Memory CD8 T-Cell Differentiation during Viral Infection (original) (raw)
Memory CD8 T cells are an important component of protective immunity against viral infections, and understanding their development will aid in the design of optimal vaccines. Recent work has shed light on the complex differentiation process that occurs during a CD8 T-cell response to viral infection. Dramatic cellular changes occur as T cells transition through the three characteristic phases of an antiviral response, initial activation and expansion, the death phase, and the formation of memory T cells. Each of these three phases of the T-cell response is accompanied by extensive transcriptional and functional changes that result in naïve T cells expanding and gaining effector functions, survival of 5 to 10% of the effectors through the death phase, and the gradual acquisition of memory properties by the surviving virus-specific T cells. This review will discuss our current understanding of how functional and protective CD8 T-cell responses are generated and maintained following different types of infections.
Viral infections can be largely divided into two types: (i) acute infections, where virus is eliminated; and (ii) chronic infections, where virus persists. This second type of infection may be further classified into latent infections and those in which there is persistent viral replication. While acute infections usually result in effective antiviral immune responses, chronic infections can be associated with suboptimal T-cell function. We will first focus on acute infections and on recent work that has led to our current understanding of the CD8 T-cell differentiation program that occurs when antigen is eliminated following initial infection and then discuss how CD8 T-cell responses can be altered and impaired during chronic infections when virus persists.
MEMORY CD8 T-CELL DIFFERENTIATION DURING ACUTE VIRAL INFECTION
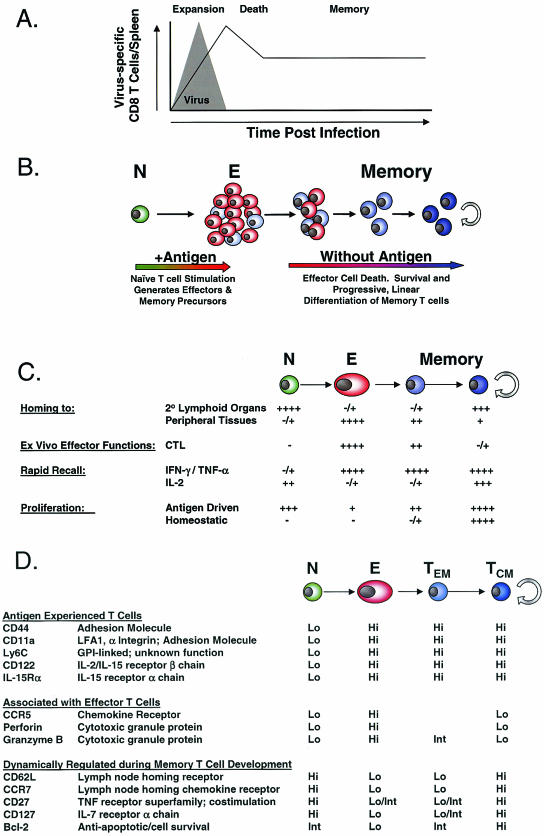
During a CD8 T-cell response to infection, there are three characteristic phases: a period of initial activation and expansion, a contraction or death phase, and the establishment and maintenance of memory (Fig. 1A) (60). The analysis of CD8 T-cell responses has been greatly facilitated by the recent introduction of major histocompatibility complex (MHC) tetramer technology that allows accurate enumeration and phenotypic characterization of antigen-specific T cells by flow cytometry (5, 85). It has been estimated that, in the naïve CD8 T-cell pool in the mouse, T cells specific for a given peptide/MHC complex exist at a frequency of ∼1/105 T cells (17), with similar estimates for humans (8). During the initial phase of a CD8 T-cell response to infection, this population, which corresponds to ∼102 cells/spleen in the mouse, can expand to greater than 107 cells/spleen (17, 85). Robust CD8 T-cell responses have also been observed for humans during the acute phase of viral infections (22). This 104- to 105-fold expansion indicates that, at the peak of proliferation, antigen-specific CD8 T cells can divide approximately every 6 to 8 h (85). Along with this dramatic proliferation, CD8 T cells also undergo activation and differentiation. These cells acquire antiviral effector functions, including the ability to rapidly produce cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). Upon antigenic stimulation, they upregulate the expression of cytotoxic granule proteins, such as granzymes and perforin, and become cytolytic, and they gain the ability to enter nonlymphoid tissues (9, 25, 47, 58, 60, 88, 130). In addition to changes in the expression of these effector molecules, the overall pattern of gene expression is dramatically altered during this activation phase, and a complex pattern of genetic regulation accompanies T-cell activation and expansion (58). One of the more interesting recent observations is that the key components of this activation and expansion can be initiated by brief exposure to antigen. CD8 T-cell stimulation for as little as 24 h resulted in activation and expansion of naïve CD8 T cells, and the proliferation and differentiation of these T cells into effector T cells proceeded without the need for additional antigen (57, 81, 124, 134). An important finding of these studies was that, once the parental naïve CD8 T cell was sufficiently activated, the program of proliferation and differentiation passed on to the daughter cells proceeded without further antigenic stimulation. Thus, a T-cell autonomous program of differentiation and expansion can be initiated following brief antigenic stimulation, and this differentiation program can proceed in an antigen-independent manner, resulting in the acquisition of effector properties and subsequently the formation of memory T cells (57, 81, 124, 134). It will be important to determine whether there is a minimal period of antigenic stimulation that leads to the induction of this developmental program and how further exposure to antigen and other signals, including costimulation, cytokines, and CD4 help, modify the CD8 T-cell activation and functional development.
FIG. 1.
(A) The dynamics of a CD8 T-cell response to acute infection. A CD8 T-cell response to an acute viral infection undergoes an expansion phase, culminating in the generation of effector CD8 T cells and viral clearance. The expansion phase is followed by a death phase, when 90 to 95% of the effector T cells die. The surviving 5 to 10% of the effector CD8 T-cell pool further differentiates and generates a memory T-cell population that is maintained long term in the absence of antigen. (B) Memory CD8 T-cell generation is linear and progressive. Antigenic stimulation causes naïve CD8 T cells to proliferate and acquire effector functions. The effector T cells that survive the death phase further differentiate, giving rise to memory T cells that continue to differentiate in the absence of antigen and acquire the ability to persist in the absence of antigen via homeostatic turnover. (C) Memory CD8 T-cell properties that change during the naïve → effector → memory transition are listed. (D) Phenotypic changes that occur during the naïve → effector → memory transition are listed, including differences between the effector memory (TEM) and central memory (TCM) subsets of memory CD8 T cells. Int, intermediate.
During the 2 to 3 weeks following the peak of CD8 T-cell expansion, the majority (90 to 95%) of the activated effector CD8 T cells die by apoptosis (10, 60, 85). The remaining antigen-specific CD8 T cells eventually populate a pool of long-lived memory T cells. However, additional changes in phenotype, function, and gene expression accompany this transition (43, 44, 58, 130). Importantly, the cells that survive the death phase are not simply preformed memory CD8 T cells constituting a minor population within the effector pool (58). Rather, a small proportion of T cells with full effector qualities undergoes additional differentiation before entering the memory compartment (58). Indeed, recent work has identified a population of effector cells that expresses high levels of CD127, the interleukin 7 receptor alpha (IL-7Rα) chain, as the precursors of memory CD8 T cells (59). This population of CD127Hi effector CD8 T cells, which constitutes ∼5 to 10% of the effector pool, preferentially survives and differentiates from effector CD8 T cells with an activated phenotype, high levels of granzyme B, and low levels of Bcl-2 into memory CD8 T cells characterized by a resting phenotype, low granzyme B expression, and high levels of Bcl-2 (59). In contrast, the CD127Lo population of effector CD8 T cells largely disappears during the transition from the effector phase to the memory phase of the response.
Following the effector and death phases, a memory CD8 T-cell population is established and maintained in the absence of antigen (60, 69, 86). A characteristic feature of the memory phase, in contrast to the effector phase when CD8 T-cell numbers are increasing and the death phase when CD8 T-cell numbers are decreasing, is that essentially constant CD8 T-cell numbers are maintained over long periods of time (69, 85). This homeostasis of the memory CD8 T-cell pool is achieved by the slow but steady division of memory CD8 T cells and is termed homeostatic turnover. An important component of this homeostatic turnover is that there is no net increase in CD8 T-cell numbers, resulting in maintenance of the memory CD8 T-cell pool at a constant size. The cytokines IL-7 and IL-15 are primarily responsible for this homeostatic turnover of memory CD8 T cells (14, 41, 64, 100, 101, 109). In particular, in the absence of IL-15 signals, memory CD8 T cells can be generated, but these cells fail to undergo homeostatic division, and their numbers decline over time (14). In comparison, IL-7 signals during the memory phase appear more important for memory CD8 T-cell survival (41, 100). The ability to persist long term in the absence of antigen is a defining property of memory T cells. The acquisition of responsiveness to homeostatic signals (IL-7 and IL-15) during the transition from effector to memory cells is one of the key changes that results in the formation of long-lived, antigen-independent memory CD8 T cells.
Several recent studies of mice using both gene expression profiling and functional characterization have demonstrated that memory CD8 T-cell development is a gradual process (58, 59, 130). The essence of these studies is that CD8 T-cell differentiation following an acute infection or vaccination is linear and progressive (Fig. 1B). Upon initial activation, naïve CD8 T cells become activated and expand and differentiate into effector T cells. While memory CD8 T-cell precursors are present within the effector pool, as discussed above, these cells have not yet acquired memory CD8 T-cell properties (58, 59). Rather, CD8 T cells that survive the death phase continue to differentiate, gradually losing some effector qualities while acquiring memory CD8 T-cell characteristics (58, 59, 130). Figures 1C and D illustrate some of the properties and phenotypic markers that change during the naïve (N) to effector (E) to memory (M) transition (N→E→M). First, while naïve cells have a low potential to perform effector functions, effector CD8 T cells have acquired the ability to rapidly lyse infected cells and secrete antiviral cytokines in response to antigenic stimulation (9, 25, 47, 58, 60, 88, 130). As effector T cells differentiate into memory cells (E→ M), they acquire a resting phenotype but retain the potential to rapidly produce IFN-γ and TNF-α and to quickly reacquire cytotoxic activity when exposed to antigen (11, 20a, 28, 58, 60, 85, 130). This capacity to rapidly perform effector functions upon restimulation is one property that distinguishes naïve and memory CD8 T cells and confers on memory CD8 T cells a greater ability to mediate protective immunity. Several important CD8 T-cell properties undergo significant changes during the E→M transition. First, the ability to produce IL-2 is low in effectors but increases progressively during memory CD8 T-cell differentiation (129, 130). Second, proliferative potential in response to either antigen or homeostatic signals gradually increases as CD8 T cells differentiate into memory T cells (58, 130). The ability to undergo homeostatic turnover in response to the cytokines IL-7 and IL-15 is a property that is acquired by fully differentiated memory CD8 T cells but not by early-transitional memory CD8 T cells. Third, the ability to efficiently home to lymph nodes via high endothelial venules is lost as naïve CD8 T cells downregulate expression of CD62 ligand (CD62L) and CCR7 and become effector T cells. Reciprocally, effector CD8 T cells acquire the ability to preferentially localize to nonlymphoid organs, and this property is maintained to some extent early into the memory pool when the memory CD8 T cells remain CD62LLo and CCR7Lo (128, 130). As these memory T cells gradually reacquire CD62L and CCR7, however, they regain the ability to localize to central lymphoid organs (130).
MEMORY CD8 T-CELL SUBSETS
Molecules involved in homing to lymphoid tissue have received significant recent attention, since they can be used to subdivide T-cell populations (6, 23, 26, 35, 48, 76, 90, 97, 111, 115, 119). The CD62LHiCCR7Hi population of memory CD8 T cells is found in the spleen, blood, and lymph nodes, while the CD62LLoCCR7Lo subset is also found in the spleen and blood but not in lymph nodes. Rather, the CD62LLoCCR7Lo population is enriched in nonlymphoid locations (72, 135). Initial studies suggested that CD62LLoCCR7Lo memory T cells were more efficient producers of IFN-γ and TNF-α following stimulation than the CD62LHiCCR7Hi subset, while it was predominantly the CD62LHiCCR7Hi memory T cells that had the capacity to synthesize IL-2 (97). Together with their homing properties, these characteristics led to the designation of the CD62LHiCCR7Hi subset as central memory T cells (TCM) and the CD62LLoCCR7Lo subset as effector memory T cells (TEM) (97). A model was proposed in which TEM were present at peripheral sites and provided a first line of defense against infection, while TCM present in lymphoid tissues generated a second wave of effector T cells (97). These original functional studies were performed by using predominantly polyclonal stimulation of human peripheral blood mononuclear cells (PBMC). Recent studies of both mice (117, 130) and humans (91) have demonstrated that, following restimulation with cognate antigen, both subsets are equally good at rapidly producing IFN-γ and TNF-α but that IL-2 production remains a property of TCM. Moreover, when compared directly on a per cell basis, it was the population of CD62LHiCCR7Hi TCM that conferred the more effective protective immunity following either systemic or peripheral challenge (130). The increased proliferative capacity of TCM compared to that of TEM was an important component of this enhanced protective immunity mediated by TCM because it resulted in a larger pool of secondary effectors derived from TCM than from TEM (130). It remains possible that in some inflammatory environments, such as the intestinal mucosa, a different pattern of effector functions by memory CD8 T cells may be observed (77) and that the presence of tissue-resident memory T cells may provide an advantage for protective immunity from some localized infections. It will be important to test this possibility directly using models of local infection. However, since protection from virulent infections will almost certainly require significant T-cell expansion, the advantage of TCM in proliferative potential and protective immunity indicates that the optimal induction and maintenance of this TCM subset, as well as tissue-resident memory T cells, should be an important consideration for vaccine design.
There has been considerable recent interest in understanding the lineage relationship between memory CD8 T-cell subsets (12, 26, 76, 89, 97, 111). Over time there is a conversion of the memory CD8 T-cell pool from CD62LLoCCR7Lo to CD62LHiCCR7Hi (92, 113, 130), and this conversion correlates with enhanced protective immunity, IL-2 production, and homeostatic turnover resulting in long-term antigen-independent maintenance (130). Two models are possible to account for memory T-cell subsets and the changes that occur over time. First, these subsets may arise as distinct lineages during priming and one, the CD62LHiCCR7Hi subset, persists while the other, the CD62LLoCCR7Lo subset, does not. A second model is that these are related subsets and that over time one converts directly into the other. It has been difficult to address memory T-cell lineage development in vivo in humans since one cannot distinguish the loss of one subset and the outgrowth of another from direct conversion of one cell type to another. To address this question directly using labeled cells that could be tracked in vivo, we recently investigated memory CD8 T-cell differentiation in vivo following viral or bacterial infection of mice. Following acute infection with lymphocytic choriomeningitis virus (LCMV) or Listeria monocytogenes, an obligate intracellular bacterium, the phenotypic and functional changes that occurred within the memory compartment were, in fact, the result of differentiation of CD62LLoCCR7Lo memory CD8 T cells directly into CD62LHiCCR7Hi cells (130). Importantly, this conversion of transitory CD62LLoCCR7Lo memory CD8 T cells to the self-renewing CD62LHiCCR7Hi population occurs in the memory pool in the absence of antigen (130). Thus, memory CD8 T-cell subsets do not arise as separate lineages during priming in vivo but rather are related cell types along a continuum of differentiation. The ultimate result of this linear differentiation is the formation of a cell type (CD62LHiCCR7Hi memory CD8 T cells) that can rapidly respond to antigen during secondary infections and that has acquired the stem cell-like property of self-renewal via homeostatic turnover necessary for long-term antigen-independent maintenance.
ALTERATIONS IN CD8 T-CELL RESPONSES DURING CHRONIC VIRAL INFECTION: HIERARCHICAL LOSS OF EFFECTOR FUNCTIONS
The memory CD8 T-cell differentiation pathway discussed above is likely the paradigm for most acute infections. It is possible, however, that under certain conditions (76), especially chronic infections where antigen persists (1, 6, 26, 133), one may see a different pattern of memory T-cell differentiation. When compared directly, several aspects of a normal CD8 T-cell response are altered during a chronic infection. First, the pattern of immunodominance, the hierarchy between responses that are high frequency, or immunodominant, and those of lower magnitude, or subdominant, can be dramatically skewed. For example, during chronic LCMV infection of mice, subdominant specificities often come to predominate the LCMV-specific CD8 T-cell response (37, 122, 129, 138). A similar pattern of inverted immunodominance has been observed during persisting mouse hepatitis virus infection (16) and simian immunodeficiency virus (SIV) infection of macaques (33). Second, the tissue distribution of antigen-specific CD8 T cells can differ from that observed following acute infections, with a large number of virus-specific CD8 T cells present in nonlymphoid tissues (50, 83, 129), driven by antigen localized in these compartments or differences in the expression of homing molecules expressed by the CD8 T-cell populations generated during chronic infection compared to those generated during acute infection. Third, chronic infections can result in severely impaired T-cell function (functional exhaustion) and/or the physical elimination of responding T cells (deletion). Murine models of CD8 T-cell responses to persisting viruses, including LCMV (37, 38, 67, 129, 138, 139), murine gammaherpesvirus (24, 74, 120), mouse hepatitis virus (16), and polyomavirus (84), have been useful for investigating the altered patterns of effector functions and T-cell persistence during chronic infections. The impact of persisting infection or antigen on normal CD8 T-cell differentiation, however, is not restricted to these murine models, since similar functional exhaustion and deletion have been observed in primate models of SIV infection (125, 136), during human infections with human immunodeficiency virus (HIV) (40, 53, 66, 73, 103), hepatitis B virus (HBV) (99), hepatitis C virus (HCV) (46, 70, 118, 127), or human T lymphotropic virus (HTLV) (45), and during malignant melanoma (71).
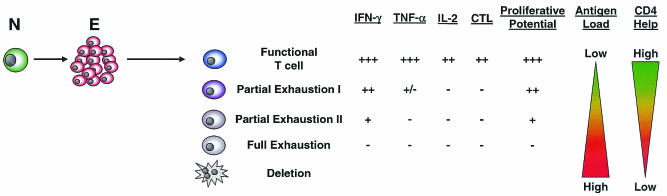
One of the key properties of memory CD8 T cells generated following acute infection is that they maintain the ability to reactivate antiviral effector functions upon antigenic stimulation (e.g., cytokine production). In contrast, during chronic LCMV infection there is a hierarchical loss of the ability to perform effector functions, starting during the effector phase and becoming progressively more severe as the infection progresses (129). This exhaustion of effector functions can be characterized by several stages (Fig. 2). First, functional CD8 T-cell populations, resembling the memory CD8 T cells that develop following acute infections, can coexist with persisting virus if viral antigen expression is low or antigen encounter by T cells is infrequent, which may be the case when antigen is anatomically separated from the immune system or during some latent infections (2, 18, 36, 94). In other circumstances when virus persists, virus-specific CD8 T cells may become partially exhausted, a state characterized by the loss of a subset of effector functions. IL-2 production appears most sensitive to inactivation, followed by TNF-α synthesis, while IFN-γ production appears more resistant to this early functional loss (129). This second stage, partial exhaustion I, may occur during early periods of chronic infection or when the antigen load is not exceptionally high and is characterized by CD8 T cells that are either IFN-γ+TNF-α+IL-2− or IFN-γ+TNF-α−IL-2− following antigenic stimulation (129). Ex vivo cell-mediated cytotoxicity and perforin expression can also be impaired at a stage when IFN-γ production remains relatively intact but TNF-α production is compromised (7, 129). The third stage, partial exhaustion II, exists when functions more resistant to loss, such as IFN-γ production, begin to be impaired. At this point, no TNF-α- or IL-2-producing cells are found and only a fraction of the CD8 T cells identified by using MHC/peptide tetramers can make IFN-γ, while the remainder have become functionally inert, unable to synthesize IL-2, TNF-α, or IFN-γ (37, 79, 129, 138). Finally, cells may enter a state of full functional exhaustion, the complete loss of all effector functions. Cells in this state are incapable of ex vivo cytotoxicity and IL-2 and TNF-α production and have now also completely lost the ability to synthesize IFN-γ in response to peptide stimulation (37, 70, 71, 129, 138). In addition, CD8 T cells in this state express activation markers suggesting frequent T-cell receptor (TCR) stimulation in vivo (138). If antigen load in the form of peptide/MHC complexes presented in vivo is high, epitope-specific CD8 T cells can be physically deleted. During chronic LCMV infection, this is the case for two dominant responses (Db/NP396 and Kb/GP34) (37, 122, 129, 138, 139). These stages of functional exhaustion likely represent a continuum of inactivation, with loss of function becoming progressively worse as either viral load or the duration of infection increases. It will be important to determine how reversible these defects are in response to control of infection and therapeutic intervention.
FIG. 2.
Model for hierarchical exhaustion of CD8 T-cell functions during chronic viral infections. Persisting virus can result in various levels of CD8 T-cell function during chronic infections. If the level or frequency of antigenic stimulation of T cells is low and CD4 help is sufficient, functional CD8 T cells can coexist with persisting virus. However, as the level or frequency of antigenic stimulation increases and/or CD4 help decreases, virus-specific CD8 T cells progressively lose functional properties. Partial exhaustion I is the stage when CD8 T cells have lost the capacity to produce IL-2 and when TNF-α production begins to be impaired. Ex vivo lytic capacity may also be reduced at this stage. Partial exhaustion II occurs when CD8 T cells begin to lose the ability to synthesize IFN-γ and fail to produce IL-2 or TNF-α following antigenic stimulation. Full exhaustion, a state when CD8 T cells lack all effector functions (IFN-γ, TNF-α, IL-2, and ex vivo lytic activity) following antigenic stimulation, can occur when the antigen load is high and/or CD4 help is low. Finally, physical deletion of antigen-specific T cells can occur if epitope presentation to T cells is high and/or sustained. T-cell proliferative potential in response to antigenic stimulation also likely decreases during the outlined stages of functional exhaustion as other functions are impaired. This hierarchical loss of function is dramatically influenced by the level and/or duration of antigenic stimulation experienced by T cells during chronic infections. Further, the stage of exhaustion is also related to CD4 help, since the absence of CD4 help results in more extreme loss of function by CD8 T cells during chronic infections.
In addition to the impairment of effector functions, antigen-driven proliferation appears to be negatively impacted by chronic infection. The proliferative potential of HIV-specific CD8 T cells is substantially lower in patients with progressive disease (and high viral loads) than in long-term nonprogressors (82), and this low proliferative potential correlates with phenotypic markers such as CD7 and CD57 during human chronic viral infections (1, 20). Importantly, while these cells appear to retain the capacity to synthesize IFN-γ, their lack of proliferation is associated with a defect in perforin expression (82). These results suggest that the impairment of proliferative potential may occur at an early stage during the hierarchical loss of effector functions. Given the importance of proliferative potential for protective immunity discussed above, the reduced ability of virus-specific CD8 T cells to proliferate to antigen during chronic infections may have important consequences during viral rebound or when the individual is exposed to related strains of the same virus. Indeed, superinfection of HIV-infected patients with new strains of HIV has been reported (3, 55), suggesting that the level of immunity present in some chronically infected individuals is not sufficient to protect from virulent challenge. It will be important to analyze the level of responsiveness of the virus-specific T-cell populations prior to superinfection in these cases to determine what factors may contribute to ineffective immunity.
One of the more complex and controversial issues regarding the development and maintenance of CD8 T-cell responses during chronic infections is the relationship between virus levels and CD8 T-cell functions. As highlighted in Fig. 2, a diverse range of functional phenotypes can arise during persisting infections. There are at least four major factors that need to be considered when evaluating how antigen levels impact CD8 T-cell function during a persistent infection: (i) the replication pattern of different viruses (e.g., latent versus persistently replicating), (ii) viral tropism, (iii) the stimulatory capacity of individual epitopes, and (iv) CD4 T-cell help.
(i) Viral replication patterns in vivo range from acute infections (where virus is eliminated), to latent infections that undergo periodic reactivation (e.g., herpes simplex virus [HSV], varicella-zoster virus [VZV], and Epstein-Barr virus [EBV]), to “smoldering” chronic infections such as cytomegalovirus (CMV), to chronic infections with high viremia (e.g., HCV, HBV, and HIV/AIDS). These types of infection are summarized in Table 1 with the possible effects on the development of functional CD8 T cells. Acute infections with viruses such as influenza virus and vaccinia virus do not result in viral persistence and induce functional memory T cells that develop and persist in the absence of antigen (30, 31, 49, 68, 123). The first set of persisting viruses listed in Table 1 are those that cause latent infections. After the initial infection, lack of antigen synthesis and/or inefficient antigen presentation during latency likely results in a minimal capacity for prolonged CD8 T-cell stimulation. From the standpoint of the T cell, the key feature of these infections is that there are periods of rest from antigen. In fact, repeated periods of rest from antigen and antigen reexposure upon reactivation from latency may provide effective T-cell boosts and play a role in maintaining robust and functional virus-specific T cells, as is often observed for EBV (21, 95). It will be interesting to investigate how this intermittent antigen exposure followed by rest impacts memory CD8 T-cell differentiation compared to that for a single exposure to antigen during an acute infection. Next, other persisting viruses may cause more of a smoldering infection, with pockets of ongoing viral replication. The periods of rest between antigenic stimulation are likely to be less frequent or of shorter duration and may depend on the location of viral replication (see below). CMV infection may represent an example of such a smoldering infection (105). CMV-specific CD8 T cells have been reported to fail to produce IL-2 and occasionally even TNF-α and IFN-γ upon TCR stimulation (39, 98), suggesting that the level of persisting antigen exposure during this infection may lead to a state of partial functional impairment. Finally, chronic infections with viremia are likely to lead to T-cell dysfunction (Table 1). The nearly complete loss of effector CD8 T-cell functions that has been described during chronic LCMV infection of mice (37, 129, 138) has also been reported in some HIV (40, 66, 102), HCV (46, 70) and tumor-bearing patients (71). This classification of viral infections into those that cause acute, latent, smoldering, or high-level viral replication may provide a useful framework in which to further explore the relationship between antigen levels and T-cell responses, in particular the level of T-cell rest from antigenic stimulation, during persistent infections.
TABLE 1.
Effects of different types of infection on the development of functional CD8 T cells
| Type of infection | Characteristics | CD8 T-cell differentiation | Referencesa |
|---|---|---|---|
| Acute | Virus cleared; T cells experience rest from antigen | Functional memory CD8 T cells with long-term T-cell persistence | 30, 31, 49, 68, 123 |
| Latent | Periodic reactivation; cycles of T-cell rest and restimulation | Functional CD8 T cells often at high frequency with long-term T-cell persistence | 21, 95 |
| Smoldering | Ongoing low-level viral replication; T-cell stimulation with infrequent rest | Some impaired effector functions depending on frequency of TCR stimulation | 39, 51, 98, 112 |
| Chronic with viremia | Persisting high-level viral replication; continuous TCR stimulation, no rest | Functional exhaustion and deletion | 7, 26, 27, 62, 70, 73, 80, 118 |
(ii) A second consideration regarding antigen levels and CD8 T-cell function during chronic infection is that, at similar viral loads, not all T-cell populations experience the same level of stimulation. For example, in the LCMV system some epitope-specific CD8 T-cell populations are driven to deletion while others become only partially exhausted during the same infection, and this appears to be a function of the level of individual epitopes presented in vivo (129). For example, the LCMV NP396 epitope induces deletion of Db/NP396-specific CD8 T cells and is present at higher levels in vivo than the GP33 epitope that induces functional exhaustion (129). A related issue is epitope mutation. Numerous reports have demonstrated that HIV, SIV, and HCV CD8 T-cell epitopes can be mutated in response to selective pressure by CD8 T-cell responses (34, 87, 137). Even mutations that reduce the affinity of MHC binding, TCR interaction, or the efficiency of generation rather than abolish epitope production may considerably reduce T-cell stimulation. Therefore, because of either their inherent potency or as a result of mutation, the stimulatory capacity of different epitopes presented during chronic infection will impact the properties of T cells responding to individual epitopes. As a result, during the same infection a range of functional properties is possible for T cells specific for different epitopes.
(iii) The level of viral replication during different types of infections is likely a major factor influencing CD8 T-cell function, but this factor alone is not always sufficient to explain the range of functional properties observed. Viral tropism (e.g., CD4 T cells, macrophages, hepatocytes), the anatomical location of viral replication (e.g., lymphoid tissue versus liver versus intestinal mucosa), and the amount of progeny virus produced per infected cell will all influence the apparent relationship between viral load and T-cell exhaustion. For example, if two viral infections each result in a virus level in serum of 106 viral RNA copies/ml but one virus replicates only in restricted cell types or locations while the other replicates systemically and in many cell types, the second is likely to result in substantially more T-cell stimulation. In such a simplified example, the impact on T-cell function would likely be very different in these two situations, despite the apparently similar antigen burden (viremia). In addition, the local microenvironment of viral replication and the type of cell infected may also impact T-cell responses. While HIV replicates in lymphoid tissue and in cells that can act as professional antigen-producing cells (APC) if appropriately activated, the toleragenic environment of the liver (29, 65) may have important consequences for T-cell responses to hepatatropic viruses such as HBV and HCV.
(iv) The importance of CD4 help for the maintenance of CD8 T-cell functions during chronic infections has long been appreciated (78). Many chronic infections are more severe in the absence of adequate CD4 T-cell help, and the quality of the CD8 T-cell response is often substantially worse. Loss of CD4 T cells results in a failure to control chronic LCMV infection and the complete functional inactivation of LCMV-specific CD8 T cells (13, 78, 138). Elimination of CD4 T cells also leads to the impaired long-term control of murine gammaherpesvirus infection (24). For humans, loss of CD4 T cells during HIV infection often precedes or is associated with CD8 T-cell dysfunction and AIDS progression (4, 33). Such CD4 deficiency also correlates with CD8 T-cell exhaustion during EBV-related non-Hodgkin's lymphoma (NHL) (121). Interestingly, in the LCMV system deficiency in costimulatory molecules (CD28−/−, CD40L−/−, and 41BBL−/−) can also result in more severe chronic infection and greater CD8 T-cell exhaustion (108, 110, 131, 132), which could reflect CD8 intrinsic defects but may also underscore the importance of optimal CD4 T-cell function during chronic infections. It will be important to determine whether this critical role of CD4 T cells reflects help mediated by the release of cytokines such as IL-2 (106) and/or the conditioning of APC (15, 96, 102) or CD4 T-cell antiviral effects, either directly or via help for antibody production.
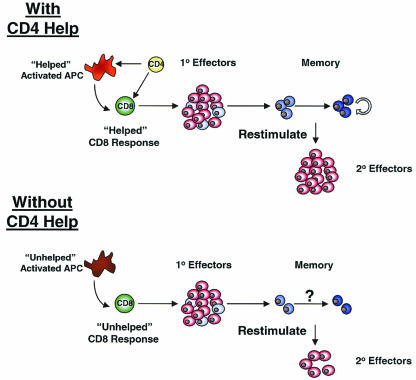
CD4 T cells also appear to play an important role in the optimal priming of CD8 T cells during acute infections (19, 54, 104, 107). The common finding of these recent reports was that the secondary expansion of memory CD8 T cells following restimulation was dramatically reduced if the CD8 T cells were originally primed in the absence of CD4 T cells (Fig. 3). These studies using acute infections of mice suggest that, as long as CD4 T cells were present during the initial priming, then CD4 help was dispensable during secondary challenge. However, recent studies with chimpanzees have demonstrated that optimal-recall CD8 T-cell responses following HCV infection may depend on the presence of CD4 T cells at the time of HCV challenge, even when the virus-specific CD8 T cells were originally primed in the presence of CD4 help (42). CD4 T-cell responses are likely important for optimal generation of memory CD8 T cells following acute infections and for sustained CD8 T-cell responses during chronic infections. However, the HCV experiments suggest that CD4 T-cell help may also be critical at the time of challenge with virulent infections. Together, these studies provide evidence that in the absence of signals from CD4 T cells the differentiation program of CD8 T cells may be altered. It will be important to determine the impact of CD4 deficiency on not only the generation of functional effector CD8 T cells but also on memory CD8 T-cell differentiation, including the transition from TEM→TCM (Fig. 3).
FIG. 3.
CD4 help for CD8 T-cell responses during acute viral infections. In the presence of adequate CD4 help during acute infection, efficient effector CD8 T-cell responses are generated and subsequently form memory CD8 T cells that can persist long term. Upon reinfection, these “helped” memory CD8 T cells undergo efficient recall responses generating a large pool of secondary effector T cells. In the absence of CD4 help during primary infection, CD8 T cells still generate a population of effector T cells, and these effectors can populate a memory T-cell pool. However, these “unhelped” CD8 T cells respond poorly to restimulation with antigen and generate a suboptimal population of secondary effectors following reinfection compared to that of helped CD8 T cells. It will be important to determine whether memory CD8 T-cell differentiation occurs normally in unhelped CD8 T cells. In addition, the importance of long-term maintenance of CD4 responses during chronic infections has long been appreciated, but precisely how CD4 T cells help ongoing CD8 T-cell responses during persisting infections is not well understood.
CHALLENGES FOR PREVENTIVE AND THERAPEUTIC VACCINES
Understanding CD8 T-cell differentiation following acute and chronic infection has important implications for vaccine design. First, the high potential of memory CD8 T cells (CD62LHiCCR7HiCD127Hi) for antigen-driven proliferation is associated with robust protective immunity (130). Thus, for many if not most vaccines, inducing memory T cells with high proliferative potential should be an important goal. In some circumstances, the presence of tissue-resident memory CD8 T cells (CD62LLoCCR7Lo) may mediate important local viral control at the site of infection, and the presence of both memory T-cell subsets may enhance the level of protection in these circumstances. However, the need for large numbers of secondary effector cells to control most virulent infections suggests that, since the proliferative capacity of the CD62LLoCCR7Lo TEM cells is lower than that of CD62LHiCCR7HiCD127Hi TCM, the presence of TCM will be important for effective protective immunity. In addition, the proliferative advantage of CD62LHiCCR7HiCD127Hi memory CD8 T cells indicates that when evaluating priming and boosting strategies perhaps one should wait until a sufficient number of memory cells have acquired this memory T-cell phenotype before giving the booster immunization. The optimal time interval between the first and second immunization is likely to vary depending on the strength of the primary vaccination (i.e., the duration of the memory T-cell differentiation). Since this differentiation process is slower following high-dose infection than following low-dose infection (130), we would predict that stronger vaccines will require a longer interval between the primer and booster vaccinations than weaker vaccines. A kinetic analysis of the rate of CD62LLoCCR7LoCD127Lo → CD62LHiCCR7HiCD127Hi conversion in the blood after vaccination may allow one to design optimal boosting regimens tailored for individual T-cell vaccines.
The ability to evaluate the differentiation state of virus-specific CD8 T cells may be particularly useful for designing therapeutic vaccination strategies. For example, the type of therapeutic vaccine administered may depend on the stage of exhaustion of the target T cells during chronic infection. It is unlikely that a vaccine designed to elicit a response to an epitope that induces deletion will be successful. Similarly, providing more antigen to CD8 T cells that are continuously stimulated in vivo and functionally impaired may, in fact, worsen the exhaustion. Indeed, with a recent notable exception (75), therapeutic vaccination has rarely provided benefit when the antigen load is high (32, 56, 61, 126), and most positive results have been achieved when viral replication is suppressed either by drug treatment or latency (52, 63, 93, 114). As with preventive vaccination, the proliferative potential of responding T cells will be an important factor determining the outcome of therapeutic vaccinations (116). Therefore, evaluating the functional status of T cells during chronic infection and understanding the nature of the defects in these partially or fully exhausted T cells should allow therapeutic approaches to be tailored to overcome these deficiencies. For example, proliferative potential may be enhanced by lowering viral load with drug therapy (i.e., providing rest from antigen) prior to therapeutic vaccination. Additionally, therapeutic approaches that provide signals that will restore function and facilitate T-cell survival may be used to overcome other T-cell defects during chronic infections. In this regard it will be important to evaluate the impact of prosurvival cytokines such as IL-2, IL-7, and IL-15 and the role of inhibitory and costimulatory signals in combination with therapeutic vaccination.
Chronic infections can result in ineffective T-cell responses, and therapeutic boosting of these responses holds promise to reduce disease or eradicate persisting infection. This will be a challenging goal, given the functional defects often observed within the target T-cell populations. However, future studies investigating the nature of the defects in these T cells and the signals that can be used to overcome inefficient effector functions and weak proliferative potential should provide opportunities to alter the course of human chronic viral infections.
Acknowledgments
We thank D. L. Barber, S. M. Kaech, and D. Masopust for helpful discussions.
This research was supported by National Institutes of Health grant AI30048 (to R.A.) and a Cancer Research Institute postdoctoral fellowship (to E.J.W.).
REFERENCES
- 1.Aandahl, E. M., J. K. Sandberg, K. P. Beckerman, K. Tasken, W. J. Moretto, and D. F. Nixon. 2003. CD7 is a differentiation marker that identifies multiple CD8 T cell effector subsets. J. Immunol. 170**:**2349-2355. [DOI] [PubMed] [Google Scholar]
- 2.Abendroth, A., and A. Arvin. 1999. Varicella-zoster virus immune evasion. Immunol. Rev. 168**:**143-156. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420**:**434-439. [DOI] [PubMed] [Google Scholar]
- 4.Altfeld, M., and E. S. Rosenberg. 2000. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12**:**375-380. [DOI] [PubMed] [Google Scholar]
- 5.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274**:**94-96. [DOI] [PubMed] [Google Scholar]
- 6.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8**:**379-385. [DOI] [PubMed] [Google Scholar]
- 7.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192**:**63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arstila, T. P., A. Casrouge, V. Baron, J. Even, J. Kanellopoulos, and P. Kourilsky. 1999. A direct estimate of the human alphabeta T cell receptor diversity. Science 286**:**958-961. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann, M. F., M. Barner, A. Viola, and M. Kopf. 1999. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29**:**291-299. [DOI] [PubMed] [Google Scholar]
- 10.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3**:**619-626. [DOI] [PubMed] [Google Scholar]
- 11.Barber, D. L., E. J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171**:**27-31. [DOI] [PubMed] [Google Scholar]
- 12.Baron, V., C. Bouneaud, A. Cumano, A. Lim, T. P. Arstila, P. Kourilsky, L. Ferradini, and C. Pannetier. 2003. The repertoires of circulating human CD8+ central and effector memory T cell subsets are largely distinct. Immunity 18**:**193-204. [DOI] [PubMed] [Google Scholar]
- 13.Battegay, M., D. Moskophidis, A. Rahemtulla, H. Hengartner, T. W. Mak, and R. M. Zinkernagel. 1994. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 68**:**4700-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker, T. C., E. J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195**:**154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett, S. R. M., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. A. P. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393**:**478-480. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163**:**3379-3387. [PubMed] [Google Scholar]
- 17.Blattman, J. N., R. Antia, D. J. D. Soudive, X. Wang, S. M. Kaech, K. Murali-Krishan, J. D. Altman, and R. Ahmed. 2002. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 195**:**657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borysiewicz, L. K., and J. G. Sissons. 1994. Cytotoxic T cells and human herpes virus infections. Curr. Top. Microbiol. Immunol. 189**:**123-150. [DOI] [PubMed] [Google Scholar]
- 19.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297**:**2060-2063. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101**:**2711-2720. [DOI] [PubMed] [Google Scholar]
- 20a.Byers, A. M., C. C. Kemball, J. M. Moser, and A. E. Lukacher. 2003. Cutting edge: rapid in vivo CTL activity by polyomavirus-specific effector and memory CD8+ T cells. J. Immunol. 171**:**17-21. [DOI] [PubMed] [Google Scholar]
- 21.Callan, M. F. 2003. The evolution of antigen-specific CD8+ T cell responses after natural primary infection of humans with Epstein-Barr virus. Viral Immunol. 16**:**3-16. [DOI] [PubMed] [Google Scholar]
- 22.Callan, M. F., L. Tan, N. Annels, G. S. Ogg, J. D. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187**:**1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell, J. J., K. E. Murphy, E. J. Kunkel, C. E. Brightling, D. Soler, Z. Shen, J. Boisvert, H. B. Greenberg, M. A. Vierra, S. B. Goodman, M. C. Genovese, A. J. Wardlaw, E. C. Butcher, and L. Wu. 2001. CCR7 expression and memory T cell diversity in humans. J. Immunol. 166**:**877-884. [DOI] [PubMed] [Google Scholar]
- 24.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184**:**863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerwenka, A., T. M. Morgan, and R. W. Dutton. 1999. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J. Immunol. 163**:**5535-5543. [PubMed] [Google Scholar]
- 26.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410**:**106-111. [DOI] [PubMed] [Google Scholar]
- 27.Chisari, F.V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13**:**29-60. [DOI] [PubMed] [Google Scholar]
- 28.Cho, B. K., C. Wang, S. Sugawa, H. N. Eisen, and J. Chen. 1999. Functional differences between memory and naive CD8 T cells. Proc. Natl. Acad. Sci USA 96**:**2976-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crispe, I. N. 2003. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3**:**51-62. [DOI] [PubMed] [Google Scholar]
- 30.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171**:**4969-4973. [DOI] [PubMed] [Google Scholar]
- 31.Demkowicz, W. E., Jr., R. A. Littaua, J. Wang, and F. A. Ennis. 1996. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 70**:**2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dikici, B., A. G. Kalayci, F. Ozgenc, M. Bosnak, M. Davutoglu, A. Ece, T. Ozkan, T. Ozeke, R. V. Yagci, and K. Haspolat. 2003. Therapeutic vaccination in the immunotolerant phase of children with chronic hepatitis B infection. Pediatr. Infect. Dis. J. 22**:**345-349. [DOI] [PubMed] [Google Scholar]
- 33.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76**:**2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15**:**883-895. [DOI] [PubMed] [Google Scholar]
- 35.Faint, J. M., N. E. Annels, S. J. Curnow, P. Shields, D. Pilling, A. D. Hislop, L. Wu, A. N. Akbar, C. D. Buckley, P. A. Moss, D. H. Adams, A. B. Rickinson, and M. Salmon. 2001. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. J. Immunol. 167**:**212-220. [DOI] [PubMed] [Google Scholar]
- 36.Favoreel, H. W., H. J. Nauwynck, and M. B. Pensaert. 2000. Immunological hiding of herpesvirus-infected cells. Arch. Virol. 145**:**1269-1290. [DOI] [PubMed] [Google Scholar]
- 37.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170**:**477-486. [DOI] [PubMed] [Google Scholar]
- 38.Gallimore, A., A. Glithero, A. Godkin, A. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187**:**1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillespie, G. M., M. R. Wills, V. Appay, C. O'Callaghan, M. Murphy, N. Smith, P. Sissons, S. Rowland-Jones, J. I. Bell, and P. A. Moss. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J. Virol. 74**:**8140-8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74**:**10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldrath, A. W., P. V. Sivakumar, M. Glaccum, M. K. Kennedy, M. J. Bevan, C. Benoist, D. Mathis, and E. A. Butz. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195**:**1515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302**:**659-662. [DOI] [PubMed] [Google Scholar]
- 43.Grayson, J. M., K. Murali-Krishna, J. D. Altman, and R. Ahmed. 2001. Gene expression in antigen-specific CD8+ T cells during viral infection. J. Immunol. 166**:**795-799. [DOI] [PubMed] [Google Scholar]
- 44.Grayson, J. M., A. J. Zajac, J. D. Altman, and R. Ahmed. 2000. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 164**:**3950-3954. [DOI] [PubMed] [Google Scholar]
- 45.Greten, T. F., J. E. Slansky, R. Kubota, S. S. Soldan, E. M. Jaffee, T. P. Leist, D. M. Pardoll, S. Jacobson, and J. P. Schneck. 1998. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc. Natl. Acad. Sci. USA 95**:**7568-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75**:**5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamann, A., K. Klugewitz, F. Austrup, and D. Jablonski-Westrich. 2000. Activation induces rapid and profound alterations in the trafficking of T cells. Eur. J. Immunol. 30**:**3207-3218. [DOI] [PubMed] [Google Scholar]
- 48.Hamann, D., P. Baars, M. Rep, B. Hooibrink, S. Kerkhof-Garde, M. Klein, and R. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 9**:**1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9**:**1131-1137. [DOI] [PubMed] [Google Scholar]
- 50.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96**:**5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He, X. S., K. Mahmood, H. T. Maecker, T. H. Holmes, G. W. Kemble, A. M. Arvin, and H. B. Greenberg. 2003. Analysis of the frequencies and of the memory T cell phenotypes of human CD8+ T cells specific for influenza A viruses. J. Infect. Dis. 187**:**1075-1084. [DOI] [PubMed] [Google Scholar]
- 52.Hel, Z., D. Venzon, M. Poudyal, W. P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. Shearer, J. D. Altman, D. Watkins, N. Bischofberger, A. Abimiku, P. Markham, J. Tartaglia, and G. Franchini. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 6**:**1140-1146. [DOI] [PubMed] [Google Scholar]
- 53.Islam, S. A., C. M. Hay, K. E. Hartman, S. He, A. K. Shea, A. K. Trocha, M. J. Dynan, N. Reshamwala, S. P. Buchbinder, N. O. Basgoz, and S. A. Kalams. 2001. Persistence of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte clones in a subject with rapid disease progression. J. Virol. 75**:**4907-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421**:**852-856. [DOI] [PubMed] [Google Scholar]
- 55.Jost, S., M. C. Bernard, L. Kaiser, S. Yerly, B. Hirschel, A. Samri, B. Autran, L. E. Goh, and L. Perrin. 2002. A patient with HIV-1 superinfection. N. Engl. J. Med. 347**:**731-736. [DOI] [PubMed] [Google Scholar]
- 56.Jung, M. C., N. Gruner, R. Zachoval, W. Schraut, T. Gerlach, H. Diepolder, C. A. Schirren, M. Page, J. Bailey, E. Birtles, E. Whitehead, J. Trojan, S. Zeuzem, and G. R. Pape. 2002. Immunological monitoring during therapeutic vaccination as a prerequisite for the design of new effective therapies: induction of a vaccine-specific CD4+ T-cell proliferative response in chronic hepatitis B carriers. Vaccine 20**:**3598-3612. [DOI] [PubMed] [Google Scholar]
- 57.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2**:**415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaech, S. M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111**:**837-851. [DOI] [PubMed] [Google Scholar]
- 59.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4**:**1191-1198. [DOI] [PubMed] [Google Scholar]
- 60.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2**:**251-262. [DOI] [PubMed] [Google Scholar]
- 61.Kakimi, K., M. Isogawa, J. Chung, A. Sette, and F. V. Chisari. 2002. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J. Virol. 76**:**8609-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188**:**2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keadle, T. L., K. A. Laycock, J. L. Morris, D. A. Leib, L. A. Morrison, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic vaccination with vhs− herpes simplex virus reduces the severity of recurrent herpetic stromal keratitis in mice. J. Gen. Virol. 83**:**2361-2365. [DOI] [PubMed] [Google Scholar]
- 64.Kieper, W. C., J. T. Tan, B. Bondi-Boyd, L. Gapin, J. Sprent, R. Ceredig, and C. D. Surh. 2002. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195**:**1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knolle, P. A., and G. Gerken. 2000. Local control of the immune response in the liver. Immunol. Rev. 174**:**21-34. [DOI] [PubMed] [Google Scholar]
- 66.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31**:**677-686. [DOI] [PubMed] [Google Scholar]
- 67.Kristensen, N. N., J. P. Christensen, and A. R. Thomsen. 2002. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J. Gen. Virol. 83**:**2123-2133. [DOI] [PubMed] [Google Scholar]
- 68.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186**:**859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lau, L. L., B. D. Jamieson, T. Somasundaram, and R. Ahmed. 1994. Cytotoxic T-cell memory without antigen. Nature 369**:**648-652. [DOI] [PubMed] [Google Scholar]
- 70.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191**:**1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee, P. P., C. Yee, P. A. Savage, L. Fong, D. Brockstedt, J. S. Weber, D. Johnson, S. Swetter, J. Thompson, P. D. Greenberg, M. Roederer, and M. M. Davis. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5**:**677-685. [DOI] [PubMed] [Google Scholar]
- 72.Lefrancois, L., and D. Masopust. 2002. T cell immunity in lymphoid and non-lymphoid tissues. Curr. Opin. Immunol. 14**:**503-508. [DOI] [PubMed] [Google Scholar]
- 73.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98**:**1667-1677. [DOI] [PubMed] [Google Scholar]
- 74.Liu, H., S. Andreansky, G. Diaz, T. Hogg, and P. C. Doherty. 2002. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J. Immunol. 168**:**3477-3483. [DOI] [PubMed] [Google Scholar]
- 75.Lu, W., X. Wu, Y. Lu, W. Guo, and J. M. Andrieu. 2003. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 9**:**27-32. [DOI] [PubMed] [Google Scholar]
- 76.Manjunath, N., P. Shankar, J. Wan, W. Weninger, M. A. Crowley, K. Hieshima, T. A. Springer, X. Fan, H. Shen, J. Lieberman, and U. H. von Andrian. 2001. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Investig. 108**:**871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291**:**2413-2417. [DOI] [PubMed] [Google Scholar]
- 78.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68**:**8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKay, P. F., J. E. Schmitz, D. H. Barouch, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, D. A. Gorgone, and N. L. Letvin. 2002. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 168**:**332-337. [DOI] [PubMed] [Google Scholar]
- 80.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410**:**980-987. [DOI] [PubMed] [Google Scholar]
- 81.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165**:**6833-6839. [DOI] [PubMed] [Google Scholar]
- 82.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3**:**1061-1068. [DOI] [PubMed] [Google Scholar]
- 83.Moniuszko, M., C. Brown, R. Pal, E. Tryniszewska, W. P. Tsai, V. M. Hirsch, and G. Franchini. 2003. High frequency of virus-specific CD8+ T cells in the central nervous system of macaques chronically infected with simian immunodeficiency virus SIVmac251. J. Virol. 77**:**12346-12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moser, J. M., J. D. Altman, and A. E. Lukacher. 2001. Antiviral CD8+ T cell responses in neonatal mice: susceptibility to polyomavirus-induced tumors is associated with lack of cytotoxic function by viral antigen-specific T cells. J. Exp. Med. 193**:**595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8**:**177-187. [DOI] [PubMed] [Google Scholar]
- 86.Murali-Krishna, K., L. L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286**:**1377-1381. [DOI] [PubMed] [Google Scholar]
- 87.O'Connor, D., T. Friedrich, A. Hughes, T. M. Allen, and D. Watkins. 2001. Understanding cytotoxic T-lymphocyte escape during simian immunodeficiency virus infection. Immunol. Rev. 183**:**115-126. [DOI] [PubMed] [Google Scholar]
- 88.Oehen, S., and K. Brduscha-Riem. 1998. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J. Immunol. 161**:**5338-5346. [PubMed] [Google Scholar]
- 89.Opferman, J. T., B. T. Ober, and P. G. Ashton-Rickardt. 1999. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283**:**1745-1748. [DOI] [PubMed] [Google Scholar]
- 90.Ostler, T., T. Hussell, C. D. Surh, P. Openshaw, and S. Ehl. 2001. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur. J. Immunol. 31**:**2574-2582. [DOI] [PubMed] [Google Scholar]
- 91.Ravkov, E. V., C. M. Myrick, and J. D. Altman. 2003. Immediate early effector functions of virus-specific CD8+CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J. Immunol. 170**:**2461-2468. [DOI] [PubMed] [Google Scholar]
- 92.Razvi, E., R. Welsh, and H. McFarland. 1995. In vivo state of antiviral CTL precursors. J. Immunol. 154**:**620-632. [PubMed] [Google Scholar]
- 93.Richards, C. M., R. Case, T. R. Hirst, T. J. Hill, and N. A. Williams. 2003. Protection against recurrent ocular herpes simplex virus type 1 disease after therapeutic vaccination of latently infected mice. J. Virol. 77**:**6692-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rickinson, A. B., S. P. Lee, and N. M. Steven. 1996. Cytotoxic T lymphocyte responses to Epstein-Barr virus. Curr. Opin. Immunol. 8**:**492-497. [DOI] [PubMed] [Google Scholar]
- 95.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15**:**405-431. [DOI] [PubMed] [Google Scholar]
- 96.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393**:**474-478. [DOI] [PubMed] [Google Scholar]
- 97.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401**:**708-712. [DOI] [PubMed] [Google Scholar]
- 98.Sandberg, J. K., N. M. Fast, and D. F. Nixon. 2001. Functional heterogeneity of cytokines and cytolytic effector molecules in human CD8+ T lymphocytes. J. Immunol. 167**:**181-187. [DOI] [PubMed] [Google Scholar]
- 99.Schlaak, J. F., G. Tully, H. F. Lohr, G. Gerken, and K. H. Meyer zum Buschenfelde. 1999. The presence of high amounts of HBV-DNA in serum is associated with suppressed costimulatory effects of interleukin 12 on HBV-induced immune response. J. Hepatol. 30**:**353-358. [DOI] [PubMed] [Google Scholar]
- 100.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1**:**426-432. [DOI] [PubMed] [Google Scholar]
- 101.Schluns, K. S., K. Williams, A. Ma, X. X. Zheng, and L. Lefrancois. 2002. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168**:**4827-4831. [DOI] [PubMed] [Google Scholar]
- 102.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393**:**480-483. [DOI] [PubMed] [Google Scholar]
- 103.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 96**:**3094-3101. [PubMed] [Google Scholar]
- 104.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300**:**337-339. [DOI] [PubMed] [Google Scholar]
- 105.Sissons, J. G., A. J. Carmichael, N. McKinney, J. H. Sinclair, and M. R. Wills. 2002. Human cytomegalovirus and immunopathology. Springer Semin. Immunopathol. 24**:**169-185. [DOI] [PubMed] [Google Scholar]
- 106.Su, H. C., L. P. Cousens, L. D. Fast, M. K. Slifka, R. D. Bungiro, R. Ahmed, and C. A. Biron. 1998. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. J. Immunol. 160**:**5007-5017. [PubMed] [Google Scholar]
- 107.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300**:**339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 167**:**5565-5573. [DOI] [PubMed] [Google Scholar]
- 109.Tan, J. T., B. Ernst, W. C. Kieper, E. LeRoy, J. Sprent, and C. D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195**:**1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan, J. T., J. K. Whitmire, K. Murali-Krishna, R. Ahmed, J. D. Altman, R. S. Mittler, A. Sette, T. C. Pearson, and C. P. Larsen. 2000. 4-1BB costimulation is required for protective anti-viral immunity after peptide vaccination. J. Immunol. 164**:**2320-2325. [DOI] [PubMed] [Google Scholar]
- 111.Tomiyama, H., T. Matsuda, and M. Takiguchi. 2002. Differentiation of human CD8+ T cells from a memory to memory/effector phenotype. J. Immunol. 168**:**5538-5550. [DOI] [PubMed] [Google Scholar]
- 112.Topp, M. S., S. R. Riddell, Y. Akatsuka, M. C. Jensen, J. N. Blattman, and P. D. Greenberg. 2003. Restoration of CD28 expression in CD28-CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J. Exp. Med. 198**:**947-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tripp, R. A., S. Hou, and P. C. Doherty. 1995. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J. Immunol. 154**:**5870-5875. [PubMed] [Google Scholar]
- 114.Tryniszewska, E., J. Nacsa, M. G. Lewis, P. Silvera, D. Montefiori, D. Venzon, Z. Hel, R. W. Parks, M. Moniuszko, J. Tartaglia, K. A. Smith, and G. Franchini. 2002. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J. Immunol. 169**:**5347-5357. [DOI] [PubMed] [Google Scholar]
- 115.Tussey, L., S. Speller, A. Gallimore, and R. Vessey. 2000. Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur. J. Immunol. 30**:**1823-1829. [DOI] [PubMed] [Google Scholar]
- 116.Tussey, L. G., U. S. Nair, M. Bachinsky, B. H. Edwards, J. Bakari, K. Grimm, J. Joyce, R. Vessey, R. Steigbigel, M. N. Robertson, J. W. Shiver, and P. A. Goepfert. 2003. Antigen burden is major determinant of human immunodeficiency virus-specific CD8+ T cell maturation state: potential implications for therapeutic immunization. J. Infect. Dis. 187**:**364-374. [DOI] [PubMed] [Google Scholar]
- 117.Unsoeld, H., S. Krautwald, D. Voehringer, U. Kunzendorf, and H. Pircher. 2002. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J. Immunol. 169**:**638-641. [DOI] [PubMed] [Google Scholar]
- 118.Urbani, S., C. Boni, G. Missale, G. Elia, C. Cavallo, M. Massari, G. Raimondo, and C. Ferrari. 2002. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 76**:**12423-12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Usherwood, E. J., R. J. Hogan, G. Crowther, S. L. Surman, T. L. Hogg, J. D. Altman, and D. L. Woodland. 1999. Functionally heterogeneous CD8+ T-cell memory is induced by Sendai virus infection of mice. J. Virol. 73**:**7278-7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Usherwood, E. J., K. A. Ward, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2001. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 75**:**8283-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Baarle, D., E. Hovenkamp, M. F. Callan, K. C. olthers, S. Kostense, L. C. Tan, H. G. Niesters, A. D. Osterhaus, A. J. McMichael, M. H. van Oers, and F. Miedema. 2001. Dysfunctional Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood 98**:**146-155. [DOI] [PubMed] [Google Scholar]
- 122.van der Most, R. G., K. Murali-Krishna, J. G. Lanier, E. J. Wherry, M. T. Puglielli, J. N. Blattman, A. Sette, and R. Ahmed. 2003. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology 315**:**93-102. [DOI] [PubMed] [Google Scholar]
- 123.Van Epps, H. L., M. Terajima, J. Mustonen, T. P. Arstila, E. A. Corey, A. Vaheri, and F. A. Ennis. 2002. Long-lived memory T lymphocyte responses after hantavirus infection. J. Exp. Med. 196**:**579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2**:**423-429. [DOI] [PubMed] [Google Scholar]
- 125.Vogel, T. U., T. M. Allen, J. D. Altman, and D. I. Watkins. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 75**:**2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.von Herrath, M. G., D. P. Berger, D. Homann, T. Tishon, A. Sette, and M. B. Oldstone. 2000. Vaccination to treat persistent viral infection. Virology 268**:**411-419. [DOI] [PubMed] [Google Scholar]
- 127.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169**:**3447-3458. [DOI] [PubMed] [Google Scholar]
- 128.Weninger, W., M. A. Crowley, N. Manjunath, and U. H. von Andrian. 2001. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194**:**953-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77**:**4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4**:**225-234. [DOI] [PubMed] [Google Scholar]
- 131.Whitmire, J. K., R. A. Flavell, I. S. Grewal, C. P. Larsen, T. C. Pearson, and R. Ahmed. 1999. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J. Immunol. 163**:**3194-3201. [PubMed] [Google Scholar]
- 132.Williams, M. A., T. M. Onami, A. B. Adams, M. M. Durham, T. C. Pearson, R. Ahmed, and C. P. Larsen. 2002. Cutting edge: persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J. Immunol. 169**:**5387-5391. [DOI] [PubMed] [Google Scholar]
- 133.Wills, M. R., G. Okecha, M. P. Weekes, M. K. Gandhi, P. J. Sissons, and A. J. Carmichael. 2002. Identification of naive or antigen-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J. Immunol. 168**:**5455-5464. [DOI] [PubMed] [Google Scholar]
- 134.Wong, P., and E. G. Pamer. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 166**:**5864-5868. [DOI] [PubMed] [Google Scholar]
- 135.Woodland, D. L., and R. W. Dutton. 2003. Heterogeneity of CD4+ and CD8+ T cells. Curr. Opin. Immunol. 15**:**336-342. [DOI] [PubMed] [Google Scholar]
- 136.Xiong, Y., M. A. Luscher, J. D. Altman, M. Hulsey, H. L. Robinson, M. Ostrowski, B. H. Barber, and K. S. MacDonald. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8+ T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75**:**3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu, X. N., G. R. Screaton, and A. J. McMichael. 2001. Virus infections: escape, resistance, and counterattack. Immunity 15**:**867-870. [DOI] [PubMed] [Google Scholar]
- 138.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188**:**2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou, S., R. Ou, L. Huang, and D. Moskophidis. 2002. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 76**:**829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]