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conibear E. Converging views of endocytosis in yeast and mammals. Current opinion in cell biology. 2010;22:513–8. doi: 10.1016/j.ceb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 89.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–20. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 90.Nannapaneni S, et al. The yeast dynamin-like protein Vps1:vps1 mutations perturb the internalization and the motility of endocytic vesicles and endosomes via disorganization of the actin cytoskeleton. European journal of cell biology. 2010;89:499–508. doi: 10.1016/j.ejcb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 91.Smaczynska-de R, II, et al. Yeast dynamin Vps1 and amphiphysin Rvs167 function together during endocytosis. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01311.x. [DOI] [PubMed] [Google Scholar]
- 92.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–14. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 93.Aghamohammadzadeh S, Ayscough KR. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol. 2009;11:1039–42. doi: 10.1038/ncb1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–9. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 95.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–7. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 97.Derivery E, et al. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Developmental cell. 2009;17:712–23. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 98.Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat Cell Biol. 2000;2:125–7. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- 99.Mesaki K, Tanabe K, Obayashi M, Oe N, Takei K. Fission of tubular endosomes triggers endosomal acidification and movement. PloS one. 2011;6:e19764. doi: 10.1371/journal.pone.0019764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rothman JH, Raymond CK, Gilbert T, O'Hara PJ, Stevens TH. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990;61:1063–74. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- 101.Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–75. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nature cell biology. 2011;13:1124–31. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schlunck G, et al. Modulation of Rac localization and function by dynamin. Molecular biology of the cell. 2004;15:256–67. doi: 10.1091/mbc.E03-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell. 2003;14:1085–96. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gold ES, et al. Dynamin 2 is required for phagocytosis in macrophages. The Journal of experimental medicine. 1999;190:1849–56. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baldassarre M, et al. Dynamin participates in focal extracellular matrix degradation by invasive cells. Molecular biology of the cell. 2003;14:1074–84. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ochoa GC, et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol. 2000;150:377–89. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee E, De Camilli P. Dynamin at actin tails. Proc Natl Acad Sci U S A. 2002;99:161–6. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc Natl Acad Sci U S A. 2002;99:167–72. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Unsworth KE, et al. Dynamin is required for F-actin assembly and pedestal formation by enteropathogenic Escherichia coli (EPEC). Cellular microbiology. 2007;9:438–49. doi: 10.1111/j.1462-5822.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 111.Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. The EMBO journal. 1993;12:2855–62. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gomez TS, et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nature immunology. 2005;6:261–70. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 113.Yamada H, et al. Dynasore, a dynamin inhibitor, suppresses lamellipodia formation and cancer cell invasion by destabilizing actin filaments. Biochemical and biophysical research communications. 2009;390:1142–8. doi: 10.1016/j.bbrc.2009.10.105. [DOI] [PubMed] [Google Scholar]
- 114.Tanabe K, Takei K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J Cell Biol. 2009;185:939–48. doi: 10.1083/jcb.200803153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–6. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chircop M, et al. Phosphorylation of dynamin II at serine-764 is associated with cytokinesis. Biochimica et biophysica acta. 2011;1813:1689–99. doi: 10.1016/j.bbamcr.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 117.Thompson HM, Skop AR, Euteneuer U, Meyer BJ, McNiven MA. The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis. Current biology : CB. 2002;12:2111–7. doi: 10.1016/s0960-9822(02)01390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goss JW, Toomre DK. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. The Journal of cell biology. 2008;181:1047–54. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gromley A, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 120.Morita E, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. The EMBO journal. 2007;26:4215–27. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan TC, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nature cell biology. 2003;5:701–10. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- 122.Kashatus DF, et al. RALA and RALBP1 regulate mitochondrial fission at mitosis. Nature cell biology. 2011;13:1108–15. doi: 10.1038/ncb2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nature reviews. Molecular cell biology. 2009;10:609–22. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sigismund S, et al. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Developmental cell. 2008;15:209–19. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 125.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–9. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 126.Windler SL, Bilder D. Endocytic internalization routes required for delta/notch signaling. Current biology : CB. 2010;20:538–43. doi: 10.1016/j.cub.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poodry CA. shibire, a neurogenic mutant of Drosophila. Developmental biology. 1990;138:464–72. doi: 10.1016/0012-1606(90)90212-2. [DOI] [PubMed] [Google Scholar]
- 128.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 129.Shen H, et al. Constitutive activated Cdc42-associated kinase (Ack) phosphorylation at arrested endocytic clathrin-coated pits of cells that lack dynamin. Molecular biology of the cell. 2011;22:493–502. doi: 10.1091/mbc.E10-07-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chircop M, et al. Inhibition of Dynamin by Dynole 34-2 Induces Cell Death following Cytokinesis Failure in Cancer Cells. Molecular cancer therapeutics. 2011;10:1553–62. doi: 10.1158/1535-7163.MCT-11-0067. [DOI] [PubMed] [Google Scholar]
- 131.Goldenthal KL, Pastan I, Willingham MC. Initial steps in receptormediated endocytosis. The influence of temperature on the shape and distribution of plasma membrane clathrin-coated pits in cultured mammalian cells. Exp Cell Res. 1984;152:558–64. doi: 10.1016/0014-4827(84)90658-x. [DOI] [PubMed] [Google Scholar]
- 132.Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annual review of cell and developmental biology. 2009;25:133–60. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- 133.Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shupliakov O, et al. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–63. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- 135.Sundborger A, et al. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. Journal of cell science. 2011;124:133–43. doi: 10.1242/jcs.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu J, et al. GTP-independent rapid and slow endocytosis at a central synapse. Nature neuroscience. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- 137.Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–7. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- 138.Lou X, Paradise S, Ferguson SM, De Camilli P. Selective saturation of slow endocytosis at a giant glutamatergic central synapse lacking dynamin 1. Proc Natl Acad Sci U S A. 2008;105:17555–60. doi: 10.1073/pnas.0809621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Isaka F, et al. Ectopic expression of the bHLH gene Math1 disturbs neural development. The European journal of neuroscience. 1999;11:2582–8. doi: 10.1046/j.1460-9568.1999.00699.x. [DOI] [PubMed] [Google Scholar]
- 140.Dewachter I, et al. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:3445–53. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends in neurosciences. 2001;24:659–65. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- 142.Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci U S A. 2004;101:546–51. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McPherson PS, Takei K, Schmid SL, De Camilli P. p145, a major Grb2-binding protein in brain, is co-localized with dynamin in nerve terminals where it undergoes activity-dependent dephosphorylation. J Biol Chem. 1994;269:30132–9. [PubMed] [Google Scholar]
- 144.Lu J, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–89. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carroll RC, et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14112–7. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perez-Otano I, et al. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nature neuroscience. 2006;9:611–21. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hayashi M, et al. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci U S A. 2008;105:2175–80. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Clayton EL, et al. The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7706–17. doi: 10.1523/JNEUROSCI.1976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zuchner S, et al. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet. 2005;37:289–94. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]
- 150.Bitoun M, et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nature genetics. 2005;37:1207–9. doi: 10.1038/ng1657. [DOI] [PubMed] [Google Scholar]
- 151.Durieux AC, Prudhon B, Guicheney P, Bitoun M. Dynamin 2 and human diseases. Journal of molecular medicine. 2010;88:339–50. doi: 10.1007/s00109-009-0587-4. [DOI] [PubMed] [Google Scholar]
- 152.Nicot AS, et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nature genetics. 2007;39:1134–9. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 153.Lee E, et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–6. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 154.Boumil RM, et al. A missense mutation in a highly conserved alternate exon of dynamin-1 causes epilepsy in fitful mice. PLoS genetics. 2010;6 doi: 10.1371/journal.pgen.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Di Paolo G, et al. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron. 2002;33:789–804. doi: 10.1016/s0896-6273(02)00601-3. [DOI] [PubMed] [Google Scholar]
- 156.Koch D, et al. Proper synaptic vesicle formation and neuronal network activity critically rely on syndapin I. The EMBO journal. 2011 doi: 10.1038/emboj.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Milosevic I, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fassio A, et al. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Human molecular genetics. 2011;20:2297–307. doi: 10.1093/hmg/ddr122. [DOI] [PubMed] [Google Scholar]
- 159.Patterson EE, et al. A canine DNM1 mutation is highly associated with the syndrome of exercise-induced collapse. Nature genetics. 2008;40:1235–9. doi: 10.1038/ng.224. [DOI] [PubMed] [Google Scholar]
- 160.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nature reviews. Molecular cell biology. 2011;12:362–75. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nature reviews. Molecular cell biology. 2007;8:870–9. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 162.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annual review of biochemistry. 2007;76:751–80. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 163.Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annual review of genetics. 2005;39:503–36. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 164.Westermann B. Mitochondrial fusion and fission in cell life and death. Nature reviews. Molecular cell biology. 2010;11:872–84. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 165.Chircop M, et al. Phosphorylation of dynamin II at serine-764 is associated with cytokinesis. Biochimica et biophysica acta. 2010 doi: 10.1016/j.bbamcr.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 166.Eppinga RD, et al. Increased expression of the large GTPase dynamin 2 potentiates metastatic migration and invasion of pancreatic ductal carcinoma. Oncogene. 2011 doi: 10.1038/onc.2011.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nature genetics. 2011;43:429–35. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature genetics. 2011;43:436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Veiga E, et al. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell host & microbe. 2007;2:340–51. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu J, et al. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138:549–61. doi: 10.1016/j.cell.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Orso G, et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature. 2009;460:978–83. doi: 10.1038/nature08280. [DOI] [PubMed] [Google Scholar]
- 172.Haller O, Gao S, von der Malsburg A, Daumke O, Kochs G. Dynamin-like MxA GTPase: structural insights into oligomerization and implications for antiviral activity. The Journal of biological chemistry. 2010;285:28419–24. doi: 10.1074/jbc.R110.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prakash B, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature. 2000;403:567–71. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- 174.Gu X, Verma DP. Phragmoplastin, a dynamin-like protein associated with cell plate formation in plants. The EMBO journal. 1996;15:695–704. [PMC free article] [PubMed] [Google Scholar]
- 175.Low HH, Sachse C, Amos LA, Lowe J. Structure of a bacterial dynamin-like protein lipid tube provides a mechanism for assembly and membrane curving. Cell. 2009;139:1342–52. doi: 10.1016/j.cell.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miyagishima SY, et al. A plant-specific dynamin-related protein forms a ring at the chloroplast division site. The Plant cell. 2003;15:655–65. doi: 10.1105/tpc.009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Daumke O, et al. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–927. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- 178.Delettre C, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nature genetics. 2000;26:207–10. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 179.Zuchner S, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nature genetics. 2004;36:449–51. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 180.Zhao X, et al. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nature genetics. 2001;29:326–31. doi: 10.1038/ng758. [DOI] [PubMed] [Google Scholar]
- 181.Waterham HR, et al. A lethal defect of mitochondrial and peroxisomal fission. The New England journal of medicine. 2007;356:1736–41. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 182.Baumgart T, Capraro BR, Zhu C, Das SL. Thermodynamics and mechanics of membrane curvature generation and sensing by proteins and lipids. Annual review of physical chemistry. 2011;62:483–506. doi: 10.1146/annurev.physchem.012809.103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Farsad K, De Camilli P. Mechanisms of membrane deformation. Current opinion in cell biology. 2003;15:372–81. doi: 10.1016/s0955-0674(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 184.Antonny B. Membrane deformation by protein coats. Current opinion in cell biology. 2006;18:386–94. doi: 10.1016/j.ceb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 185.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–6. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 186.Peter BJ, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–9. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 187.Frost A, et al. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–17. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shimada A, et al. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell. 2007;129:761–72. doi: 10.1016/j.cell.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 189.Wang Q, et al. Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12700–5. doi: 10.1073/pnas.0902974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rao Y, et al. Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8213–8. doi: 10.1073/pnas.1003478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Moss TJ, Daga A, McNew JA. Fusing a lasting relationship between ER tubules. Trends in cell biology. 2011;21:416–23. doi: 10.1016/j.tcb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gao S, et al. Structure of myxovirus resistance protein a reveals intra- and intermolecular domain interactions required for the antiviral function. Immunity. 2011;35:514–25. doi: 10.1016/j.immuni.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 194.Kim BH, et al. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–21. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 195.MacMicking JD. IFN-inducible GTPases and immunity to intracellular pathogens. Trends in immunology. 2004;25:601–9. doi: 10.1016/j.it.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 196.Gao H, Kadirjan-Kalbach D, Froehlich JE, Osteryoung KW. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4328–33. doi: 10.1073/pnas.0530206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Glynn JM, Miyagishima SY, Yoder DW, Osteryoung KW, Vitha S. Chloroplast division. Traffic. 2007;8:451–61. doi: 10.1111/j.1600-0854.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 198.Vater CA, Raymond CK, Ekena K, Howald-Stevenson I, Stevens TH. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992;119:773–86. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data