The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease (original) (raw)
. Author manuscript; available in PMC: 2016 Oct 1.
Published in final edited form as: Trends Immunol. 2015 Oct;36(10):578–604. doi: 10.1016/j.it.2015.08.007
Abstract
The nervous system and the immune system are the principal sensory interfaces between the internal and external environment. They are responsible for recognizing, integrating, and responding to varied stimuli, and have the capacity to form memories of these encounters leading to learned or ‘adaptive’ future responses. Here, we review the current understanding of the cross-regulation between these systems. The autonomic and somatosensory nervous systems regulate both the development and deployment of immune cells, with broad functions that impact hematopoiesis as well as priming, migration and cytokine production. In turn, specific immune cell subsets contribute to homeostatic neural circuits such as those controlling metabolism, hypertension and the inflammatory reflex. We examine the contribution of the somatosensory system to autoimmune, autoinflammatory, allergic, and infectious processes in barrier tissues and in this context, discuss opportunities for therapeutic manipulation of neuro-immune interactions.
Keywords: Neuroscience, Immunology, Homeostasis, Barrier Tissues
Introduction
The idea of mutual influences between the manifestations of neuronal activity and the body’s other organ systems can be traced back to antiquity when the Roman poet Decimus Iunius Iuvenalis (1st to 2nd Century A.D.) famously coined the phrase “_mens sana in corpore sano_” (“sound mind in a sound body”). For centuries, this concept has stimulated countless explorations by philosophers, psychologists, clinicians and biologists who traditionally have been focusing their inquiries on interactions between neuroendocrine and/or higher cerebral functions with non-neuronal organs, including the innate and adaptive immune system. However, research during the past few years has generated mounting experimental evidence for an additional functional link that is based on interactions between the peripheral nervous system (PNS) and the immune system.
Early observations of commonalities between these two systems date back to the original discovery of some of their key components. Indeed, some canonical molecules and cell types that have become a focus of research for either neuroscientists or immunologists were actually discovered in tissues whose primary function was considered the domain of the other field. A prominent example is the description by Paul Langerhans in 1868 of an epidermal subset of dendritic cells (DCs), which he hypothesized to function as sensory neurons [1]. It took over a century for biologists to recognize that what we now call Langerhans cells are actually not of neuronal but of hematopoietic origin, and that they function as prototypical antigen-presenting cells (APCs) [2, 3].
Similarly, acetylcholine, a hallmark neurotransmitter of cholinergic neurons was originally isolated from a major lymphoid organ, the spleen [4]. While acetylcholine was soon understood to have a critical role as a neurotransmitter at neuromuscular junctions and in the parasympathetic nervous system, it’s source and role in splenic immunity took decades to decipher [5–8]. Recent work has identified a subset of memory T cells (TMem) as a key source of acetylcholine in the spleen while splenic innervation is predominantly adrenergic in nature [7–9].
So, does acetylcholine belong to the nervous system and are Langerhans cells and other DCs merely of relevance in immunology? All too often, the serendipitous history of discovery of a biological process dictates its perceived relevance to a given field [10, 11]. The emergence of field-specific vocabulary and the use of buzzwords as shorthand to describe complex biological processes often stymie the uninitiated and impede information exchange. Notwithstanding, classifications are often helpful and even necessary [12–14]. However, as our understanding of interactions between the nervous system and the immune system continues to deepen, the once clear-cut material and functional boundaries have faded.
In this review, we aim to integrate classical work and thinking in the field of neuro-immunology [15] with our contemporary cellular and molecular understanding of both systems [16, 17]. We start with an overview of the peripheral nervous system and provide a toolbox (Box 1) for how neuro-immune interactions can be studied. We then discuss how the autonomic and somatosensory PNS regulates the development, deployment and homeostasis of the immune system. Finally, we explore the role of the somatosensory system in autoimmune, autoinflammatory, allergic, and infectious processes, particularly in barrier tissues, and we will contemplate opportunities for therapeutic manipulation of neuro-immune interactions.
BOX 1. Experimental Strategies to Study Neuro-Immune Interactions.
The standard techniques to investigate neuro-immune interactions include several experimental strategies. The most reductionist approach relies on exposing leukocyte subsets in vitro to specific neurotransmitters or neurotransmitter receptor agonists and/or antagonists. This strategy provides high mechanistic resolution and allows exacting investigations of the underlying cellular biology, but it typically cannot identify a neuronal source, location or physiological role. A related approach employs receptor agonists or antagonists in vivo. This strategy can provide clues that a candidate pathway is active in a physiological setting, but it can be difficult to assess if the observed effects on the immune system are direct or indirect. One approach to address this question involves technically demanding co-cultures of isolated DRG neurons with purified leukocyte subsets [38]. A more widely used in vivo strategy makes use of selective elimination of specific neuronal activities. For example, pharmacological agents, such as capsaicin and resiniferatoxin (RTX) can ablate TRPV1+ heat-sensing neurons and 6-hydroxydopamine (6-OHDA) can transiently deplete catecholamine stores by interfering with neurotransmitter recycling [39, 40]. These agents enable pharmacological interrogation of specific neuronal subsets but are typically used on a systemic level. More recently, investigators have implemented genetic models of neural ablation based on the diphtheria toxin system to either constitutively or conditionally ablate neurons by targeted expression of diphtheria toxin or its receptor in neuronal populations that are defined by expression of lineage specific markers [27, 28, 30, 41]. These systems allow for the cleanest genetic interrogation of specific neuro-immune interactions but their interpretation can be challenging in light of the complex and dynamic expression patterns of ion channels during neuronal development.
In-depth molecular understanding of the PNS together with the recent advent of chemicogenetic and optogenetic approaches affords an unprecedented opportunity to genetically target neuronal populations of interest for functional studies [42, 43]. So far, these tools have been utilized mainly to map neural circuits in fine detail by activating or inhibiting specific populations of neurons in a genetically and regionally defined fashion. Their use should also be very informative to study neuro-immune interactions.
Overview of the Peripheral Nervous System
The PNS represents the part of the nervous system that is outside of the brain and spinal cord and provides a channel of communication between the central nervous system (CNS) and peripheral tissues. The PNS is broadly divided into the somatic system and the autonomic system, which interface with the external and internal environment, respectively (Figure 1). The somatic system enables us to sense and respond to the outside world, while the autonomic system functions to maintain homeostasis.
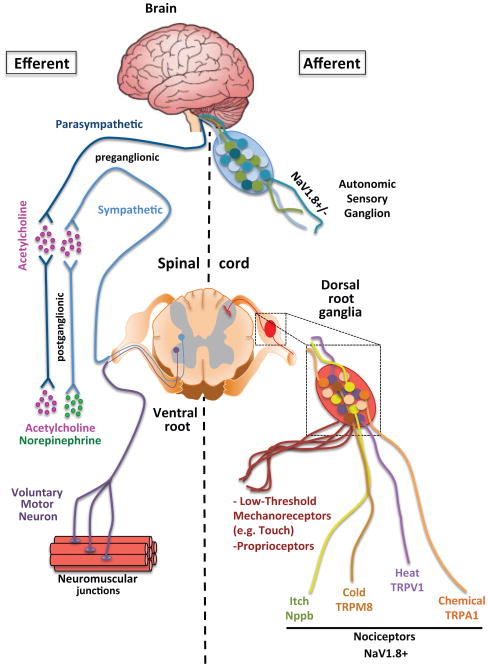
Figure 1. Efferent and Afferent Branches of the Autonomic and Somatosensory Nervous Systems.
The peripheral nervous system can be broadly divided into the autonomic and somatosensory nervous systems. It is important to note that both systems have efferent and afferent function, meaning that the autonomic nervous system has both motor and sensory neurons. While the motor neurons of the autonomic nervous system are fairly homogenous and can be subdivided into sympathetic and parasympathetic based on the post-ganglionic neurotransmitters, there is considerable sensory heterogeneity. This sensory heterogeneity is also exhibited by the somatosensory PNS which encodes specific subsets of neurons whose cell bodies reside in dorsal root ganglia (DRG) and are responsive to distinct physical, thermal, or chemical stimuli. The names of the major ion channels that are characteristic markers of discrete subpopulations of somatosensory neurons with shared function are shown.
There are two arms to each system with sensory/afferent neurons carrying information from the periphery to the CNS and motor/efferent neurons sending commands outwards to effector tissues. The afferent/sensory neurons in both the autonomic and somatic categories have bifurcated axons with cell bodies organized into discrete clusters termed sensory ganglia. Almost all sensory information from anatomic regions caudal to the neck passes through dorsal root ganglia (DRG) or vagal sensory ganglia. Somatosensory information from above the neck including the face and brain is transmitted through the trigeminal ganglia. DRG sensory neurons are considered part of the somatic system, with vagal sensory/afferent neurons being their counterpart in the autonomic system.
There are fundamental differences between the organizational principles of the efferent components of the two systems. The efferent branch of the autonomic nervous system is comprised of two anatomically, biochemically and functionally distinct subsystems: the sympathetic and parasympathetic nervous systems. The preganglionic neurons for both systems arise in the CNS, either from the thoracic and lumbar regions of the spinal cord for sympathetic neurons, or from several nuclei of the brainstem and the sacral region of the spinal cord for parasympathetic neurons. The two systems remain spatially segregated as preganglionic neurons synapse with postganglionic neurons in a ganglion that then projects to peripheral effector tissues. The specific effect induced by sympathetic and parasympathetic systems on a particular target organ depends on a multitude of factors, including the pattern and density of innervation, the type of neurotransmitter used and the type of receptor(s) on a target cell. In a nutshell, effects in different organs elicited by the sympathetic and parasympathetic systems are coordinated at a whole body level to support either a fight-or-flight response or a rest-and-digest function, respectively [18].
The sympathetic and parasympathetic systems are composed of relatively homogenous populations of pre- and post-ganglionic neurons (Figure 1). For instance, preganglionic neurons of both systems, postganglionic parasympathetic neurons and a subset of postganglionic sympathetic neurons, such as the ones innervating sweat glands, are cholinergic (acetylcholine-synthesizing), while the majority of postganglionic sympathetic neurons are adrenergic (norepinephrine-synthesizing). The sympathetic and parasympathetic innervation of peripheral tissues is therefore conveniently identified based on expression of enzymes critical for biosynthesis and vesicle loading of the corresponding neurotransmitter, e.g. tyrosine hydroxylase (TH), and dopamine beta-hydroxylase (DBH) for sympathetic neurons, and choline acetyltransferase (ChAT) for parasympathetic neurons [18]. Efferent neurons of the autonomic nervous system can also express neuropeptides, such as neuropeptide Y (NPY), yet their functional relevance is still unclear.
In contrast, the molecular characteristics of autonomic sensory and somatosensory neurons are extremely diverse [19, 20], and deciphering this heterogeneity is a topic of ongoing investigation [21, 22]. The conventional definition of sensory neurons is based on their function: nociceptors preferentially respond to noxious and pruritogenic stimuli as well as temperature; mechanoreceptors respond to innocuous tactile sensations and vibration; and proprioceptors detect joint and muscle position (Figure 1). Several systems have been proposed to classify sensory neurons based on their molecular traits. For instance, within nociceptors, peptidergic nociceptors are defined by expression of neuropeptides, such as calcitonin gene-related peptide (CGRP) and Substance P, while the majority of nonpeptidergic nociceptors express binding sites for the lectin IB4 [23, 24]). Alternatively, these same neurons can also be distinguished by their differential expression of hallmark receptors and ion channels. According to this nomenclature, the two nociceptor subsets defined by TRPV1 (transient receptor potential cation channel, subfamily V, member 1) and MrgprD (MAS-related GPR, member D) preferentially mediate thermal and mechanical nociception, respectively [25, 26].
NaV1.8 is a sodium channel expressed on the majority of sensory neurons in both the somatic and autonomic nervous systems [27, 28]. The NaV1.8+ population of somatic sensory neurons includes those involved in thermosensation (TRPV1: heat, TRPM8: cold), mechanosensation (Mrgprd), chemical nociception (TRPA1), and pruriception (Mrgpra3, Nppb) but excludes proprioceptors and most mechanoreceptors [20, 29]. Developmental deletion of TRPV1+ sensory afferents using the genetic expression of diphtheria toxin in TRPV1+ neurons has demonstrated considerable developmental overlap in subsets as these mice lose both heat and cold sensation [30]. However, adult ablation of either TRPV1 or TRPM8 neurons with diphtheria toxin injection yields mice in which either heat or cold sensation are specifically lost [31]. Furthermore, despite the fact that the neuronal subset expressing TRPV1 is required for themosensation, loss of the ion channel itself does not fully impair noxious heat sensation implying that other mechanisms can compensate [26, 32, 33]. While expression of some of these ion channels can be mutually exclusive, there is considerable overlap as in the case of TRPV1 and TRPA1 [16].
A recent study that employed a single-cell RNA-Seq approach and unbiased transcriptome clustering to identify eleven distinct subtypes of DRG neurons revealed even greater diversity [20]. Regardless of the classification scheme, different sensory modalities are generally mediated by different populations of sensory neurons each endowed with unique morphological and physiological properties [34, 35]. There are, however, notable exceptions where the sensory modality represented by a given neuronal population depends on that population’s interactions with other neurons [36, 37].
Neuro-Immune Regulation - a Historical Perspective
A dichotomy between sympathetic nerves dampening and somatosensory nerves promoting inflammation was proposed as early as 1909. It was noted that resection of the sympathetic innervation to a rabbit’s ear exacerbated experimental inflammation, while resection of sensory nerves prevented inflammation [44]. When challenged with Streptococcus pyogenes, rabbits lacking sensory innervation failed to control bacterial replication presumably due to a delayed and blunted inflammatory response [44]. While this work did not elucidate the cellular or molecular mechanisms, it laid the foundation for a rich literature investigating the neural regulation of inflammation [5, 45, 46].
Predating the discovery of the cellular and molecular underpinnings of adaptive immunity, the innervation of primary and secondary lymphoid organs had been well documented using histological techniques [45, 47]. In the 1980s, work by Felten and others using anterograde and retrograde labeling techniques mapped the circuitry of sympathetic and peptidergic innervation in primary, secondary, and mucosa-associated lymphoid tissues [6, 9, 48–50]. This work established potential conduits for the regulation of immune responses by the PNS.
Historical studies of neuro-immune interactions have also addressed adaptive immune responses in primary and secondary lymphoid organs. Functional experiments using chemical denervation demonstrated that norepinephrine from sympathetic nerve terminals negatively regulates antibody responses in the spleen [6]. Nils Jerne in his Nobel Prize acceptance speech explained how, “when we place a population of lymphocytes from [...] an animal in appropriate tissue culture fluid, and when we add an antigen, the lymphocytes will produce specific antibody molecules in the absence of any nerve cells.” [51, 52] However, in the original description of a splenic cell-culture system for studying antibody responses, the authors noted that the “in vitro response does not appear to be limited by whatever mechanisms regulate the in vivo response.”[52] One plausible explanation is that in vitro splenic cultures are deprived of neural inputs, primarily from sympathetic [6] but also from sensory fibers, which regulate immune responses in vivo [49, 53, 54]. Functional studies using chemical denervation described how norepinephrine released from sympathetic nerve terminals in spleen negatively regulated antibody responses [6]. Thus, this demonstrated neural regulation over the initiation of adaptive immunity [6], and the regulation of one complex system by another.
Role of the PNS in the Development, Deployment, and Homeostatic Regulation of the Immune System
The production, distribution, and activation state of leukocytes are all subject to variation and alterations during immune responses but typically remain within a definable range, akin to many homeostatic processes [55]. Recent studies have begun to characterize the specific subsets of cells in the peripheral nervous system that regulate hematopoiesis and the numbers of leukocytes present in circulation or in tissues (Figure 2). Peripheral neurons also play a role in determining the activation state and polarization of lymphocytes during immune responses. Furthermore, immune cells have been found to complete circuits that were previously thought to be the sole domain of the PNS. Another important mechanism, beyond the scope of this review, is the neuroendocrine modulation of inflammation and immunity through the action of the adrenal gland [56].
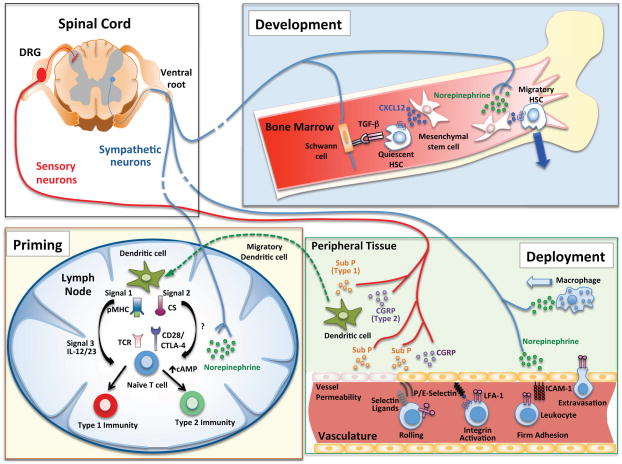
Figure 2. Neural signals that regulate hematopoiesis, priming and migration of immune cells.
Development (Bone Marrow): Hematopoiesis is regulated by the sympathetic nervous system. Sympathetic neural outflow leads to increased norepinephrine release which acts on nestin+ mesenchymal stem cells (MSCs) to reduce levels of CXCL12, which normally keeps HSCs resident in the niche. When CXCL12 levels drop, HSCs can egress from the niche. Furthermore, Schwann cells which surround autonomic neurons provide a source of TGF-β which keeps HSCs in a quiescent state. Priming (Lymph Node): Dendritic cell priming of T cells requires Signal 1 through the TCR, Signal 2 via co-stimulatory molecules, and Signal 3 which is provided via soluble cytokines. Sensory neurons, via Substance P and CGRP, can regulate the production of Signal 3 cytokines from DCs, which subsequently impacts T cell polarization and effector cytokine production. Furthermore, sympathetic neurons can produce norepinephrine, which negatively regulates Type 1 responses and promotes Type 2 responses by increasing levels of intracellular cAMP. Deployment (Peripheral Tissue): Peripheral neurons associated with vessels can impact leukocyte recruitment into tissues. Neuropeptides from sensory neurons can act on endothelial cells to increase vascular permeability (classically termed neurogenic inflammation) but also regulate translocation of P-selectin and expression of E-selectin, which are important in leukocyte rolling. Once leukocytes are rolling within vessels, they receive chemotactic signals that trigger integrin activation. ICAM-1, an important endothelial ligand for the integrin LFA-1, is regulated by norepinephrine from sympathetic neurons in some vascular beds. Finally, firmly adherent leukocytes extravasate into the surrounding tissue. Norepinephrine from sympathetic neurons is chemotactic for macrophages and sensory neuron-derived Substance P and CGRP can bias cytokine production by dendritic cells.
Hematopoiesis
During adult hematopoiesis leukocytes arise in the bone marrow (BM) from HSCs which reside in the stem cell niche, a unique microenvironment that regulates HSC quiescence and migration [57]. Three-dimensional image analysis of bone has revealed heterogeneous distribution of HSCs in distinct niches [58, 59]. HSCs maintain constant populations of lymphoid, myeloid, and erythroid cells as well as platelets through progressive differentiation into lineage committed progenitors. The magnitude and composition of the cellular output from the BM is responsive to environmental perturbations such as hemorrhage and infection which modulate HSC activity [60].
Early work characterizing the innervation of primary and secondary lymphoid organs identified sympathetic nerves as the predominant neural constituents in the BM [49, 50, 61]. These observations prompted investigations into how sympathetic tone affects the production and release of leukocytes from BM [61]. Indeed, the sympathetic nervous system has recently emerged as a key component and regulator of the stem cell niche [62].
Work by the Frenette group identified how norepinephrine released by sympathetic neurons triggers HSC mobilization in response to granulocyte colony-stimulating factor (G-CSF) administration [63]. This process depends on factors that regulate nerve myelination [63]. HSCs are physiologically retained in the BM by CXCL12, a chemokine that is produced by BM stroma cells and binds CXCR4 on HSCs. Experiments with β2 adrenergic agonists and with mice in which sympathetic neurons were deficient in catecholamine production demonstrated that norepinephrine release in BM is required to reduce CXCL12 production by BM stromal cells, which resulted in HSC mobilization into the blood [63] (Figure 2).
Catecholamines can also act directly on human and mouse HSCs and progenitor cells to enhance their motility, proliferation, and release from BM [64]. Transient peripheralization of HSCs is often induced clinically to harvest donor HSCs for BM transplantation (BMT). Moreover, recent work has demonstrated the importance of sympathetic neuronal regulation of CXCL12 dependent HSCs function in the context of psychosocial stress [65]. Thus, sympathetic nerves regulate not only acute drug-induced HSC trafficking, but apparently also long-term HSC homeostasis.
As circadian rhythms are well known to modulate the sympathetic nervous system[18], it has been proposed that normal diurnal fluctuations in sympathetic tone regulate HSC mobilization [66]. Indeed, HSCs are preferentially released from BM and peak in the circulation during periods of inactivity in mice (e.g. daytime). These circadian oscillations are entrained via central signals transmitted through norepinephrine secretion from sympathetic nerves resulting in rapid downregulation of CXCL12 [66]. One benefit of this physiologic HSC mobilization is to provide a source for “on-the-spot” hematopoiesis during infection as blood-borne HSCs are able not only to return to the BM, but also to access and survey peripheral tissues as well as secondary lymphoid organs [67].
Beyond mobilization, HSC cycling is also regulated by cellular niche components, including some of neural origin [68]. Recently, a relationship between nestin+ mesenchymal stem cells (MSCs) and HSCs was identified [69]. Nestin+ MSCs relay signals from sympathetic nerves to HSCs which regulate their maintenance in BM. Sympathetic nerves in the BM are ensheathed by non-myelinating Schwann cells which have been postulated to provide a source of TGF-β to maintain HSC quiescence [70] (Figure 2). Loss of sympathetic neurons, nestin+ MSCs or Schwann cells all lead to a decrease in HSCs in the BM due to enhanced mobilization and reduced quiescence [69, 70]. Regulation of hematopoiesis is not the sole domain of the sympathetic nervous system as genetic loss of NK-1R (tachykinin receptor, for substance P and hemokinin-1) from HSCs is important for lymphocyte generation after BMT [71].
While the HSC niche has been intensively studied, more committed progenitors of lymphoid and myeloid lineages inhabit distinct regions of the BM [72]. Recent experiments have uncovered that the contribution of HSCs to steady-state hematopoiesis is actually minimal [73]. Instead, the lineage-restricted progenitors appear to generate the bulk of lymphoid, myeloid, or erythroid cells [73]. The impact of neural inputs on this essential activity of committed hematopoietic progenitors remains to be determined. However, adrenergic signaling is known to modulate myelopoiesis. Chemical sympathectomy in mice was found to boost myeloid cell numbers after BMT, implying that the sympathetic nervous system might attenuate myelopoiesis [74]. However, it should be cautioned that extra-myeloid effects of systemic sympathectomy could also play a role. For example, the spleen functions as a major reservoir for inflammatory monocytes whose release requires angiotensin signaling through the AT-1 receptor and is controlled by noradrenergic neurons [75, 76]. Consequently, catecholamine depletion has been found to enhance monocyte recruitment to sites of bacterial infection [76], presumably due to exacerbated splenic release rather than altered myelopoiesis. Furthermore, there is also a pool of inflammatory monocytes in the BM that can be released upon TLR sensing by MSCs which induces MCP-1 (CCL2) expression and CCR2-dependent monocyte emigration [77]. How the convergence of microbial stimuli and neural derived signals on niche cells regulates myelopoiesis and monocyte release from BM is not currently understood.
Priming of Adaptive Immunity
The detection of distinct microbial patterns and cytokines by specialized APCs instructs both the magnitude and quality of a subsequent adaptive immune response [17]. We broadly distinguish between Type 1 immunity, which is primed in response to viruses, bacteria, and fungi, and Type 2 immunity, which is engaged by helminth infections, envenomation, and encounters with allergens. When a pathogen has breached an epithelial barrier, DCs are among the first APCs to encounter the microbe [17]. After pathogen detection and phagocytosis, DCs migrate to regional LNs via afferent lymphatics [78]. The two main determinants of the type of immune response that ensues are the DC subset(s) involved and the specific pattern recognition receptors (PRRs) in those DCs that are triggered by the pathogen [17, 79].
DC migration and motility is influenced by sympathetic and sensory neural input. Using confocal and two-photon microscopy, studies in skin, lung, and gut have found DCs located in close proximity to sensory neurons [80–84]. Immature DCs are responsive to both norepinephrine and neuropeptides [85, 86]. For example, CGRP can exert directional chemotactic activity on immature DCs in vitro, whereas norepinephrine appears to enhance the overall motility of DCs, perhaps aiding in their sampling and acquisition of antigen in tissues [85, 86].
In addition to PRR signaling, DCs and other myeloid leukocytes integrate signals from neuropeptide, which can influence cytokine profiles [87] (Figure 2). The pro-inflammatory activities of substance P were first mechanistically linked to enhanced production of IL-1, TNF-α, and IL-6 by monocytes [88]. The effect of CGRP on Langerhans cells was initially thought to be immunosuppressive, however, subsequent work by the same investigators demonstrated that CGRP-treated Langerhans cells preferentially stimulate Type 2 immunity [80, 89]. Consequently, deficiency in the receptor for CGRP enhances DC expression of CD80 and IL-12 and promotes delayed-type hypersensitivity (DTH), an inflammatory response linked to Type 1 immunity [90, 91]. Conversely, in vitro studies indicate that Substance P and signaling through the neurokinin-1 receptor on myeloid cells leads to enhanced Type 1 immunity by promoting the production of IL-12 and IL-23 (refs. [92, 93]). Interestingly, peptidergic sensory neurons typically co-express and can selectively secrete both CGRP and substance P, but the mechanisms that lead to the release of none, one, or both neuropeptides are currently not understood.
Some neuropeptides also act directly on T cells. The priming of antigen-specific naive T cells involves serial contacts with DCs in lymphoid tissues, whereby the T cells must integrate Signal 1 (through the TCR), Signal 2 (co-stimulatory molecules), and Signal 3 (cytokines). The strength and combinatorial activity of these inputs determines the magnitude and quality of the ensuing T cell response [17, 94, 95]. In particular, the strength and duration of TCR signaling dictates the receptiveness of T cells to the inflammatory milieu [96] and their ability to form long-lived memory [97]ment. These distinct signals converge on common signaling and metabolic pathways within T cells, which are regulated, in part, by neurotransmitters and neuropeptides [98] (Figure 2). Indeed, sympathetic neurons negatively regulate DC-dependent priming of CD8+ T cells during a primary viral infection [99]. Moreover, sympathetic activity and norepinephrine production negatively regulate Type 1 responses by reducing IL-12 production by DCs and by acting directly on T cells, which express the β2-adrenergic receptor [100, 101]. Catecholamine induced signaling through this receptor results in elevation of cyclic-AMP (cAMP) levels in T cells [54, 101]. By inhibiting mTOR (mammalian target of rapamycin), a key integrator of cellular metabolic state and effector T cell polarization, elevated cAMP levels favor the generation of Tregs and CD8+ memory T cells [98].
Most mechanistic experiments exploring neuropeptide effects on DCs and T cells have been performed in vitro, or by treating animals with neurotransmitter receptor agonists or antagonists. In vivo, the relevant neural subsets involved in immune regulation and the precise anatomic context in which this regulation occurs remain to be elucidated. It is also still unclear how combinatorial signals from sympathetic and sensory neurons are integrated at the level of individual APCs and T cells.
Immune Cell Trafficking
One of the defining features of most cellular constituents of the immune system is their migratory capacity [102]. This allows for the deployment of recently-primed T and B cells to the appropriate anatomical location to eliminate pathogen-bearing cells. Once priming has occurred in a LN or other lymphoid tissue, the activated lymphocytes exit into efferent lymphatics, which discharge migratory cells via the thoracic duct into the blood circulation. The egress from LNs is regulated by sphingosine-1 phosphate (S1P) signaling; T cells express a S1P receptor, S1P1, to sense gradients of extracellular S1P which is extremely low in the LN parenchyma, but high within lymph conduits [103]. For egress to occur, the S1P signal must overcome competing signals from CCR7 and CXCR4, whose ligands are highly expressed in the LN parenchyma and promote T cell retention. β2-adrenergic receptors on lymphocytes can modulate this process by enhancing the activity of CCR7 and CXCR4, thus antagonizing the egress signal and prolonging T cell sequestration [104].
Once activated T cells have become blood-borne, they need to travel to their target(s) in peripheral tissues where microvessels and peripheral neurons are intimately associated. Classical studies of neurogenic inflammation highlight the involvement of “vaso-dilators” (sensory fibers) and “vaso-constrictors” (sympathetic inputs) and their role in regulating tissue perfusion and vascular permeability [46]. However there is also a developmental link between these two cell types. Sensory nerves produce CXCL12 and VEGF-a, which are required for arteriogenesis and vascular branching, thus laying the groundwork for the vascular highways that lymphocytes use to patrol the body [105, 106]. Lymphocytes flow through arteries and arterioles and pass through capillaries before entering post-capillary venules, which are lined by specialized endothelial cells that support leukocyte adhesion and emigration [102]. Leukocyte interactions with the venular wall require the sequential engagement of adhesion and chemoattractant receptors, which allow the blood-borne cells to roll, arrest, and eventually emigrate into the extravascular compartment (Figure 2).
This multi-step adhesion cascade allows for tissue-specific “area-codes” that preferentially recruit tissue-tropic lymphocytes within the appropriate vascular bed. Lymphocytes can be imprinted to express specific tissue homing receptors during priming by DCs. These imprinting signals are often embodied by tissue-restricted environmental mediators, such as retinoic acid, a metabolite of dietary vitamin A that promotes lymphocyte homing to the small intestine, or 1,25 di-hydroxy vitamin D3, which promotes epidermal trafficking [107, 108]. It is tempting to speculate that tissue restricted neurotransmitters might also contribute to this imprinting of tissue-tropic lymphocytes, but this question has not yet been explored.
Beyond regulating blood flow and vascular permeability, adrenergic nerves have been identified as regulators of leukocyte recruitment to and within tissues. For example, neuropeptides can regulate P-selectin surface translocation from Weibel-Palade bodies and E-selectin synthesis on cutaneous venules, which directly impacts leukocyte trafficking [109]. Norepinephrine has chemotactic effects on migrating extravascular monocytes and macrophages [110]. Moreover, the sympathetic tone regulates leukocyte abundance and distribution, presumably to provide anticipatory immunity during periods of peak activity when individuals are at a greater risk for pathogen exposure [111–113]. Sympathetic nerves transmitting circadian cues from the CNS entrain circadian rhythmicity on tissues by connecting the central clock of the suprachiamsatic nucleus with the peripheral clock in each tissue [114]. This has functional effects on leukocyte numbers in the circulation and in tissues by regulating levels of endothelial adhesion molecules, such as ICAM-1 in skeletal muscle and P- and E-selectin in the BM [113] (Figure 2). This circadian regulation of leukocyte recruitment has functional consequences during inflammation, as mice are more susceptible to septic shock during periods of peak hepatic ICAM-1 expression when the number of neutrophils in liver parenchyma is elevated [113]. While the circadian regulation of physiological processes through sympathetic neural outflow has been well characterized, recent work also highlights that expression of the key clock gene BMAL1 in inflammatory monocytes intrinsically regulates diurnal rhythms [112]. It will be of interest to determine how extrinsic and intrinsic circadian factors converge to regulate the regional and systemic distribution of leukocytes.
Sharing of Homeostatic Circuits by the Nervous and Immune Systems
Homeostatic processes such as metabolic thermogenesis, the regulation of blood pressure, and intestinal motility involve interactions between sensory afferents, central neural regulators, the autonomic nervous system and parenchymal cells in target organs [55]. Recent studies have uncovered a key role for immune cells in these regulatory processes.
Temperature maintenance in endotherms depends on somatosensory and autonomic signals that control classical effector responses such as shivering and catecholamine induced sweating. Recent work has shown that macrophage derived catecholamines also contribute to thermoregulation [115]. In mammals, adaptive thermogenesis is driven by the metabolic activity of uncoupled mitochondrial respiration in brown adipose tissue (BAT) by altering levels of UCP1 [116]. Afferent peripheral sensation of warmth and cooling of the skin is transmitted via TRPV1+ and TRPM8+ sensory fibers, respectively, and converge on the hypothalamus [31, 116]. The balance of these signals determines the efferent sympathetic outflow to both white adipose tissue (WAT) and BAT. While efferent sympathetic nerves in BAT produce norepinephrine which acts on adipocyte adrenergic receptors, alternatively activated macrophages are required for thermogenesis in cold environments [115]. IL-4 signaling is essential for alternative macrophages activation, which drives the expression of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis [115]. Norepinephrine production by adipose tissue macrophages increases UCP1 levels and stimulates lipolysis in WAT.
In addition to BAT, thermogenesis can also be regulated by beiging of WAT, a process that is also regulated by immune cells [117, 118]. Eosinophil derived IL-4 and production of IL-5 and IL-13 by type 2 innate lymphoid cells (ILC2) drive the differentiation of pre-adipocytes to beige adipocytes as well as catecholamine production by macrophages [117–119]. Thus, eosinophils, ILC2s, and alternatively activated macrophages form an efferent loop linking neural thermosensation with thermogenesis [120]. The specific neural circuitry that stimulates cytokine production by eosinophils and ILC2 in adipose tissue remains to be determined, but studies in the intestine indicate that neurotransmitters can act directly on ILC2 to stimulate IL-5/IL-13 production promoting eosinophil production and activity [121]. Another aspect of bi-directional neuro-immune interactions is the resetting of the body’s internal core temperature to the febrile range and the downstream effects on host defense [122]. The details of this process have been reviewed recently [123].
The regulation of vascular tone represents another neuro-immune circuit [124]. Mice that lack the receptor for CGRP, a neuropeptide released by somatosensory neurons, are spontaneously hypertensive, suggesting that the sympathetic and sensory nervous systems may play opposing roles in regulating vascular tone although the source of CGRP that mediates vascular relaxation is unknown [125]. A role for T cells in hypertension was suggested by experiments in Rag-1−/− mice, which were resistant to experimentally induced chronic hypertension, while repletion of the T cell compartment rendered the animals susceptible [126]. A recent study identified a link between the nervous system and a splenic T cell reservoir during chronic hypertension induced by angiotensin-II infusion [127]. Angiotensin II increases norepinephrine release from splenic nerve fibers originating in the celiac ganglion. Norepinephrine then acts on marginal zone macrophages to produce placental growth factor (PIGF), which promotes T cell mobilization to sites of damage in the hypertensive state [127]. Consequently, sympathectomy inhibits PIGF release and subsequent T cell mobilization [127]. Intriguingly, angiotensin-II also regulates the release of splenic inflammatory monocytes that mediate atherosclerotic progression [75]. Thus, adrenergic neurons emanating from the celiac ganglion regulate at least two splenic immune cell subsets that contribute to hypertension and cardiovascular disease, processes that are often associated with the metabolic syndrome.
Many metabolic diseases initiate from an imbalance in caloric intake relative to expenditure and fundamentally impact lifespan. Host metabolism is monitored and modulated by both the nervous and immune systems [120, 128, 129]. TRPV1−/− mice have enhanced lifespan and improved metabolic parameters due to decreased CGRP release and enhanced pancreatic insulin production [130]. The relevant neuronal or non-neuronal cell type(s) that express TRPV1 and regulate(s) systemic metabolism and longevity are not well understood, however, at least in the NOD mouse, a model of juvenile autoimmune diabetes, a precise balance of TRPV1 signaling in sensory neurons is essential to maintain the health of insulin producing beta cells [131].
The enteric nervous system senses and responds to changes in the composition of intestinal content to regulate nutrient absorbtion. Its development and function is not only entrained by intrinsic genetic programs but also is sensitive to the intestinal inflammatory state and microbial communities, similar to the mucosal immune system [132, 133]. A recent study has shown how the presence of the microbiota is important for the development of enteric glial cells after weaning and for the continued repopulation of glial cells in intestinal villi [134]
Macrophages play an important role in completing an enteric nervous system circuit regulating colonic muscular contraction [135]. Bacterial products translocate across the epithelium and lamina propria into the myenteric plexus and profoundly impact peristalsis [135]. These microbial products acted directly on enteric neurons to stimulate macrophage colony stimulating factor (CSF1) secretion [135]. In mice treated with broad-spectrum antibiotics, levels of CSF1 were decreased resulting in a reduction in the number of muscularis macrophages producing BMP2, which feeds back onto enteric neurons. Through this feedback loop, the antibiotic-induced loss of macrophage derived BMP2 resulted in reduced neuronal stimulation of muscular contractility and intestinal peristalsis [135].
Neuro-Immune Circuits that Sense Inflammation
Peripheral inflammatory signals are important in regulating host defense responses orchestrated by the CNS. Pioneering work by the Besedovsky group in the 1980s has mapped how peripheral inflammation, resulting in enhanced levels of circulating IL-1β, is perceived in the hypothalamus [122, 136]. Several years later, Watkins and colleagues showed that the vagus nerve was required for the fever response to low doses of intraperitoneal IL-1β, suggesting that in settings of localized inflammation, vagal sensory afferents are important to detect and report on peripheral immune responses [137]. Conversely, efferent signal from the CNS are critical in regulating inflammatory responses, including sepsis [4]. The Tracey group observed that electrical stimulation of the vagus nerve suppresses splenic and hepatic cytokine responses to systemic LPS challenge via acetylcholine action on macrophages [138]. This work led to the concept of the inflammatory reflex whereby the vagus nerve is thought to constitute both an afferent sensory and efferent suppressive arc [4].
Of note, the spleen does not receive direct vagal innervation and instead is innervated primarily by noradrenergic efferents from the superior mesenteric celiac ganglion [48]. Norepinephrine released from these fibers stimulates a splenic memory T cell subset to produce acetylcholine that then acts on macrophages to suppress TNF-α production [7, 8]. The idea of the inflammatory reflex has served as an excellent model to continually revise and refine the specifics of the underlying neuro-immune circuitry [139, 140]. This has also prompted unexpected discoveries, such as a link between electroacupuncture in the sciatic nerve leading to vagal activation and adrenal synthesis of dopamine, a pathway that can dampen the NLRP3 inflammasome by enhancing cAMP levels in cells expressing the dopamine D1 receptor [141, 142]. Systemic dopamine release has also shown protective activity during polymicrobial and LPS-mediated sepsis [141, 142]. The recent characterization of specific subsets of vagal sensory afferents and the generation of optogenetic techniques to manipulate their activity will undoubtedly help to trace the specifics of the afferent and efferent arcs in this and other anti-inflammatory circuits [4, 19].
Role of the PNS in Barrier Tissues
Detection
Cells of the immune system have evolved to mount innate and adaptive responses to noxious challenge. In particular, their strategic positioning in barrier tissues is critical for the maintenance of tolerance of commensals and for the induction to protective inflammatory responses against invading pathogens. These protective defense mechanisms can become dysregulated in the context of autoimmune, autoinflammatory, or allergic inflammation. A growing body of literature suggests a key role of nociceptors in driving these pathological processes. Furthermore, a direct dialogue between microbes and neurons exists with profound implications for infectious disease progression.
When faced with disease, organisms can utilize three distinct and complementary mitigation strategies: avoidance, resistance, and tolerance [111, 143, 144]. The sensory nervous system can protect a host by avoiding environments that are potentially injurious or contain pathogenic microorganisms [111]. Work in C. elegans has described the importance of neural circuits in discriminating between pathogenic bacteria versus harmless species that can be utilized as a food source [145, 146]. Avoidance behavior in humans can result from both innate and learned behavioral cues mediated by tactile, olfactory, visual and gustatory inputs [111, 147]. Interestingly, it has been speculated that Type 2 host defense, particularly IgE-mediated mast-cell degranulation may be a way of storing “memories” to potentially injurious agents that would otherwise not trigger sensory neurons [148, 149]. However, in a modern environment, this form of anticipatory host defense can result in allergy or anaphylaxis triggered in response to innocuous or sub-threshold levels of harmless antigens [148].
In the following, we will discuss how the PNS interacts with the immune system in three major barrier tissues: the lung, gut and skin (Figure 3, Key Figure).
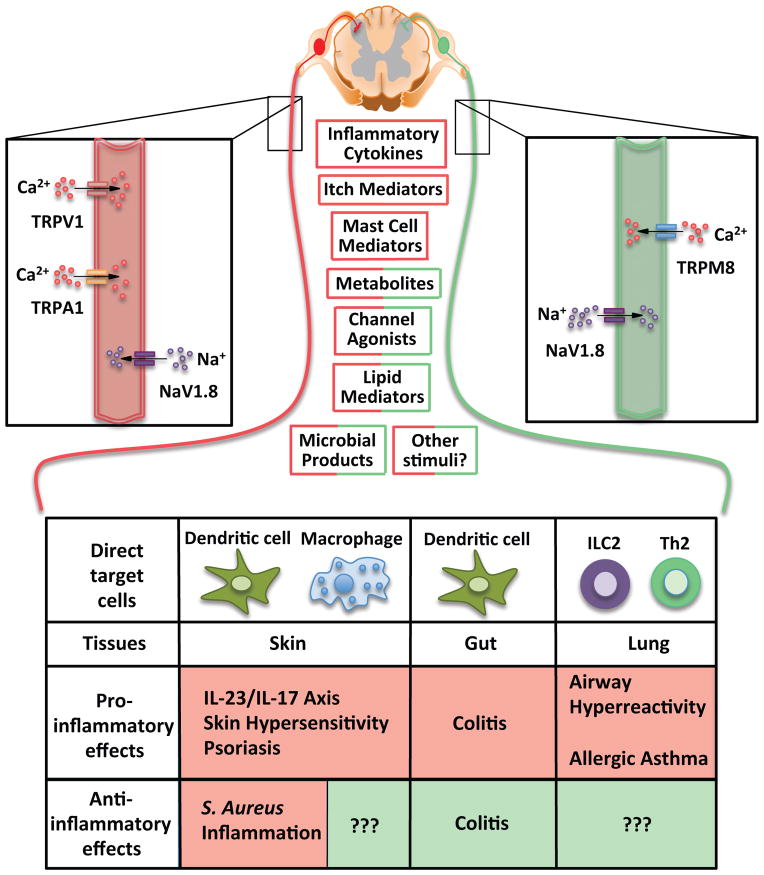
Figure 3(Key Figure). An integrative view of neural signals in regulation of immune responses at barrier tissues.
Certain commonalities have emerged in the neural regulation of immune responses at barrier tissues, such as the skin, gut and lung. Neuronal activation can occur via inflammatory cytokines, itch mediators, mast-cell derived products, metabolites, canonical ion channel ligands, lipid mediators, microbial products, and as-yet characterized stimuli. The available evidence suggests that primarily TRPV1+ TRPA1+ sensory neurons (depicted in red) promote Type 1 inflammation in the skin and gut, and Type 2 inflammation in the lung. In the skin, NaV1.8+ neurons, which encompass TRPV1+ TRPA1+ neurons, appear to have contextual anti-inflammatory properties during microbial infections, particularly with S. aureus. The mechanisms of neuronal activation and whether the regulation of immune cells occurs via single and/or combinatorial neuropeptides as well as other pathways remains to be firmly determined for most of these conditions. However, recent discoveries shed light on cytokines such as TSLP and IL-5 playing a key role in the skin and lung, respectively, in triggering sensory neurons. TRPM8+ sensory neurons have been identified as serving anti-inflammatory roles in the gut via CGRP release, however, the role of these neurons in skin and lung inflammation remains to be determined. Sensory neurons exert their effects via direct communication with several target immune cells of which only a few are highlighted here.
Lung
Both sensory and sympathetic nerves, by interacting with immune and parenchymal cells, profoundly influence lung pathology. Here, we will focus mainly on neural effects on asthmatic responses. For a comprehensive overview of neural regulation of airway and lung function we refer the reader to an excellent recent review on this subject [150].
In a series of imaging studies utilizing confocal-microscopy on fixed pulmonary tissue and two-photon microscopy on ex-vivo lung slices, Braun and co-workers noted that ~10% of pulmonary DCs are located in immediate proximity (within 0.3 μm) to CGRP+ sensory neurons [82, 151]. Activation of sensory neurons by electric field stimulation triggered increased motility of DCs by an unknown mechanism [152]. During acute allergen-induced asthma in ovalbumin (OVA) sensitized mice, DC motility slowed significantly, and DCs appeared in direct contact with proliferating T cells suggesting a potential neural regulation of T cell responses in asthmatic lungs through DCs [151, 152].
The cardinal features of asthma include the formation of a pulmonary inflammatory infiltrate and airway constriction. Combinations of pharmacological and genetic tools to inhibit or delete neuronal subsets have been used to address the PNS’ impact on this pathology. These strategies have implicated the vagus nerve, the TRPA1 but not TRPV1 ion channel, and TRPV1+ vagal sensory afferents [153–155]. A meta-analysis of the current literature suggests a pathway by which the triggering of antigen-specific IgE-bearing mast cells induces serotonin release which acts on 5-HT receptors on TRPA1+TRPV1+ vagal sensory afferents [153, 156] (Figure 3, Key Figure).
TRPA1−/− but not TRPV1−/− OVA sensitized mice showed a diminished inflammatory infiltrate after OVA challenge as well as decreased airway hyperreactivity [154]. This effect could not be explained by altered OVA-specific IgE levels, but was associated with reduced levels of Type 2 cytokines and chemokines in the absence of TRPA1. Specific developmental or post-developmental ablation of TRPV1+ neurons (using TRPV1-DTA mice or treating TRPV1-DTR mice with DTX, respectively) led to loss of both TRPV1 and TRPA1 vagal sensory neurons and ameliorated airway hyperreactivity [31, 155]. Surprisingly, this was not associated with a reduced inflammatory infiltrate but only with a reduction in airway hyperreactivity [155]. Conversely, a recent study from the Woolf lab elucidates a potential cascade of neuro-immune interactions with influences over both airway and inflammatory parameters. Either pharmacological silencing using a charged derivative of lidocaine or ablation of NaV1.8-lineage neurons (which targets TRPV1+ and TRPA1+ neurons amongst others) decreased eosinophilia and macrophage accumulation in broncheoalveolar lavage fluid [157]. The study also reported that IL-5, a cytokine produced by ILC2s, can act directly on TRPA1+ vagal sensory afferents triggering the release of VIP, which stimulates further IL-5 and IL-13 production from ILC2s and CD4+ Th2 cells [157]. Further work will be required to understand how complete absence of NaV1.8-lineage neurons leads to reduced inflammation and hyperreactivity while specific loss of TRPV1+ TRPA1-bearing vagal sensory afferents uncouples airway hyperreactivity from inflammation [154, 155, 157].
Gut
The gut is innervated by the pseudo-autonomous enteric nervous system that is partially tuned by the sympathetic nervous system, as well as separate vagal and spinal sensory inputs [158]. Neurons with sensory function exist in the enteric, autonomic, and DRG-derived intestinal nerves but their precise source is often unclear [159, 160]. The density of TRPV1+ CGRP+ nerve fibers increases along the cephalocaudal axis of the colon [161]. Enteric innervation can modify intestinal inflammation and has been implicated in the pathogenesis of the two major manifestations of inflammatory bowel disease (IBD), Crohn’s disease and ulcerative colitis. Early suggestions of this connection came from the finding that acute ablation of GFAP+ enteric glial cells in adult mice leads to jejuno-ileitis [162]. One major challenge for the field will be to determine which sensory branch is activated during IBD as this determines the efferent motor circuitry engaged and the type of neuropeptides released.
TRP channels and the neuronal subsets that express them can both promote and inhibit intestinal inflammation. Several studies have employed either capsaicin or RTX to ablate both vagal and spinal TRPV1+ sensory afferent neurons, but the effects of these compounds on the enteric nervous system remain ambiguous [26, 163]. In a model of TNBS-colitis, capsaicin-denervation promoted disease while sympathectomy was protective, suggesting that TRPV1+ vagal or spinal sensory afferents are net anti-inflammatory while adrenergic neural transmission exacerbates disease [164]. Accordingly, TRPV1−/− mice subjected to DNBS colitis had increased inflammation suggesting that TRPV1 function on sensory neurons was protective, at least in this model of IBD [165]. However, others observed no differences between wildtype and TRPV1−/− mice in TNBS-colitis [165]. Moreover, RTX mediated ablation of TRPV1+ sensory neurons ameliorated, rather than exacerbated, colitis in other settings, including in a T cell transfer model [166] as well as in TNBS- and DSS-colitis, which was attributed to loss of pro-inflammatory Substance P [167]. Clearly, the role of TRPV1 and the cells that express this ion channel in intestinal inflammation requires further investigation.
Both TRPA1 and the cold-sensing TRPM8 ion channel, which is expressed on a neuronal subset distinct from that expressing TRPV1+ [31], have been implicated in intestinal inflammation albeit with opposing effects. TRPA1−/− mice were protected from TNBS colitis as compared to WT controls [167]. TNBS and other haptens can bind and activate TRPA1 directly and stimulate the production of pro-inflammatory lipid mediators which can also be sensed through TRPA1 [167, 168]. Two recent studies have shown that TRPM8 inhibits colitis. In one study, a TRPM8 agonist ameliorated both TNBS- and DSS-colitis, but the disease course in TRPM8−/− mice was comparable to that in control mice [169]. A subsequent study by other investigators found that TRPM8-deficiency in nonhematopoieitic cells (presumably sensory neurons) worsened DSS-induced colitis [83]. In this model, TRPM8−/− afferent sensory neurons could not release anti-inflammatory CGRP, which allowed nearby CD11c+ DCs to produce more pro-inflammatory cytokines [83, 91].
In aggregate, the current literature on murine models of colitis indicates that TRPA1+ TRPV1+ vagal and/or spinal sensory neurons exert net pro-inflammatory effects in IBD by releasing Substance P, whereas CGRP released from TRPM8+ afferent spinal sensory neurons confers protection [170] (Figure 3, Key Figure). It should also be noted that even neurons that may not be actively driving the disease process are sensitive to the inflammatory state of the gut. Colitis often leads to gastrointestinal dysfunction due to death of enteric neurons. An elegant study has unraveled a mechanism for this neurotoxic effect in colitis, whereby elevated levels of extracellular ATP trigger direct neuronal cell death via the purinergic receptor P2X7 and pannexin-1 [171].
Mucosal IgA secretion is considered a major mechanism by which the immune system regulates intestinal microbial communities and keeps pathogens at bay [172]. Initial in vitro studies of the effects of gut-associated neuropeptides found that Substance P could increase IgA secretion from gut-associated lymphocytes while vasointestinal peptide (VIP) had variable effects depending on the location [173]. In an in vitro study of class-switching be activated B cells, VIP promoted switching to IgA in synergy with the CD40 pathway [174]. Intriguingly, IgA+ B cells are usually located in close proximity to peptidergic sensory neurons in gut lamina propria, suggesting that switching to and synthesis of IgA may be regulated by sensory neurons _in vivo_[175].
Skin
The skin is arguably the most extensively studied tissue with regards to neuro-immune interactions to date [176]. The skin is the body’s largest sensory interface with the environment and discriminates between a plethora of harmless and noxious stimuli using specialized somatosensory neurons [16, 177]. The skin-resident immune system also provides a diverse repertoire of cell subsets with dedicated roles in host defense [3, 178]. Autoinflammatory and allergic diseases of the skin, such as atopic dermatitis and psoriasis, are characterized by symptoms of discomfort, pain and itch which are transmitted via dedicated nociceptive or pruriceptive neurons, yet this is not the case in other cutaneous autoimmune diseases, such as vitiligo which is usually painless [31, 156, 179, 180].
Much of the literature predating the recent molecular genetic characterization of neuronal subsets in the skin, has focused on the role of neuropeptides in the modulation of classic DTH reactions by capsaicin denervation [176]. In a nutshell, these studies mirror observations in other barrier tissues in that Substance P is pro-inflammatory, whereas CGRP has anti-inflammatory properties [87]. We refer the interested reader to the original literature and comprehensive reviews on this topic [87, 176, 181–184].
Psoriasis is characterized by keratinocyte hyperplasia and a characteristic neutrophilic infiltrate which is driven by the IL-23/IL-17 axis [185, 186]. A role for neurons in this disease was first suggested by the finding that enhanced neuronal turnover occurs in pre-psoriatic lesions [187]. In this seminal study, Weddelland colleagues applied innovative histological techniques to study the innervation pattern in uninvolved and involved psoriatic skin, noting enhanced neural turnover in healthy skin before the development of a plaque and increased axonal entry into the epidermis accompanied by Schwann cells [187]. The authors speculated that the neurites and Schwann cells may promote lesion development. This idea was further substantiated by clinical reports indicating that interruption of innervation to affected psoriatic skin led to remission and that the return of sensation was accompanied by plaque progression [188–190]. In a genetic mouse model of chronic psoriasiform dermatitis accompanied by enhanced cutaneous innervation, Ward and colleagues showed that surgical denervation ameliorated epidermal hyperplasia and some inflammatory parameters [191]. In this model, Substance P promoted the accumulation of CD11c+ leukocytes, CGRP was important for epidermal thickening, and blockade of neuropeptide release by local injection of botulinum neurotoxin could ameliorate disease [191, 192].
These clinical and laboratory findings prompted further investigations into the mechanisms by which specific neuro-immune interactions regulate psoriasiform lesions. In a recent study of imiquimod-induced psoriasiform dermatitis, we explored the effect of both RTX-mediated ablation of TRPV1+ nociceptors and genetic deletion of NaV1.8+ sensory neurons (which also eliminates most TRPV1+ nociceptors) on the magnitude and quality of the inflammatory response. Indeed, TRPV1+ NaV1.8+ nociceptors regulate the production of the dermal DC derived instructive cytokine IL-23 as well as the downstream effector cytokines IL-17 and IL-22, which were secreted by a subset of dermal γδT cells, the γδT17 cells [81, 193, 194] (Figure 3, Key Figure). Confocal and intravital multi-photon microscopy showed that dermal DCs, the principal source of IL-23, were in close and dynamic contact with dermal NaV1.8+ nociceptors [81]. In the absence of TRPV1+ nociceptors, both dermal DC-derived IL-23 and γδT17 cell-derived cytokines were markedly reduced. This defect in imiquimod-induced inflammation in RTX treated mice could be rescued by direct injection of IL-23, indicating that RTX sensitive neurons are needed for IL-23 production, but not for the downstream inflammatory sequelae [81, 193, 194]). This study did not identify the specific communication signals between nociceptors and dermal DCs. Emergent technologies for unbiased single-cell interrogation in both the nervous and immune system may help to clarify the underlying mechanisms [20, 195].
Atopic dermatitis is a Type 2 inflammatory disease that results from a combination of epidermal barrier breakdown and the synthesis of thymic stromal lymphopoietin (TSLP), a key instructive cytokine in keratinocytes released due to barrier disruption[196]. The most bothersome symptom for patients is intractable itch [197]. Chronic atopic dermatitis is thought to result from a severely disrupted epidermal barrier which leads to sensitization to a variety of allergens and altered microbial communities as well as microbial translocation beneath the epidermis [198, 199].
Clinical studies have also documented an increase in innervation within atopic dermatitis lesions and amelioration of disease in areas of skin with diminished neural input [200, 201]. The TRPA1 channel, but not TRPV1, on cutaneous nociceptors is required for the development of both inflammation and itch in allergic contact dermatitis to oxazolone and urushiol (poison ivy) [168]. The combined action of TSLP on DCs, local effector T cells, ILC2 cells and prurireceptors contributes to disease progression and itch [196, 202–204]. TSLP stimulates IL-4 and IL-13 production by CD4+ Th2 cells and IL-5 and IL-13 expression in ILC2 cells [202–204]. The Bautista group identified a connection between TSLP and TRPA1 in atopic dermatitis by demonstrating the TSLP-receptor (TSLPR and IL7Ra) on a subset of sensory neurons [196]. TRPA1 was required to induce itch, while TRPV1 was dispensable, yet RTX pre-treatment ablated TSLP-evoked itch, suggesting that TRPV1+TRPA1+ sensory neurons conduct itch sensations in this system in a TRPA1 dependent fashion [196]. These sensory neurons are largely distinct from the traditionally described histamine and chloroquine responsive prurireceptors [37, 156].
While lymphocytes and mast cells are not required for acute TSLP-driven itch [196], the cellular mechanisms of chronic itch sensation may be more complex. A functional role for TRPA1 on mast cells and keratinocytes in chronic atopic dermatitis driven by elevated IL-13 has been proposed [205]. Furthermore, Th2 cell-derived IL-31 acts on IL-31RA on TRPA1+TRPV1+ sensory neurons which can produce a “memory” of itch independent of TSLP [206]. Unlike the proposed linear model in psoriasis, where TRPV1+NaV1.8+ nociceptors regulate DC expression of IL-23 with downstream effects on other immune cells [81], epithelial cell-derived TSLP is thought to act simultaneously on both immune cells and sensory neurons.
Another neuro-immune interface that has long been recognized in barrier tissues is that between peptidergic neurons and mast cells [207–209]. The presence of cutaneous sensory nerves is essential for mediating classic reactions such as IgE-driven passive-cutaneous anaphylaxis [210]. Mast cells release the small molecules histamine and serotonin which can act directly on TRPV1+ sensory neurons to induce itch as well as granule-associated proteases such as tryptase which can cleave proteins and generate small pruriogenic peptides that are sensed by protease-activated receptor 2 (PAR2) [156]. Itch can be mediated by many distinct agonists which may act on unique and/or overlapping subsets of sensory neurons [156]. Recent characterization of the three-dimensional localization of skin-resident leukocytes has highlighted that mast cells are actually closely apposed to ILC2 cells [211, 212]. It will be important to determine how this putative “mast cell-nerve functional unit” communicates with ILC2 cells [209, 212].
Neuro-Immune Interactions during Infection and Disease
Some bacterial and viral infections trigger intense pain in the host. In this section, we will discuss how neuropeptides can function in host defense, the mechanisms through which bacterial infections can cause or modulate pain, and how viral infections are counteracted by the intrinsic immune capacity of neurons. We will also discuss recent evidence suggesting that sensory and autonomic neuropathies can result in immunodeficiency.
Bacterial and Viral Infection
In the African clawed frog (Xenopus laevis), specialized neuroepithelial glands are present in the skin which, upon norepinephrine signaling, discharge a mixture of antimicrobial neuropeptides onto the animal’s skin [213]. Neuropeptides exhibit commonalities with canonical antimicrobial peptides with respect to their size, cationic charge, and amphipathic nature. Recent evidence suggests this defense strategy may not be restricted to amphibians, but utilized by mammals as well [213, 214]. In fact, chemosensory cells in the upper respiratory tract can release antimicrobial peptides in response to sensing of secreted bacterial products by bitter taste receptors [215].
What are the afferent neural signaling circuits that mediate neuropeptide release during an infection? Infection associated inflammatory pain has long been considered to be secondary to signals derived from immune cells acting on nociceptors [17, 46, 216]. However recent evidence suggests that infection associated pain can also be mediated by direct action of bacteria on nociceptors.
Adding to the observation that bacterial cell lysates can directly trigger calcium flux in DRG neurons [217], pioneering work in the Woolf laboratory extended this work on bacterial activation of nociceptors. The group demonstrated in vivo that pain in response to S. aureus infection is not secondary to inflammation mediated by pattern recognition (TLR2/MyD88), lymphocytes (T and B cells), or myeloid cells (neutrophils and monocytes), but rather triggered directly by bacterial N-formylated peptides and α-haemolysin, a pore-forming toxin [38]. Chiu and colleagues further showed that these bacterial products act directly on TRPV1+NaV1.8+ nociceptors. Interestingly, LPS can activate TRPA1+ sensory neurons in vitro in a TLR4-independent but TRPA1-dependent fashion, suggesting that nociceptor recognition of bacterial products may be a common event during infections [218].
Despite the generalized view of dermal nociceptors as having a pro-inflammatory role, subcutaneous _S. aureu_s challenge in mice that lacked NaV1.8+ nociceptors actually resulted in enhanced local tissue swelling and lymphadenopathy as compared to WT mice [38]. The proposed explanation for this paradoxical effect invoked a direct anti-inflammatory effect of NaV1.8+ nociceptor-derived CGRP to suppress macrophage production of TNFα. An alternative explanation could be that deletion of NaV1.8+ cells may not only eliminate cutaneous nociceptors, but also sensory neurons within the vagus nerve where this ion channel is broadly expressed [28]. It will be of interest to dissect the circuits modulating immune cell proliferation in response to S. aureus infection, if there are any shared components with the prototypical vagal anti-inflammatory reflex, and how the presence or absence of bacterial-sensing nociceptors affects bacterial clearance and viability at different stages of infection [4, 38].
It has long been assumed that pruritis in atopic dermatitis was primarily due to IgE mediated activation of mast cells resulting in histamine release. However, recent evidence suggests a second pruritogenic mechanism whereby certain bacteria trigger itch without requiring IgE. For example, delta-toxin derived from S. aureus, which is commonly found in eczematous lesions, can directly induce mast cell degranulation [198]. This intriguing capacity of S. aureus to activate nociceptors and mast cells, which both play a central role in atopic dermatitis, raises the possibility that direct activation of neural and immune system components by S. aureus precipitates and/or sustains at least some eczematous lesions.
Bacteria can also hijack elements of the nervous system to avoid detection and establish permanent residence by diminishing pain. Mycobacterium leprae and M. ulcerans both lead to alterations in pain sensation either by causing nerve demyelation through infecting Schwann cells or by secreted mycolactone which hyperpolarizes neurons [219, 220]. Furthermore, M. leprae actually reprograms Schwann cells to a mesenchymal stem cell-like state in order to promote their survival and dissemination [221]. The consequences of this mycobacterial neuromodulation for anti-bacterial immune responses are largely unexplored.
Many viruses exhibit exquisite neurotropism and utilize the peripheral nervous system to access the CNS and/or establish latent reservoirs in ganglia [222]. Herpes and rabies viruses are two classes that are particularly insidious in human populations. After subcutaneous inoculation of vesicular stomatitis virus (VSV), a relative of rabies virus that is usually transmitted by insect bites, VSV gains access to the CNS by infecting neurons that innervate the peripheral LNs draining the infection site [223]. Usually, subcapsular sinus macrophages, which function as a cellular “flypaper” in lymph nodes, trap lymph-borne VSV and rapidly produce type I IFN which acts on neighboring nerves to prevent viral spread [223]. This sentinel function of subcapsular sinus macrophages is not shared by the other major macrophage subset in the LN medulla because, unlike their medullary brethren, subcapsular sinus macrophages constantly interact with lymphotoxin expressing follicular B cells. The lymphotoxin signal is needed to induce and maintain the ability of subcapsular sinus macrophages to protect against neuroinvasion by VSV [224]. In the absence of this key macrophage subset, VSV gains entry into peripheral neurons and ascends to disseminate within the CNS causing lethal neuropathology.
Primary and Secondary Immunodeficiency
Except for some rare types of neurons, the nervous system lacks the regenerative capacity of other tissues. Most neurons would be irreplaceably lost if they were killed by a viral infection or by anti-viral immune cells, potentially resulting in permanent neurological defects [225]. Thus, neurons utilize a broad array of intrinsic innate defense mechanisms to combat viral infections while maintaining cell viability [226]. For example, during HSV-1 infection, DRG neurons resist cytotoxic killing, exhibit low sensitivity to the antiviral and pro-apoptotic effects of type I IFN and employ the autophagy pathway as a means to control viral replication and to resist cell death [227].
When HSV-1 reaches the CNS, it will establish a latent reservoir which may occasionally become reactivated to produce lesions known as cold sores. However, in a small fraction of infected HSV-1 carriers, infection can manifest as herpes simplex encephalitis (HSE)[226]. HSE is usually a consequence of a primary immune deficiency that has been genetically linked to defects in TLR3, UNC-93B, TRIF, TBK1, and STAT1, leading to the hypothesis that TLR3 production of IFN-α/β or IFN-λ is essential to restrict CNS replication of HSV-1 [226]. Indeed, Notarangelo and colleagues showed that functional TLR3 signaling in neurons and oligodendrocytes is essential to restrict HSV-1 infection in vitro while astrocytes and neural stem cells utilize other pathways for protection [226]. This study highlights how a canonical PRR of the immune system, TLR3, mediates intrinsic immunity in the CNS. By contrast, less is known about how peripheral sensory neurons respond to HSV-1 infection, but recent progress in differentiation protocols to generate nociceptors in vitro may enable such investigations in the near-term [228]. Such studies will need to take into account that a subset of resident T memory cells in skin and sensory ganglia provides a critical local layer of control to prevent viral reactivation in chronically HSV-1 infected individuals [229].
Stroke-induced neurological defects can manifest as transient systemic immunodeficiency predisposing individuals to bacterial infections. The Kubes group reported that norepinephrine signaling to hepatic iNKT cells plays an important role for altered bacterial susceptibility after a stroke [230]. This effect depended on the induced production of immunosuppressive IL-10 and a systemic shift away from a protective Type 1 response [230].
Congenital defects in neural development have been linked to immunodeficiency. For example, patients with mutations in SCN9A (NaV1.7) are insensitive to pain due to impaired generation of action potentials in nociceptors [231], and mutations in NGFB or TRKA (the NGF receptor) lead to hereditary sensory and autonomic neuropathies (HSAN 4 or 5) [232]. A patient with a mutation in TRKA presented with recurrent infections secondary to hypogammaglobulinemia [233]. This may have been due, at least in part, to the role of NGF as a survival factor for memory B cells [234]. However, an elegant study by Floto and colleagues recently identified another key role for NGF-B in human immunity to S. aureus infection [232]. NGF-B has structural homology with drosophila Spaetzle, a Toll ligand, and patients with mutations in NGF-B and TRKA suffer frequent severe S. aureus infections. NGF-B was found to be released from macrophages in response to S. aureus infection in an NLR-dependent fashion, which mediated autocrine effects such as enhanced phagocytosis and neutrophil recruitment [232]. As the spectrum of genetically described primary immunodeficiencies rapidly expands beyond defects in immune cell subsets, it will be important to explore how alterations in neural development or function influence immunity.
Other Diseases
Other relevant disease areas in which neuro-immune interactions have been implicated include rheumatoid arthritis (RA) and cancer. As we cannot expound on these topics in depth within the scope of this article, we provide below only a brief overview highlighting a few examples and otherwise refer the reader to recent reviews [62, 235].
In RA, both sensory afferents and sympathetic efferents play complex, time-sensitive roles in the regulation of arthritis by interacting with endothelial, immune, and parenchymal cells in affected joints. Sympathetic and TRPV1+ sensory neurons exert pro-inflammatory activity during disease initiation, while established RA results in a loss of immunomodulatory sympathetic fibers and outgrowth of sensory innervation, which may contribute to disease chronicity [236–242].
The outgrowth or regression of autonomic neurons can also influence the oncogenic process [62]. In the case of prostate cancer, a solid tumor, autonomic nerves were found to influence both tumor development and metastasis with stromal β2 and β3 adrenergic receptors promoting tumor development and cholinergic signaling via type 1 muscarinic receptors leading to metastasis [243]. Conversely, sympathetic neuropathy results in the progression of myeloproliferative malignancy due to the importance of sympathetic neurons in maintaining nestin+ MSCs and Schwann cells to form a stable niche for HSCs [66, 69, 244, 245]. Current standard of care for leukemia includes the use of neurotoxic chemotherapeutics such as cisplatin and vincristine which reduce the ability of BM transplants to engraft due to a loss of sympathetic neurons and niche constituents [246]. The inclusion of neuroprotective agents during chemotherapy could improve the clinical recovery from BMT by preserving the stem cell niche [246].
Therapeutic Targeting of Neuro-Immune Interactions
Research in recent years has generated an increasing body of data that suggest that the immune and nervous systems, assumed to work in a independent fashion for decades, collaborate to maintain homeostasis and to protect the host against infection and diseases. However, as this partnership can also lead to exacerbated inflammation and collateral tissue damage, therapeutic approaches to inhibit, modulate or otherwise harness the neuro-immune axis should be considered. One striking recent example is the finding that implantation of an electric vagal nerve stimulator decreased inflammation and pain in two thirds of RA patients who were resistant to methotrexate (Clinical trial # NCT01552941).
More conventional drug discovery efforts, beyond “electroceuticals”, have been inspired by observations such as outgrowth of sensory nerves and increased neuropeptide levels in a number of pathologic inflammatory diseases [247–249] For example, neurokinin receptor (NKr) antagonists have been tested clinically based on encouraging results in multiple animal models. These compounds have demonstrated efficacy in the treatment of emesis and depression, but their use in other areas, particularly in respiratory diseases has been mostly disappointing. Several compounds that target multiple NKrs showed some efficacy in reducing bronchial hyperresponsiveness in asthmatics, but this effect was only transient [250, 251].
Despite the discrepancy in efficacy of many drugs in clinical settings versus animal models, there is abundant evidence from both realms to support the idea that sensory nerve fibers contribute to chronic inflammation. A complicating factor is that human patients are heterogeneous and show a broad range of responses to anti-inflammatory drugs that could not be anticipated based on experiments carried out in inbred mice. Efforts by academic, governmental and pharmaceutical entities are ongoing to identify genetic traits that correlate with defined inflammatory phenotypes, which would allow clinical investigators to stratify patient cohorts. These activities are still at an early stage, but they offer the opportunity to correlate parameters such as genetic polymorphisms in hallmark sensory neuropeptides or other neuronal markers with biochemical and functional (e.g. nerve conduction) measurements to establish correlations with specific inflammatory biomarkers and inflammatory disease symptoms, and outcomes.
Concluding Remarks
Most readers, like the authors, are undoubtedly familiar with “feeling sick”, an all-too-common experience in our daily lives. Few individuals, immunologists and neuroscientists included, may realize that this sensation usually reflects an activation of the sensory nervous system by localized or systemic immune processes. Regardless of the impact of such internal immunological processes on our conscious experience, it is apparent that a continuous concerted monitoring of the immune system’s activities and responding by the somatosensory and autonomic nervous systems is important to maintain health and that a balance of afferent and efferent neuronal activity must be maintained to avoid pathology.
In this article, we have discussed some of the mounting evidence describing how sensory perception and both local and global responses by the PNS exert context-dependent effects on diverse immunological modalities. Much remains to be learned (Outstanding Questions Box). For example, the nervous and immune systems both share the unique capacity to form memories of earlier encounters leading to learned or ‘adaptive’ responses that may differ in both quality and magnitude from a primary response. It will be important to understand how these different biological experiences are integrated to generate “neuro-immune memory” and how these processes might be manipulated for therapeutic or prophylactic intervention.
Outstanding Questions Box.
- What key molecularly defined neuronal subsets are activated in distinct inflammatory settings? Utilizing genetically encoded sensor of neuronal activity driven in specific neuronal subsets will allow investigators to determine which populations are activated in vivo.
- What are the inputs that are sensed during inflammatory conditions? Understanding the repertoire of ligands which are sensed will be informative in determining the specific or generalized roles that each neuronal subset can play.
- What are the combinatorial outputs produced in vivo by each subset? The spatiotemporal release of neuropeptides is poorly mimicked by in vitro culture of leukocytes with neuropeptides so the ability to manipulate neural activity in vivo should allow investigators to determine the place and location where neural activity can affect immune cell behavior. Some modes of communication may be mediated through non-synaptic signaling.
- Is there a modular logic to neuroimmune interactions? Given the different types of inflammation (e.g. Type 1 and Type 2) and the distinct modalities of peripheral nervous sensation, do signals detected by neurons guide subsequent neuronal outputs that instruct different arms of immune effector responses?
- What is the translatability of findings in mouse models, often dealing with acute disease settings, to human chronic conditions? Fine-tuning of neural activity may affect the development of disease, but determining whether and how electroceuticals and pharmaceuticals targeting neurons impact inflammation in humans will be of utmost importance.
The recent description of cell subsets in both the nervous and immune systems combined with tools for specific ablation and modulation of function should soon allow investigators to address these issues and to map fine circuits in much greater detail. For example, a rigorous dissection of complex homeostatic circuits, such as thermoregulation, which is thought to involve sensory afferents, centrally integrating neurons, and sympathetic outflow to multiple effector tissues with target cells including ILC2s, macrophages, and adipocytes, may not only provide basic biological insight but could also yield novel treatment opportunities for metabolic diseases. Many other questions remain to be answered as well, such as the seemingly opposite effects of cutaneous nociceptors in psoriasiform dermatitis and during infection with S. aureus. Exploring the signals that trigger nociceptor activity during these processes may aid in solving this paradox. To date, we have only just scratched the surface.
TRENDS Box.
- Homeostasis: Macrophages have been identified as key components of neurally-mediated circuits in various tissues including the gut, where they contribute to motility, and in adipose tissue, where they aid in thermogenesis.
- Barrier Tissues: Dendritic cells are found in close proximity to sensory neurons in the skin, gut and lung and evidence suggests they integrate signals from distinct neuronal subsets with either pro- or anti-inflammatory outcomes.
- Infection: Pain during some cutaneous bacterial infections can be a direct result of bacterial products acting on sensory neurons rather than secondary to inflammatory processes.
- Subsets: An enhanced molecular understanding of neural and immune cell ontogeny and function has led to the generation of tools for ablation or functional modulation of specific cell subsets.
Acknowledgments
The authors wish to thank Ms. E. Nigro for secretarial support and Dr. G. Cheng and L. Jones for technical assistance. This work was supported by funds from the Ragon Institute of MGH, MIT and Harvard and by NIH grants AR068383, AI112521, AI069259, AI111595 and AI095261 (to U. H. v. A.) and AR063546 (to J.O.-M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jolles S. Paul Langerhans. J Clin Pathol. 2002;55:243. doi: 10.1136/jcp.55.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 4.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JM, Peterson RG, Shea PA, Schmedtje JF, Bauer DC, Felten DL. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res Bull. 1981;6:83–94. doi: 10.1016/s0361-9230(81)80072-x. [DOI] [PubMed] [Google Scholar]
- 7.Pena G, Cai B, Ramos L, Vida G, Deitch EA, Ulloa L. Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis. J Immunol. 2011;187:718–725. doi: 10.4049/jimmunol.1100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon A, Tusche MW, Pavlov VA, Andersson U, Chevan S, et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. 118–121. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 10.Maestroni G. Immunology needs the mind. Nat Immunol. 2004;5:763. doi: 10.1038/ni0804-763. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein AM. Cultures and identities. Nat Immunol. 2004;5:1005. doi: 10.1038/ni1004-1005. [DOI] [PubMed] [Google Scholar]
- 12.Serafeim A, Gordon J. The immune system gets nervous. Curr Opin Pharmacol. 2001;1:398–403. doi: 10.1016/s1471-4892(01)00069-8. [DOI] [PubMed] [Google Scholar]
- 13.Steinman L. Connections between the immune system and the nervous system. Proc Natl Acad Sci U S A. 1993;90:7912–7914. doi: 10.1073/pnas.90.17.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepherd AJ, Downing JE, Miyan JA. Without nerves, immunology remains incomplete -in vivo veritas. Immunology. 2005;116:145–163. doi: 10.1111/j.1365-2567.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ader R, Cohen N, Felten DL. Brain, behavior, and immunity. Brain Behav Immun. 1987;1:1–6. doi: 10.1016/0889-1591(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 16.Le Pichon CE, Chesler AT. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat. 2014;8:21. doi: 10.3389/fnana.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCorry LK. Physiology of the autonomic nervous system. Am J Pharm Educ. 2007;71:78. doi: 10.5688/aj710478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 21.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 22.Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012;35:373–381. doi: 10.1016/j.tins.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195–205. doi: 10.1016/0306-4522(88)90018-8. [DOI] [PubMed] [Google Scholar]
- 24.Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- 25.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli mrgprd. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 28.Gautron L, Sakata I, Udit S, Zigman JM, Wood JN, Elmquist JK. Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J Comp Neurol. 2011;519:3085–3101. doi: 10.1002/cne.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, Lou S, Bryman GS, Roberson DP, Ghasemlou N, et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife. 2014;3 doi: 10.7554/eLife.04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. Embo J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caterina MJ. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 33.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109:5–12. doi: 10.1152/jn.00457.2012. [DOI] [PubMed] [Google Scholar]
- 36.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159:1417–1432. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci. 2013;16:910–918. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. The Journal of Clinical Investigation. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacological Reviews. 1974;26:199–288. [PubMed] [Google Scholar]
- 41.Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012;153:2017–2030. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 42.Farrell MS, Roth BL. Pharmacosynthetics: Reimagining the pharmacogenetic approach. Brain Res. 2013;1511:6–20. doi: 10.1016/j.brainres.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapman LF, Goodell H. The Participation of the Nervous System in the Inflammatory Reaction. Ann N Y Acad Sci. 1964;116:990–1017. doi: 10.1111/j.1749-6632.1964.tb52564.x. [DOI] [PubMed] [Google Scholar]
- 45.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacological Reviews. 2000;52:595–638. [PubMed] [Google Scholar]
- 46.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Von Euler US, Hillarp NA. Evidence for the presence of noradrenaline in submicroscopic structures of adrenergic axons. Nature. 1956;177:44–45. doi: 10.1038/177044b0. [DOI] [PubMed] [Google Scholar]
- 48.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989;3:291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 49.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 50.Weihe E, Nohr D, Michel S, Muller S, Zentel HJ, Fink T, Krekel J. Molecular anatomy of the neuro-immune connection. Int J Neurosci. 1991;59:1–23. doi: 10.3109/00207459108985446. [DOI] [PubMed] [Google Scholar]
- 51.Jerne NK. The generative grammar of the immune system. Science. 1985;229:1057–1059. doi: 10.1126/science.4035345. [DOI] [PubMed] [Google Scholar]
- 52.Mishell RI, Dutton RW. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967;126:423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 54.Straub RH, Westermann J, Scholmerich J, Falk W. Dialogue between the CNS and the immune system in lymphoid organs. Immunol Today. 1998;19:409–413. doi: 10.1016/s0167-5699(98)01297-3. [DOI] [PubMed] [Google Scholar]
- 55.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157:41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webber RH, DeFelice R, Ferguson RJ, Powell JP. Bone marrow response to stimulation of the sympathetic trunks in rats. Acta Anat (Basel) 1970;77:92–97. doi: 10.1159/000143531. [DOI] [PubMed] [Google Scholar]
- 62.Hanoun M, Maryanovich M, Arnal-Estape A, Frenette PS. Neural Regulation of Hematopoiesis, Inflammation, and Cancer. Neuron. 2015;86:360–373. doi: 10.1016/j.neuron.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 64.Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 65.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 67.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 71.Berger A, Frelin C, Shah DK, Benveniste P, Herrington R, Gerard NP, Zuniga-Pflucker JC, Iscove NN, Paige CJ. Neurokinin-1 receptor signalling impacts bone marrow repopulation efficiency. PLoS One. 2013;8:e58787. doi: 10.1371/journal.pone.0058787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maestroni GJ, Conti A. Modulation of hematopoiesis via alpha 1-adrenergic receptors on bone marrow cells. Exp Hematol. 1994;22:313–320. [PubMed] [Google Scholar]
- 75.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seeley EJ, Barry SS, Narala S, Matthay MA, Wolters PJ. Noradrenergic neurons regulate monocyte trafficking and mortality during gram-negative peritonitis in mice. J Immunol. 2013;190:4717–4724. doi: 10.4049/jimmunol.1300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Igyártó Botond Z, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson Brian T, Zurawski Sandra M, Malissen B, Zurawski G, Berman J, et al. Skin-Resident Murine Dendritic Cell Subsets Promote Distinct and Opposing Antigen-Specific T Helper Cell Responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 81.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veres TZ, Rochlitzer S, Shevchenko M, Fuchs B, Prenzler F, Nassenstein C, Fischer A, Welker L, Holz O, Muller M, et al. Spatial interactions between dendritic cells and sensory nerves in allergic airway inflammation. Am J Respir Cell Mol Biol. 2007;37:553–561. doi: 10.1165/rcmb.2007-0087OC. [DOI] [PubMed] [Google Scholar]
- 83.de Jong PR, Takahashi N, Peiris M, Bertin S, Lee J, Gareau MG, Paniagua A, Harris AR, Herdman DS, Corr M, et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunol. 2015;8:491–504. doi: 10.1038/mi.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kradin R, MacLean J, Duckett S, Schneeberger EE, Waeber C, Pinto C. Pulmonary response to inhaled antigen: neuroimmune interactions promote the recruitment of dendritic cells to the lung and the cellular immune response to inhaled antigen. Am J Pathol. 1997;150:1735–1743. [PMC free article] [PubMed] [Google Scholar]
- 85.Maestroni GJ. Dendritic cell migration controlled by alpha 1b-adrenergic receptors. J Immunol. 2000;165:6743–6747. doi: 10.4049/jimmunol.165.12.6743. [DOI] [PubMed] [Google Scholar]
- 86.Dunzendorfer S, Kaser A, Meierhofer C, Tilg H, Wiedermann CJ. Cutting edge: peripheral neuropeptides attract immature and arrest mature blood-derived dendritic cells. J Immunol. 2001;166:2167–2172. doi: 10.4049/jimmunol.166.4.2167. [DOI] [PubMed] [Google Scholar]
- 87.Madva EN, Granstein RD. Nerve-derived transmitters including peptides influence cutaneous immunology. Brain Behav Immun. 2013;34:1–10. doi: 10.1016/j.bbi.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 89.Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181:6020–6026. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mikami N, Sueda K, Ogitani Y, Otani I, Takatsuji M, Wada Y, Watanabe K, Yoshikawa R, Nishioka S, Hashimoto N, et al. Calcitonin gene-related peptide regulates type IV hypersensitivity through dendritic cell functions. PLoS One. 2014;9:e86367. doi: 10.1371/journal.pone.0086367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holzmann B. Modulation of immune responses by the neuropeptide CGRP. Amino Acids. 2013;45:1–7. doi: 10.1007/s00726-011-1161-2. [DOI] [PubMed] [Google Scholar]
- 92.Janelsins BM, Sumpter TL, Tkacheva OA, Rojas-Canales DM, Erdos G, Mathers AR, Shufesky WJ, Storkus WJ, Falo LD, Jr, Morelli AE, et al. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12. Blood. 2013;121:2923–2933. doi: 10.1182/blood-2012-07-446054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cunin P, Caillon A, Corvaisier M, Garo E, Scotet M, Blanchard S, Delneste Y, Jeannin P. The tachykinins substance P and hemokinin-1 favor the generation of human memory Th17 cells by inducing IL-1beta, IL-23, and TNF-like 1A expression by monocytes. J Immunol. 2011;186:4175–4182. doi: 10.4049/jimmunol.1002535. [DOI] [PubMed] [Google Scholar]
- 94.Grossman Z, Paul WE. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol. 2015;33:677–713. doi: 10.1146/annurev-immunol-032712-100027. [DOI] [PubMed] [Google Scholar]
- 95.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 96.van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henrickson SE, Perro M, Loughhead SM, Senman B, Stutte S, Quigley M, Alexe G, Iannacone M, Flynn MP, Omid S, et al. Antigen availability determines CD8(+) T cell-dendritic cell interaction kinetics and memory fate decisions. Immunity. 2013;39:496–507. doi: 10.1016/j.immuni.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc Natl Acad Sci U S A. 2009;106:5300–5305. doi: 10.1073/pnas.0808851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. The Journal of Clinical Investigation. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997;158:4200–4210. [PubMed] [Google Scholar]
- 102.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. The New England Journal of Medicine. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 103.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 104.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 106.Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou YR, Nagasawa T, Mukouyama YS. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 108.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 109.Smith CH, Barker JN, Morris RW, MacDonald DM, Lee TH. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151:3274–3282. [PubMed] [Google Scholar]
- 110.Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000;67:553–558. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- 111.Pacheco-Lopez G, Bermudez-Rattoni F. Brain-immune interactions and the neural basis of disease-avoidant ingestive behaviour. Philos Trans R Soc Lond B Biol Sci. 2011;366:3389–3405. doi: 10.1098/rstb.2011.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.del Toro R, Mendez-Ferrer S. Autonomic regulation of hematopoiesis and cancer. Haematologica. 2013;98:1663–1666. doi: 10.3324/haematol.2013.084764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brestoff JR, Artis D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 123.Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tracey KJ. Hypertension: an immune disorder? Immunity. 2014;41:673–674. doi: 10.1016/j.immuni.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 125.Tsujikawa K, Yayama K, Hayashi T, Matsushita H, Yamaguchi T, Shigeno T, Ogitani Y, Hirayama M, Kato T, Fukada S, et al. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:16702–16707. doi: 10.1073/pnas.0705974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity. 2014;41:737–752. doi: 10.1016/j.immuni.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 128.Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161:133–145. doi: 10.1016/j.cell.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mansuy-Aubert V, Gautron L, Lee S, Bookout AL, Kusminski C, Sun K, Zhang Y, Scherer PE, Mangelsdorf DJ, Elmquist JK. Loss of the liver X receptor LXRalpha/beta in peripheral sensory neurons modifies energy expenditure. Elife. 2015;4 doi: 10.7554/eLife.06667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 131.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 132.Rakoff-Nahoum S, Kong Y, Kleinstein SH, Subramanian S, Ahern PP, Gordon JI, Medzhitov R. Analysis of gene-environment interactions in postnatal development of the mammalian intestine. Proc Natl Acad Sci U S A. 2015;112:1929–1936. doi: 10.1073/pnas.1424886112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kabouridis PS, Pachnis V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. The Journal of Clinical Investigation. 2015;125:956–964. doi: 10.1172/JCI76308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Besedovsky H, del Rey A, Sorkin E, Da Prada M, Burri R, Honegger C. The immune response evokes changes in brain noradrenergic neurons. Science. 1983;221:564–566. doi: 10.1126/science.6867729. [DOI] [PubMed] [Google Scholar]
- 137.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol. 2001;280:R929–934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 138.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 139.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–69. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 140.Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180–1185. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 141.Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 143.Rivas FV, Chervonsky AV, Medzhitov R. ART and immunology. Trends Immunol. 2014;35:451. doi: 10.1016/j.it.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 144.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meisel JD, Kim DH. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 2014;35:465–470. doi: 10.1016/j.it.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 146.Reddy KC, Andersen EC, Kruglyak L, Kim DH. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science. 2009;323:382–384. doi: 10.1126/science.1166527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Costa-Pinto FA, Basso AS, Russo M. Role of mast cell degranulation in the neural correlates of the immediate allergic reaction in a murine model of asthma. Brain Behav Immun. 2007;21:783–790. doi: 10.1016/j.bbi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 150.McGovern AE, Mazzone SB. Neural regulation of inflammation in the airways and lungs. Auton Neurosci. 2014;182:95–101. doi: 10.1016/j.autneu.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 151.Veres TZ, Shevchenko M, Krasteva G, Spies E, Prenzler F, Rochlitzer S, Tschernig T, Krug N, Kummer W, Braun A. Dendritic cell-nerve clusters are sites of T cell proliferation in allergic airway inflammation. Am J Pathol. 2009;174:808–817. doi: 10.2353/ajpath.2009.080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Voedisch S, Rochlitzer S, Veres TZ, Spies E, Braun A. Neuropeptides control the dynamic behavior of airway mucosal dendritic cells. PLoS One. 2012;7:e45951. doi: 10.1371/journal.pone.0045951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J Immunol. 2009;182:7430–7439. doi: 10.4049/jimmunol.0900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. 2014;17:175–182. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, Laedermann C, Foster SL, Tran JV, Lai N, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Udit S, Gautron L. Molecular anatomy of the gut-brain axis revealed with transgenic technologies: implications in metabolic research. Front Neurosci. 2013;7:134. doi: 10.3389/fnins.2013.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ben-Horin S, Chowers Y. Neuroimmunology of the gut: physiology, pathology, and pharmacology. Curr Opin Pharmacol. 2008;8:490–495. doi: 10.1016/j.coph.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 160.Engel MA, Becker C, Reeh PW, Neurath MF. Role of sensory neurons in colitis: increasing evidence for a neuroimmune link in the gut. Inflamm Bowel Dis. 2011;17:1030–1033. doi: 10.1002/ibd.21422. [DOI] [PubMed] [Google Scholar]
- 161.Engel MA, Khalil M, Mueller-Tribbensee SM, Becker C, Neuhuber WL, Neurath MF, Reeh PW. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J Gastroenterol. 2012;47:256–265. doi: 10.1007/s00535-011-0495-6. [DOI] [PubMed] [Google Scholar]
- 162.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 163.Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res. 1997;746:195–206. doi: 10.1016/s0006-8993(96)01222-x. [DOI] [PubMed] [Google Scholar]
- 164.McCafferty DM, Wallace JL, Sharkey KA. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Physiol. 1997;272:G272–280. doi: 10.1152/ajpgi.1997.272.2.G272. [DOI] [PubMed] [Google Scholar]
- 165.Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med (Berl) 2006;84:142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 166.Gad M, Pedersen AE, Kristensen NN, de Fernandez CF, Claesson MH. Blockage of the neurokinin 1 receptor and capsaicin-induced ablation of the enteric afferent nerves protect SCID mice against T-cell-induced chronic colitis. Inflamm Bowel Dis. 2009;15:1174–1182. doi: 10.1002/ibd.20902. [DOI] [PubMed] [Google Scholar]
- 167.Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipovic MR, Izydorczyk I, Eberhardt M, Kichko TI, Mueller-Tribbensee SM, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 168.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. Faseb J. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, Ghosh S, McKemy DD, Vergnolle N, Beck PL, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A. 2013;110:7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Assas BM, Miyan JA, Pennock JL. Cross-talk between neural and immune receptors provides a potential mechanism of homeostatic regulation in the gut mucosa. Mucosal Immunol. 2014;7:1283–1289. doi: 10.1038/mi.2014.80. [DOI] [PubMed] [Google Scholar]
- 171.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Macpherson AJ, Geuking MB, McCoy KD. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33:160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 173.Stanisz AM, Befus D, Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer’s patches, mesenteric lymph nodes, and spleen. J Immunol. 1986;136:152–156. [PubMed] [Google Scholar]
- 174.Fujieda S, Waschek JA, Zhang K, Saxon A. Vasoactive intestinal peptide induces S(alpha)/S(mu) switch circular DNA in human B cells. The Journal of Clinical Investigation. 1996;98:1527–1532. doi: 10.1172/JCI118944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shibata M, Hisajima T, Nakano M, Goris RC, Funakoshi K. Morphological relationships between peptidergic nerve fibers and immunoglobulin A-producing lymphocytes in the mouse intestine. Brain Behav Immun. 2008;22:158–166. doi: 10.1016/j.bbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 176.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 177.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 178.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 179.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, Zhou Y, Deng A, Hunter CA, Luster AD, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra223. doi: 10.1126/scitranslmed.3007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Girolomoni G, Tigelaar RE. Capsaicin-sensitive primary sensory neurons are potent modulators of murine delayed-type hypersensitivity reactions. J Immunol. 1990;145:1105–1112. [PubMed] [Google Scholar]
- 182.Beresford L, Orange O, Bell EB, Miyan JA. Nerve fibres are required to evoke a contact sensitivity response in mice. Immunology. 2004;111:118–125. doi: 10.1111/j.1365-2567.2003.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Veronesi B, Williams WC, Smialowicz RJ, Sailstad DM, Doerfler D, Selgrade MJ. Neuropeptide denervation alters both the elicitation and induction phases of contact hypersensitivity in mice. Toxicol Appl Pharmacol. 1998;153:243–249. doi: 10.1006/taap.1998.8539. [DOI] [PubMed] [Google Scholar]
- 184.Banvolgyi A, Palinkas L, Berki T, Clark N, Grant AD, Helyes Z, Pozsgai G, Szolcsanyi J, Brain SD, Pinter E. Evidence for a novel protective role of the vanilloid TRPV1 receptor in a cutaneous contact allergic dermatitis model. J Neuroimmunol. 2005;169:86–96. doi: 10.1016/j.jneuroim.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 185.Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- 186.Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013;34:174–181. doi: 10.1016/j.it.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weddell G, Cowan MA, Palmer E, Ramaswamy S. Psoriatic Skin. Arch Dermatol. 1965;91:252–266. doi: 10.1001/archderm.1965.01600090060012. [DOI] [PubMed] [Google Scholar]
- 188.Joseph T, Kurian J, Warwick DJ, Friedmann PS. Unilateral remission of psoriasis following traumatic nerve palsy. Br J Dermatol. 2005;152:185–186. doi: 10.1111/j.1365-2133.2005.06330.x. [DOI] [PubMed] [Google Scholar]
- 189.Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220–221. [PubMed] [Google Scholar]
- 190.Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. International Journal of Dermatology. 1990;29:418–420. doi: 10.1111/j.1365-4362.1990.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 191.Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530–1538. doi: 10.1038/jid.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ward NL, Kavlick KD, Diaconu D, Dawes SM, Michaels KA, Gilbert E. Botulinum neurotoxin A decreases infiltrating cutaneous lymphocytes and improves acanthosis in the KC-Tie2 mouse model. J Invest Dermatol. 2012;132:1927–1930. doi: 10.1038/jid.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wohn C, Ober-Blobaum JL, Haak S, Pantelyushin S, Cheong C, Zahner SP, Onderwater S, Kant M, Weighardt H, Holzmann B, et al. Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc Natl Acad Sci U S A. 2013;110:10723–10728. doi: 10.1073/pnas.1307569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Satija R, Shalek AK. Heterogeneity in immune responses: from populations to single cells. Trends Immunol. 2014;35:219–229. doi: 10.1016/j.it.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84–91. doi: 10.1111/dth.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21:660–668. doi: 10.1016/j.tim.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tobin D, Nabarro G, Baart de la Faille H, van Vloten WA, van der Putte SC, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol. 1992;90:613–622. doi: 10.1016/0091-6749(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 201.Azimi E, Lerner EA, Elmariah SB. Altered manifestations of skin disease at sites affected by neurological deficit. Br J Dermatol. 2015;172:988–993. doi: 10.1111/bjd.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, Li M. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun. 2013;4:2847. doi: 10.1038/ncomms3847. [DOI] [PubMed] [Google Scholar]
- 204.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, Zheng T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol. 2013;191:5371–5382. doi: 10.4049/jimmunol.1300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Naukkarinen A, Harvima IT, Aalto ML, Harvima RJ, Horsmanheimo M. Quantitative analysis of contact sites between mast cells and sensory nerves in cutaneous psoriasis and lichen planus based on a histochemical double staining technique. Arch Dermatol Res. 1991;283:433–437. doi: 10.1007/BF00371778. [DOI] [PubMed] [Google Scholar]
- 209.Forsythe P, Bienenstock J. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem Immunol Allergy. 2012;98:196–221. doi: 10.1159/000336523. [DOI] [PubMed] [Google Scholar]
- 210.Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol. 2008;121:955–961. doi: 10.1016/j.jaci.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 211.Tong PL, Roediger B, Kolesnikoff N, Biro M, Tay SS, Jain R, Shaw LE, Grimbaldeston MA, Weninger W. The Skin Immune Atlas: Three-Dimensional Analysis of Cutaneous Leukocyte Subsets by Multiphoton Microscopy. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–564. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- 214.El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200:11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 215.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. The Journal of Clinical Investigation. 2014;124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ochoa-Cortes F, Ramos-Lomas T, Miranda-Morales M, Spreadbury I, Ibeakanma C, Barajas-Lopez C, Vanner S. Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299:G723–732. doi: 10.1152/ajpgi.00494.2009. [DOI] [PubMed] [Google Scholar]
- 218.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Marion E, Song OR, Christophe T, Babonneau J, Fenistein D, Eyer J, Letournel F, Henrion D, Clere N, Paille V, et al. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell. 2014;157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 220.Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296:927–931. doi: 10.1126/science.1067631. [DOI] [PubMed] [Google Scholar]
- 221.Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell. 2013;152:51–67. doi: 10.1016/j.cell.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, von Andrian UH. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, Fu YX, Hacohen N, von Andrian UH. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity. 2012;36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Swanson PA, 2nd, McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lafaille FG, Pessach IM, Zhang SY, Ciancanelli MJ, Herman M, Abhyankar A, Ying SW, Keros S, Goldstein PA, Mostoslavsky G, et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yordy B, Iijima N, Huttner A, Leib D, Iwasaki A. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe. 2012;12:334–345. doi: 10.1016/j.chom.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wainger BJ, Buttermore ED, Oliveira JT, Mellin C, Lee S, Saber WA, Wang AJ, Ichida JK, Chiu IM, Barrett L, et al. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat Neurosci. 2015;18:17–24. doi: 10.1038/nn.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 230.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 231.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hepburn L, Prajsnar TK, Klapholz C, Moreno P, Loynes CA, Ogryzko NV, Brown K, Schiebler M, Hegyi K, Antrobus R, et al. Innate immunity. A Spaetzle-like role for nerve growth factor beta in vertebrate immunity to Staphylococcus aureus. Science. 2014;346:641–646. doi: 10.1126/science.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kilic SS, Ozturk R, Sarisozen B, Rotthier A, Baets J, Timmerman V. Humoral immunodeficiency in congenital insensitivity to pain with anhidrosis. Neurogenetics. 2009;10:161–165. doi: 10.1007/s10048-008-0165-x. [DOI] [PubMed] [Google Scholar]
- 234.Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 235.Pongratz G, Straub RH. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat Rev Rheumatol. 2013;9:117–126. doi: 10.1038/nrrheum.2012.181. [DOI] [PubMed] [Google Scholar]
- 236.Borbely E, Botz B, Bolcskei K, Kenyer T, Kereskai L, Kiss T, Szolcsanyi J, Pinter E, Csepregi JZ, Mocsai A, et al. Capsaicin-sensitive sensory nerves exert complex regulatory functions in the serum-transfer mouse model of autoimmune arthritis. Brain Behav Immun. 2015;45:50–59. doi: 10.1016/j.bbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miller LE, Justen HP, Scholmerich J, Straub RH. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. Faseb J. 2000;14:2097–2107. doi: 10.1096/fj.99-1082com. [DOI] [PubMed] [Google Scholar]
- 238.Harle P, Mobius D, Carr DJ, Scholmerich J, Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 2005;52:1305–1313. doi: 10.1002/art.20987. [DOI] [PubMed] [Google Scholar]
- 239.Levine JD, Dardick SJ, Roizen MF, Helms C, Basbaum AI. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986;6:3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Levine JD, Moskowitz MA, Basbaum AI. The contribution of neurogenic inflammation in experimental arthritis. J Immunol. 1985;135:843s–847s. [PubMed] [Google Scholar]
- 241.Levine JD, Clark R, Devor M, Helms C, Moskowitz MA, Basbaum AI. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984;226:547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- 242.Stangenberg L, Burzyn D, Binstadt BA, Weissleder R, Mahmood U, Benoist C, Mathis D. Denervation protects limbs from inflammatory arthritis via an impact on the microvasculature. Proc Natl Acad Sci U S A. 2014;111:11419–11424. doi: 10.1073/pnas.1410854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 244.Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, Lacombe J, Armstrong SA, Duhrsen U, Frenette PS. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15:365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntion S, Tzeng YS, Lai DM, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 246.Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, Tessarollo L, Frenette PS. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. 2013;19:695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Naukkarinen A, Nickoloff BJ, Farber EM. Quantification of cutaneous sensory nerves and their substance P content in psoriasis. J Invest Dermatol. 1989;92:126–129. doi: 10.1111/1523-1747.ep13071340. [DOI] [PubMed] [Google Scholar]
- 248.Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. e1004. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 249.Mostafa GA, Reda SM, Abd El-Aziz MM, Ahmed SA. Sputum neurokinin A in Egyptian asthmatic children and adolescents: relation to exacerbation severity. Allergy. 2008;63:1244–1247. doi: 10.1111/j.1398-9995.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 250.Joos GF, Vincken W, Louis R, Schelfhout VJ, Wang JH, Shaw MJ, Cioppa GD, Pauwels RA. Dual tachykinin NK1/NK2 antagonist DNK333 inhibits neurokinin A-induced bronchoconstriction in asthma patients. Eur Respir J. 2004;23:76–81. doi: 10.1183/09031936.03.00101902. [DOI] [PubMed] [Google Scholar]
- 251.Schelfhout V, Louis R, Lenz W, Heyrman R, Pauwels R, Joos G. The triple neurokinin-receptor antagonist CS-003 inhibits neurokinin A-induced bronchoconstriction in patients with asthma. Pulm Pharmacol Ther. 2006;19:413–418. doi: 10.1016/j.pupt.2005.10.007. [DOI] [PubMed] [Google Scholar]