Genome-editing Technologies for Gene and Cell Therapy (original) (raw)
Abstract
Gene therapy has historically been defined as the addition of new genes to human cells. However, the recent advent of genome-editing technologies has enabled a new paradigm in which the sequence of the human genome can be precisely manipulated to achieve a therapeutic effect. This includes the correction of mutations that cause disease, the addition of therapeutic genes to specific sites in the genome, and the removal of deleterious genes or genome sequences. This review presents the mechanisms of different genome-editing strategies and describes each of the common nuclease-based platforms, including zinc finger nucleases, transcription activator-like effector nucleases (TALENs), meganucleases, and the CRISPR/Cas9 system. We then summarize the progress made in applying genome editing to various areas of gene and cell therapy, including antiviral strategies, immunotherapies, and the treatment of monogenic hereditary disorders. The current challenges and future prospects for genome editing as a transformative technology for gene and cell therapy are also discussed.
The realization of the genetic basis of hereditary disease led to the early concept of gene therapy in which “exogenous ‘good' DNA be used to replace the defective DNA in those who suffer from genetic defects”.1 More than 40 years of research since this proposal of gene therapy has shown the simple idea of gene replacement to be much more challenging and technically complex to implement both safely and effectively than originally appreciated. Many of these challenges centered on fundamental limitations in the ability to precisely control how genetic material was introduced to cells. Nevertheless, the technologies for addition of exogenous genes have made remarkable progress during this time and are now showing promising clinical results across a range of strategies and medical indications.2 However, several challenges still remain. Integrating therapeutic genes into the genome for stable maintenance in replicating cells can have unpredictable effects on gene expression and unintended effects on neighboring genes.3 Moreover, some therapeutic genes are too large to be readily transferred by available delivery vectors. Finally, the addition of exogenous genes cannot always directly address dominant mutations or remove unwanted genetic material such as viral genomes or receptors. To address these fundamental limitations of conventional methods for gene addition, the field of gene editing has emerged to make precise, targeted modifications to genome sequences. Here we review the recent exciting developments in the ease of use, specificity, and delivery of gene-editing technologies and their application to treating a wide variety of diseases and disorders.
Mechanisms of Gene Editing
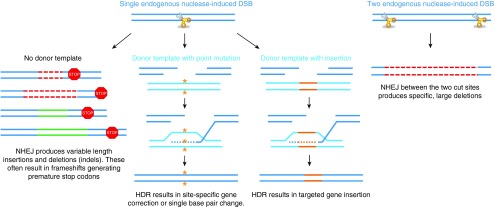
Foundational to the field of gene editing was the discovery that targeted DNA double strand breaks (DSBs) could be used to stimulate the endogenous cellular repair machinery. Breaks in the DNA are typically repaired through one of two major pathways—homology-directed repair (HDR) or nonhomologous end-joining (NHEJ) (Figure 1).4 HDR relies on strand invasion of the broken end into a homologous sequence and subsequent repair of the break in a template-dependent manner.5 Seminal work from the lab of Maria Jasin demonstrated that the efficiency of gene targeting through homologous recombination in mammalian cells could be stimulated by several orders of magnitude by introducing a DSB at the target site.6,7,8 Alternatively, NHEJ functions to repair DSBs without a template through direct religation of the cleaved ends.9 This repair pathway is error-prone and often results in insertions and/or deletions (indels) at the site of the break. Stimulation of NHEJ by site-specific DSBs has been used to disrupt target genes in a wide variety of cell types and organisms by taking advantage of these indels to shift the reading frame of a gene.10,11,12,13,14 Armed with the ability to harness the cell's endogenous DNA repair machinery, it is now possible to engineer a wide variety of genomic alterations in a site-specific manner.
Figure 1.
Mechanisms of double-strand break repair.
Gene knockout/mutation
This simplest form of gene editing utilizes the error-prone nature of NHEJ to introduce small indels at the target site. Classical NHEJ directly religates unprocessed DNA ends whereas alternative-NHEJ (also known as microhomology-mediated end joining, or MMEJ) requires end-resection followed by annealing of short single-stranded regions of microhomology and subsequent DNA end ligation.15 Active during all stages of the cell cycle, both of these NHEJ pathways repair DNA with a high frequency of mutagenesis resulting in the formation of indels at the site of the break.15,16
When the nuclease target site is placed in the coding region of a gene, the resulting indels will often cause frameshifts. In diseases such as Duchenne muscular dystrophy (DMD), where gene deletions result in frameshifts and subsequent loss of protein function, targeted NHEJ-induced indels can be used to restore the correct reading frame of the gene.17 However, the most common application of targeted mutagenesis involves inducing frameshift mutations for the purpose of gene knockout. In contrast to traditional gene therapy, which is limited to the addition of exogenous sequence into the genome, the ability to knockout endogenous genes opens a new avenue of therapeutic treatment in which gene function can be permanently disrupted. One application of this approach is to target dominant gain-of-function mutations, such as those found in Huntington's disease. This disease is caused by a repeat expansion on one allele of the huntingtin (HTT) gene, leading to the production of a toxic mutant HTT protein. Eliminating this mutant allele by NHEJ-based gene editing could provide clinical benefit to Huntington's patients.18,19 In other diseases, it may sometimes be therapeutic to remove the normal function of a gene. The most prominent example of this is the gene-editing approach currently in clinical trials for the treatment of HIV, in which knockout of CCR5, the major HIV coreceptor, prohibits viral infection of modified T cells.20,21,22 Finally, rather than directly targeting the human genome, knockout of critical genes in invading bacteria or DNA-based viruses could serve as effective anti-microbial treatments.23,24
Gene deletion
In addition to the relatively minor indels resulting from NHEJ, it is possible to delete large segments of DNA by flanking the sequence with two DSBs. Indeed, it has been shown that simultaneous introduction of two targeted breaks can give rise to genomic deletions up to several megabases in size.25,26,27,28,29 This approach is useful for therapeutic strategies that may require the removal of an entire genomic element, such as an enhancer region, as has been proposed for the treatment of hemoglobinopathies by deletion of the BCL11A erythroid-specific enhancer region.30,31 Additionally, in diseases such as DMD where different internal gene deletions can shift the gene out of frame, the intentional deletion of one or more exons can correct the reading frame and restore the expression of truncated, but partially functional, protein.32,33,34,35,36,37
Gene correction
As opposed to the unpredictable mutations resulting from NHEJ, targeted DSBs can induce precise gene editing by stimulating HDR with an exogenously supplied donor template. Active mainly during the S and G2 phases of the cell cycle, HDR naturally utilizes the sister chromatid as a template for DNA repair.15,16,38 However, an exogenously supplied donor sequence may also be used as a repair template.39 Thus the codelivery of targeted nucleases along with a targeting vector containing DNA homologous to the break site enables high-efficiency HDR-based gene editing.6,7,8 Any sequence differences present in the donor template can thus be incorporated into the endogenous locus to correct disease-causing mutations, as has been demonstrated in many proof-of-concept studies.40,41,42,43,44,45,46,47,48,49,50 While plasmids have traditionally been the most commonly used source of donor DNA, recent studies have shown that single stranded oligonucleotides (ssODNs), with as little as 80 base pairs of homology, can serve as efficient donor templates for HDR.51,52,53 For cells that are difficult to transfect, viral vectors such as integrase-deficient lentivirus or adeno-associated virus (AAV) can also be used as a source of donor DNA.54,55,56,57 In fact, the naturally recombinogenic nature of AAV, especially when combined with the particularly efficient hybrid serotypes such as AAV-DJ, makes them attractive vectors for delivery of the donor template.54,56,58,59,60,61,62
Gene insertion
Although traditional gene therapy has successfully used viral vectors to introduce exogenous genes into the genome, the inability to control the integration site of these viruses raises serious concerns of insertional mutagenesis, as was underscored in the early clinical trials that used murine retroviral vectors.63,64,65 The use of a donor template, in which the desired genetic insert is flanked by homology arms including sequences identical to the nuclease cut site, enables site-specific DNA insertion through DSB-induced HDR.66 Targeted insertion of therapeutic transgenes into predetermined sites in the genome, such as “safe harbor” loci, alleviates risks of insertional mutagenesis and enables high levels of ubiquitous gene expression.67,68,69 To maintain control of gene expression by natural regulatory elements, a wild type copy of the disease-causing gene may be inserted into the corresponding endogenous locus and thus be under the control of its own promoter.70,71 An alternative mechanism for targeted transgene insertion is to use nuclease-induced DSBs to create compatible overhangs on the donor DNA and the endogenous site, leading to NHEJ-mediated ligation of the insert DNA sequence directly into the target locus.72
Targeted Nucleases
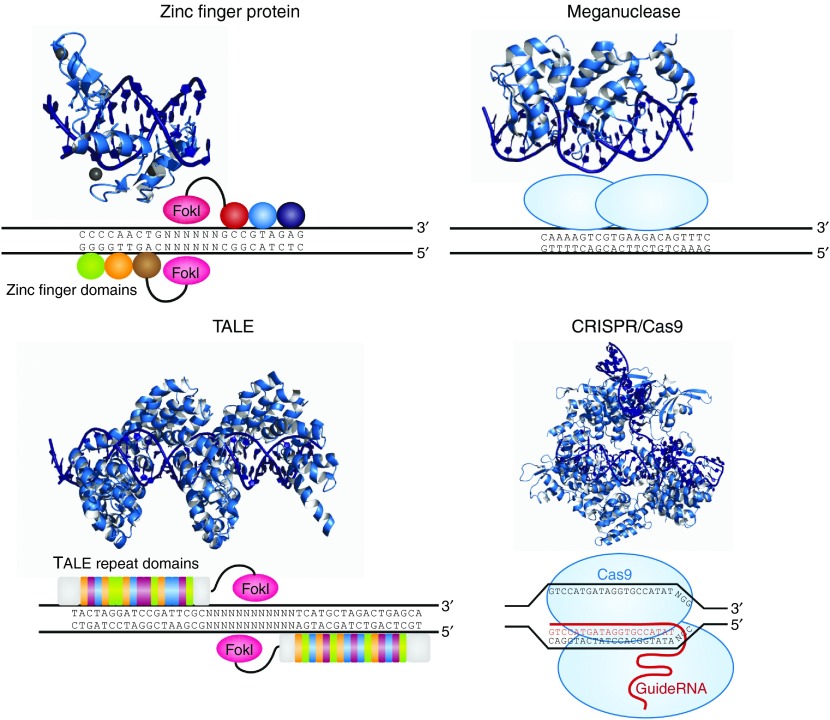
Because DSB-induced gene editing relies on the endogenous repair mechanisms of the cell, it is universally applicable to any cell type or organism that employs these methods for DNA repair. The critical element for implementing any of these gene-editing methods is the precise introduction of a targeted DSB. Four major platforms currently exist for inducing these site-specific DSBs: zinc finger nucleases (ZFNs), transcription activator-like effector (TALE)-nucleases (TALENs), meganucleases, and most recently the CRISPR/Cas system (Figure 2).
Figure 2.
Common DNA targeting platforms for genome editing.
Zinc finger nucleases
Zinc finger (ZF) proteins are the most abundant class of transcription factors and the Cys2-His2 zinc finger domain is one of the most common DNA-binding domains encoded in the human genome.73 The crystal structure of Zif268 has served as the basis for understanding DNA recognition by zinc fingers.74,75,76 In the presence of a zinc atom, the zinc finger domain forms a compact ββα structure with the α-helical portion of each finger making contact with 3 or 4 bp in the major groove of the DNA.74,77,78 Tandem fingers in a zinc finger array wrap around the DNA to bind extended target sequences such that a three-finger protein binds a 9 bp target site.
The modular structure of Zif268 suggested that these proteins might provide an attractive framework for engineering novel DNA-binding motifs.79 Initial attempts to design ZFs with unique specificities based on a simple set of rules had some success80,81; however, combinatorial libraries combined with selection-based methods proved to be a more robust approach for generating individual fingers with novel DNA-binding specificities.82,83,84,85,86,87 Following this success, the field was faced with the challenge of engineering multi-finger arrays with novel target sites long enough to be unique in a complex genome. The “modular assembly” approach relies on collections of single-finger modules, either identified in naturally occurring proteins88 or selected to bind specific three base pair target sites,89,90,91,92 which are then linked in tandem to generate novel proteins.93,94,95,96,97 Alternatively, selection-based methods, such as OPEN, may be used to select new proteins from randomized libraries.98 While significantly more labor intensive, this method takes into account context-dependent interactions between neighboring fingers within a multi-finger array.76,99,100,101 Several methods, including those used by Sangamo Biosciences and the Sigma-Aldrich CompoZr platform, combine these two approaches to assemble novel arrays using archives of multi-finger units that have been preselected to work well together.13,102,103,104
The zinc finger nuclease (ZFN) technology was made possible by the discovery that the DNA-binding domain and the cleavage domain of the FokI restriction endonuclease function independently of each other.105 By replacing the FokI DNA-binding domain with a zinc finger domain, it is possible to generate chimeric nucleases with novel binding specificities.106,107 Because the FokI nuclease functions as a dimer, two ZFNs binding opposite strands of DNA are required for induction of a DSB.108 Initial experiments showed that ZFN-induced DSBs could be used to modify the genome through either NHEJ or HDR10,109,110 and this technology has subsequently been used to successfully modify genes in human somatic40,66,98 and pluripotent stem cells.42,44,111,112,113
TALENs
The discovery of a simple one-to-one code dictating the DNA-binding specificity of TALE proteins from the plant pathogen Xanthomonas again raised the exciting possibility for modular design of novel DNA-binding proteins.114,115 Highly conserved 33–35 amino acid TALE repeats each bind a single base pair of DNA with specificity dictated by two hypervariable residues. Crystal structures of TALEs bound to DNA revealed that each repeat forms a two-helix structure connected by a loop which presents the hypervariable residue into the major groove as the protein wraps around the DNA in a superhelical structure.116,117 These modular TALE repeats can be linked together to build long arrays with custom DNA-binding specificities.118,119,120,121,122
Many platforms exist for engineering TALE arrays. The simplest methods use standard cloning techniques to assemble TALEs from archives of plasmids, each consisting of single TALE repeats.123,124 Several medium-throughput methods rely on the Golden Gate cloning system to assemble multiple pieces simultaneously in a single reaction.120,122,125,126,127,128,129 The highest-throughput methods utilize solid phase assembly130,131,132 or ligation-independent cloning techniques.133
Building off the foundation laid by a decade of ZFN-induced genome editing, the discovery of TALEs as a programmable DNA-binding domain was rapidly followed by the engineering of TALENs. Like ZFNs, TALEs were fused to the catalytic domain of the FokI endonuclease and shown to function as dimers to cleave their intended DNA target site.119,121,134,135 Also similar to ZFNs, TALENs have been shown to efficiently induce both NHEJ and HDR in human somatic119,132,134 and pluripotent stem cells.53,136
TALENs can be engineered to target virtually any sequence given that their only targeting restraint is the requirement for a 5' T, specified by the constant N-terminal domain, for each array. This unlimited targeting range, in addition to the ease of engineering new proteins, makes TALENs an attractive platform for targeted gene editing. Conversely, the large size and repetitive nature of TALE arrays presents a hurdle for in vivo delivery of these proteins. As opposed to a 30 amino acid zinc finger, which binds three bases of DNA, TALENs require 34 amino acids to specify a single base pair and this size difference can prohibit delivery of both TALEN monomers in a single viral vector with limited packaging capacity. Additionally, the unstable nature of tandem repeats, such as those present in TALENs, makes it challenging to package repetitive sequences in viral systems. Indeed, TALENs delivered by lentivirus have been shown to be susceptible to rearrangements,137 although this phenomenon may be mitigated by codon diversification between the repeats.138 Adenoviral systems have also been used to successfully deliver TALENs.139
Meganucleases
Meganuclease technology involves re-engineering the DNA-binding specificity of naturally occurring homing endonucleases. The largest class of homing endonucleases is the LAGLIDADG family, which includes the well-characterized and commonly used I-_Cre_I and I-_Sce_I enzymes.140 Through a combination of rational design and selection, these homing endonucleases can be re-engineered to target novel sequences.141,142,143,144,145,146,147,148 While many studies show promise for the use of meganucleases in genome editing,149,150,151,152 the DNA-binding and cleavage domains of homing endonucleases are difficult to separate, and the relative difficulty of engineering proteins with novel specificities has traditionally limited the use of this platform. To address this limitation, chimeric proteins comprising fusions of meganucleases, ZFs, and TALEs have been engineered to generate novel monomeric enzymes that take advantage of the binding affinity of ZFs and TALEs and the cleavage specificity of meganucleases.153,154,155,156 One potential advantage associated with meganuclease technology is that DSB-formation by these enzymes results in a 3' overhang, which may be more recombinogenic for HDR than the 5' overhang generated by FokI cleavage. Additionally, meganucleases are the smallest class of engineered nucleases, making them potentially amenable to all standard gene delivery methods. In fact, multiple meganuclease monomers could be readily packaged into single viral vectors to simultaneously create multiple DSBs.
CRISPR/Cas nucleases
CRISPR-Cas RNA-guided nucleases are derived from an adaptive immune system that evolved in bacteria to defend against invading plasmids and viruses. Decades of work investigating CRISPR systems in various microbial species has elucidated a mechanism by which short sequences of invading nucleic acids are incorporated into CRISPR loci.157 They are then transcribed and processed into CRISPR RNAs (crRNAs) which, together with a trans-activating crRNAs (tracrRNAs), complex with CRISPR-associated (Cas) proteins to dictate specificity of DNA cleavage by Cas nucleases through Watson-Crick base pairing between nucleic acids.158,159,160,161 Building off of two studies showing that the three components required for the type II CRISPR nuclease system are the Cas9 protein, the mature crRNA and the tracrRNA,162,163 Doudna, Charpentier and colleagues showed through in vitro DNA cleavage experiments that this system could be reduced to two components by fusion of the crRNA and tracrRNA into a single guide RNA (gRNA). Furthermore, they showed that re-targeting of the Cas9/gRNA complex to new sites could be accomplished by altering the sequence of a short portion of the gRNA.164 Thereafter, a series of publications demonstrated that the CRISPR/Cas9 system could be engineered for efficient genetic modification in mammalian cells.165,166,167,168 Collectively these studies have propelled the CRISPR/Cas9 technology into the spotlight of the genome-editing field.
The only sequence limitation of the CRISPR/Cas system derives from the necessity of a protospacer-adjacent motif (PAM) located immediately 3' to the target site. The PAM sequence is specific to the species of Cas9. For example, the PAM sequence 5'-NGG-3' is necessary for binding and cleavage of DNA by the commonly used Cas9 from Streptococcus pyogenes.169,170,171 However, Cas9 variants with novel PAMs may be engineered by directed evolution, thus dramatically expanding the number of potential target sequences.172,173 Cas9 complexed with the crRNA and tracrRNA undergoes a conformational change and associates with PAM motifs throughout the genome interrogating the sequence directly upstream to determine sequence complementarity with the gRNA.171,174,175,176,177 The formation of a DNA-RNA heteroduplex at a matched target site allows for cleavage of the target DNA by the Cas9-RNA complex.171
Unlike the three nuclease systems discussed above, CRISPR/Cas nucleases do not require the engineering of novel proteins for each DNA target site. The relative ease with which new sites can be targeted, simply by altering the short region of the gRNA that dictates specificity, makes this system a highly attractive method for introducing site-specific DSBs. Additionally, because the Cas9 protein is not directly coupled to the gRNA, this system is highly amenable to multiplexing through the concurrent use of multiple gRNAs to induce DSBs at several loci. Because the rich diversity of natural CRISPR systems has been largely understudied, it is reasonable to expect many new CRISPR-based gene-editing technologies to emerge, including non-Cas9 based type II systems such as the recently described RNA-guided endonuclease Cpf1 and others.178,179
Specificity of targeted nucleases
The efficacy of targeted gene editing relies on cleaving the DNA in a site-specific manner while mitigating, or ideally preventing, collateral damage to the rest of the genome. For this reason, the specificity of targeted nucleases is a major focus of the gene-editing field. Modifications to the FokI dimerization domain dramatically increased the specificity of ZFNs and TALENs by requiring two obligate heterodimers to bind the target DNA in a specific orientation and spacing.180,181,182,183 Reminiscent of the architecture of ZFNs and TALENs, the inactivation of Cas9 nuclease domains to create Cas9 nickases or Cas9-FokI fusions has increased specificity by requiring two gRNA/Cas9 complexes, each cleaving a single strand of DNA, to come together at a precise distance and orientation in order to generate a DSB.184,185,186,187 Additionally, reducing the length of complementarity between the gRNA and the target site from 20 to 17 nucleotides increases the specificity of DNA cleavage by Cas9 from S. pyogenes.188 Recently, structure-guided protein engineering has been used to develop novel Cas9 variants with increased specificity properties.189,190 These improvements have significantly alleviated initial concerns over the specificity of CRISPR/Cas nucleases.191,192,193 However, regardless of the nuclease technology, it is difficult to determine the full spectrum of off-target cleavage in a complex genome. Until recently, specificity studies were largely limited to a priori, in silico identification of potential off-target sites that could be informed by surrogate assays with purified proteins or viral integrations at double-strand breaks.194,195,196 Whole-genome sequencing of a small number of clones derived from single cells has verified the lack of off-target effects in these select populations, but cannot identify sites that are cleaved at low frequencies in bulk cell populations.197,198,199 Interrogation of DNA-binding specificity by ChIP-seq was greatly informative for understanding target site recognition, but the vast majority of the off-target binding sites were not predictive of nuclease activity.200,201,202 Recent development of methods for unbiased, genome-wide assays to determine specificity have significantly advanced the ability to characterize nuclease specificity with a degree of sensitivity that was not previously possible.195,203,204,205,206 These new methods will likely be critical to advancing targeted gene-editing nucleases as therapeutics.
Delivery of Genome-Editing Tools
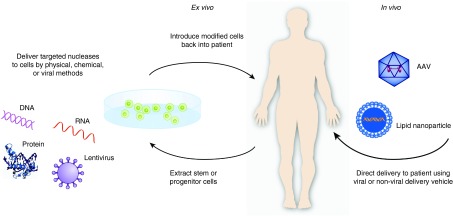
Efficient and safe delivery to target cells and tissues has been the long-standing challenge to successful gene therapy strategies (Figure 3). This challenge extends to genome-editing methods as well, where the nucleases, and in the case of the CRISPR/Cas9 system, a gRNA, must be efficiently delivered. Moreover, the dose of the donor template DNA is important to ensuring efficient homologous recombination. The duration and magnitude of nuclease expression is a critical parameter for the level of both on-target and off-target nuclease activity. Maximizing the efficiency of delivery is particularly important since gene editing is an inherently stochastic event occurring in only a fraction of the cells in which the nuclease is expressed.
Figure 3.
Ex vivo and in vivo strategies for therapeutic genome editing.
The most widely reported method for introducing nucleases into cells in proof-of-principle studies is transfection of plasmid DNA carrying nuclease and gRNA expression cassettes. Although simple and straightforward, this method is not ideal for most gene and cell therapies due to low efficiency of transfection of primary cells, DNA-related cytotoxicity, the presence of bacterial DNA sequences in plasmid backbones, and the possibility of random integration of plasmid fragments into the genome. Consequently, electroporation of mRNA encoding the nucleases and gRNAs generated through in vitro transcription has become a preferred method for ex vivo gene editing of primary cells relevant to gene therapy, such as T cells and hematopoietic stem cells (HSCs).168,207 Alternatively, the direct delivery of purified nuclease proteins or Cas9 protein-gRNA complexes has also been very successful in achieving high levels of gene editing, either by electroporation208,209 or fusion to cell-penetrating peptides, which obviates electroporation-mediated toxicity.210,211,212 Chemical modification of the gRNAs can further increase the robustness of gene editing in primary cells by increasing stability and/or decreasing innate immune responses.207 These studies have collectively shown that by restricting the duration of nuclease activity with short-lived mRNA or proteins, off-target effects can be minimized compared to plasmid-based delivery. Future efforts will likely take advantage of emerging nanoparticle formulations for efficient and nontoxic delivery.213
For many applications, viral vectors are still the optimal vehicle to maximize the efficiency of delivery while minimizing cytotoxicity.214 In particular, lentiviral vectors have been optimized for highly efficient transduction of T cells and HSCs; however these vectors also integrate into the genome and stably express their transgene cargo. In order to take advantage of the efficiency of lentiviral transduction while limiting the duration of nuclease expression in target cells, integrase-deficient lentiviral vectors have been used to transiently deliver genome-editing tools to target cells.57,111 Similarly, adenoviral systems can also achieve high levels of transduction of a variety of cell types ex vivo while providing only transient nuclease expression.20,137,139 Both lentiviral and adenoviral vectors also have the advantage of sufficient packaging capacity to carry multiple nucleases or gRNA expression cassettes for multiplex editing of several loci.215
In vivo gene editing presents additional challenges of tissue-specific targeting, distribution of the vector, and immunogenicity and biocompatibility of the carrier. Although several examples of plasmid delivery to the liver have shown important proof-of-principle of in vivo gene editing in animal models,216,217,218 translating these strategies to human therapy is not yet feasible. However, in vivo gene delivery with AAV to the liver, eye, nervous system, and skeletal and cardiac muscle has shown impressive efficacy in both preclinical models and clinical trials.219 Consequently, AAV is also a promising system for delivery of gene-editing nucleases to target tissues.220 Furthermore, the natural recombinogenic properties of AAV make it a desirable vector for delivery of DNA repair templates.56,61,62,221,222,223,224 Although some studies have shown targeted recombination of genomic loci with AAV vectors in the absence of nucleases,58,71 the efficiencies are significantly lower than reports that include nucleases. Further studies are required to understand which disease indications can be robustly addressed at lower efficiencies of gene editing.
Although AAV has shown considerable promise for in vivo gene delivery, its packaging capacity is limited to less than ~4.8 kb of DNA. This has posed a challenge for the delivery of large nucleases such as TALENs, that require two monomers each encoded by cDNAs greater than four kb in size, and the commonly used S. pyogenes Cas9 nuclease that is encoded by a ~4.2 kb cDNA. Trans-splicing vectors have been designed to recombine within cells to expand the size of transgenes delivered by AAV,225 but the efficiency of expression is significantly lower than genes delivered by a single AAV. A number of smaller Cas9 orthologs exist, and the ~3.1 kb Cas9 from S. aureus has been thoroughly characterized and shown to mediate highly efficient gene editing in vivo following AAV delivery.35,36,226,227 This important advance is critical to enabling facile and robust in vivo gene editing with the CRISPR/Cas9 system. It is particularly advantageous for developing a translatable gene therapy product that can be packaged in a single vector.
Gene Therapy Applications
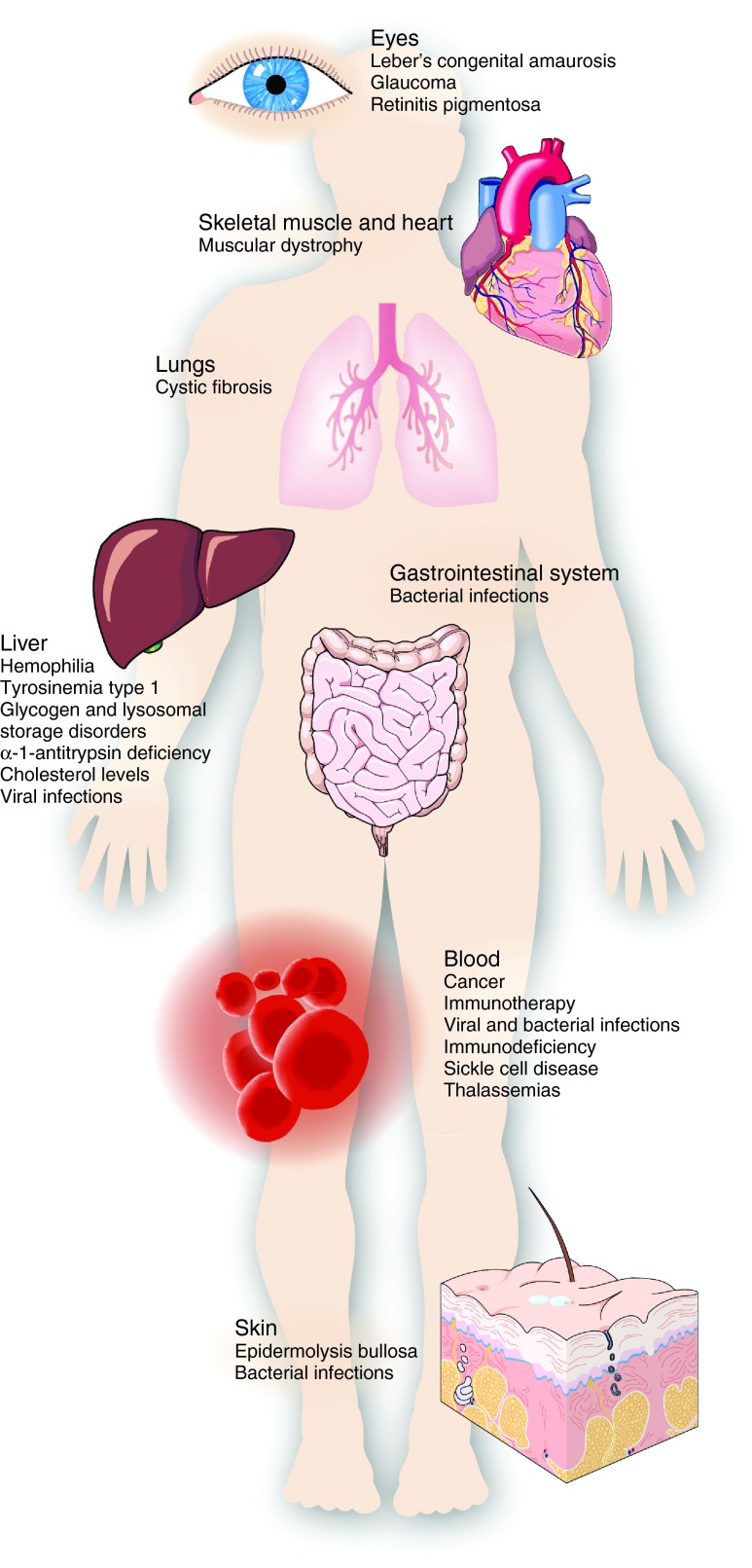
The ability to manipulate any genomic sequence by gene editing has created diverse opportunities to treating many different diseases and disorders (Figure 4). Here, we discuss the major categories of disease indications that have been pursued in preclinical models (Table 1), as well as highlight the ongoing or planned clinical trials using gene-editing strategies (Table 2).
Figure 4.
Diversity of targets for therapeutic genome editing.
Table 1. Representative preclinical studies of gene editing for gene and cell therapy.
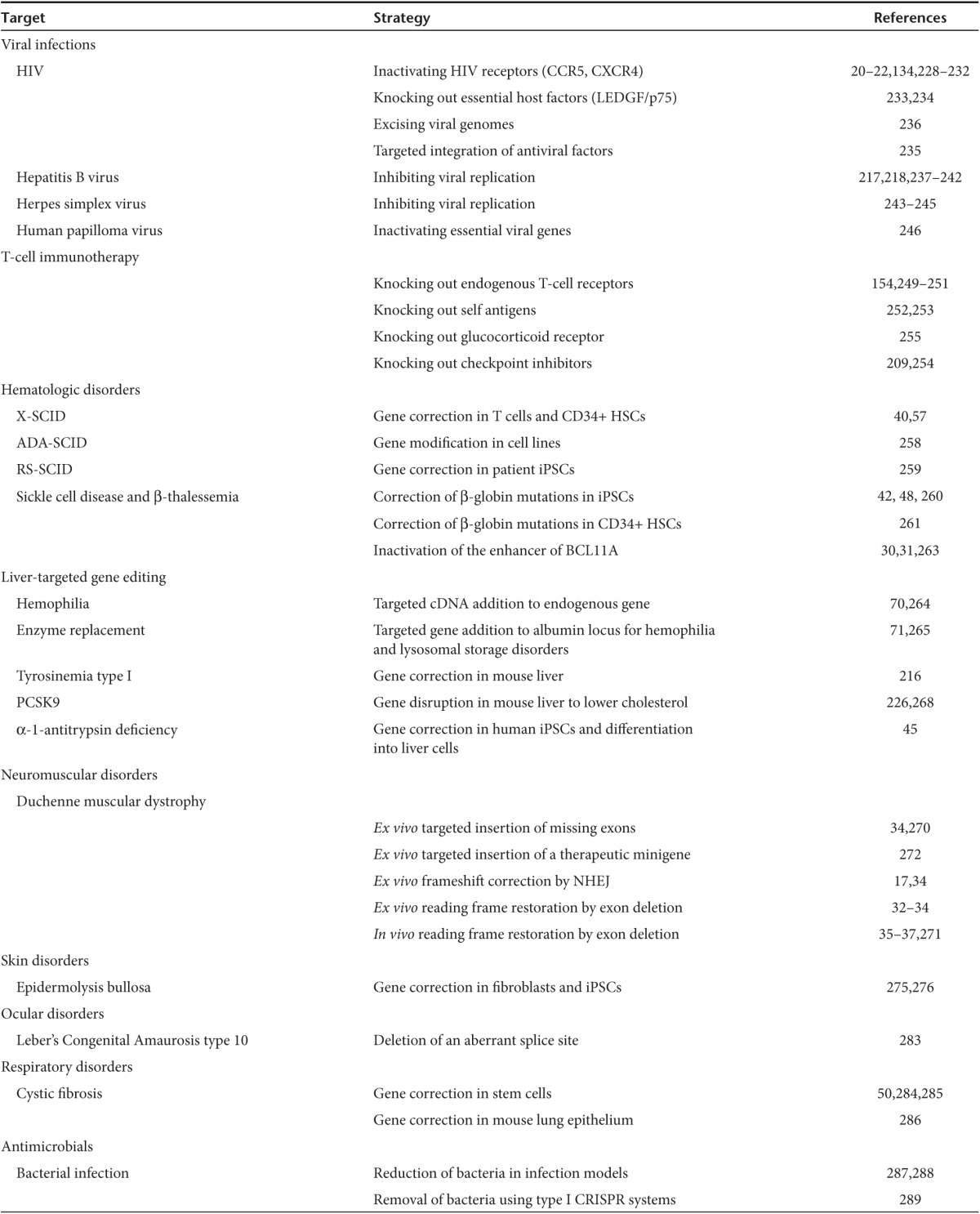
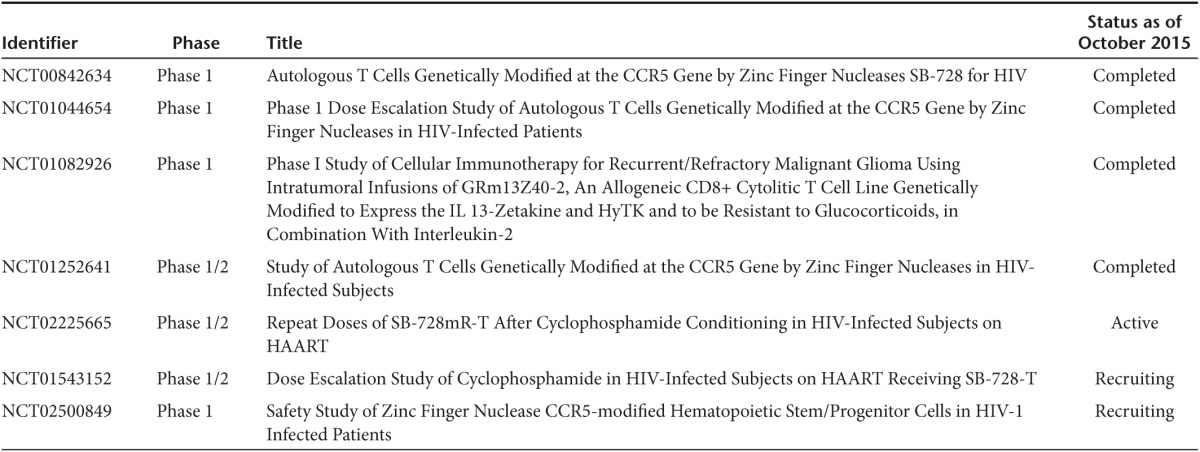
Table 2. Representative ongoing and completed gene-editing clinical trials.
Antiviral strategies
The most straightforward application of gene editing is to use the relatively efficient NHEJ mechanism to knockout genes in an ex vivo autologous cell therapy, where somatic cells can be isolated, modified, and delivered back to the patient. Moreover, one of the most compelling applications of gene editing is the prevention of viral infection or replication. Thus the most advanced gene-editing strategy to date is the ex vivo modification of T cells to knock out the CCR5 coreceptor used for primary HIV infection.20 This early study demonstrated decreased viral loads and increased CD4+ T-cell counts in HIV-infected mice engrafted with T cells in which the CCR5 gene had been knocked out by zinc finger nucleases.20 This was later followed by demonstration of similar results following gene editing and transplantation of CD34+ HSCs into irradiated mice, allowing for protection of all blood cell lineages from CCR5-tropic HIV infection.21,228 These studies have led to a series of clinical trials (Table 2) evaluating this approach in HIV-positive human patients. Thus far the studies show safe engraftment and survival of _CCR5_-modified T cells and control of viral load in some patients, providing promising proof-of-principle of a gene-editing approach in humans.22 Interestingly, data from this study showed a greater clinical efficacy in a patient that was already heterozygous for the naturally-occurring ▵32 mutation, suggesting that gene-editing efficiency may be a critical factor for success.
Building on these promising studies with ZFNs, several other efforts have developed similar gene-editing strategies to knockout CCR5 with TALENs,134,229 CRISPR/Cas9 (refs. 229, 230) and meganucleases.231 Other work has expanded beyond targeting only CCR5 to enhance resistance to HIV infection. This includes targeting the CXCR4 coreceptor232 or PSIP1 gene encoding the LEDGF/p75 protein required for HIV integration.233,234 Some studies have used targeted gene integration into the CCR5 gene by HDR to simultaneously knockout CCR5 and introduce anti-HIV factors.235 Finally, complete excision of the HIV genome from infected cells using nucleases that target sequences in the long terminal repeats (LTRs) flanking the viral genome has also been reported.236 Thus, a variety of next-generation gene-editing strategies for preventing HIV infection and replication are on the horizon.
Beyond addressing HIV infection, all of the gene-editing platforms have also been applied to various other viral pathogens23 including hepatitis B virus,217,218,237,238,239,240,241,242 herpes simplex virus,243,244,245 and human papilloma virus.246 These strategies typically involve removing viral genomes by degradation following nuclease cleavage and by targeting genes essential for genome stability, maintenance, and replication. While many of these early studies focused on proof-of-principle reduction of viral load in cell culture or following hydrodynamic plasmid DNA delivery to mice, recent studies using AAV delivery of gene-editing tools directly to the mouse liver provides a plausible path for scalability and clinical translation.238 A general challenge of antiviral therapies is the high mutability of viral targets. This is a compelling argument in favor of targeting host genes, such as CCR5, but may also be addressed by simultaneous targeting of multiple critical sites in the viral genome.
Cancer immunotherapy
Cancer immunotherapy has been widely recognized as one of the greatest advances in biomedical research in recent years.247 In particular, adoptive T-cell immunotherapy, in which autologous T cells are engineered to attack cancer antigens ex vivo and transferred back to the patient, has been impressively successful at treating some cases of lymphoma, leukemia, and melanoma.248 Despite these successes and promising ongoing clinical trials, there are several areas in which T-cell immunotherapy could be potentially improved by gene editing. Here, both the efficacy against diverse tumor types and the ability to manufacture cell products that can be applied to a broad patient population could be enhanced through gene-editing techniques. For example, a promising strategy for immunotherapy involves engineering T cells to express synthetic receptors known as chimeric antigen receptors, or CARs, that recognize epitopes on cancer cells. Such CAR T cells have been particularly successful in treating B-cell lymphoma by targeting the CD19 cell surface antigen.247,248 However, one limitation of this approach is that these modified T cells express both the endogenous T-cell receptor as well as the engineered CAR. Because these receptors function as dimers, the natural and engineered receptors can dimerize and interact, resulting in unpredictable epitope specificity and potentially reducing therapeutic potency. To address this limitation, several studies have focused on knocking out the endogenous T-cell receptors with engineered nucleases.154,249,250,251
A major challenge to the development of broadly translatable T-cell immunotherapies is the need to use autologous cells to avoid immune rejection. To address this, gene editing has been used to knockout the human leukocyte antigen (HLA) by which the immune system discriminates self and foreign cells.252 Importantly, this approach may be broadly useful for allogeneic cell therapy beyond T-cell immunotherapy. For example, similar approaches have been applied in human pluripotent cells potentially having diverse uses in regenerative medicine252 as well as in endothelial cells that could be used for allogeneic vascular grafts.253
Another major obstacle to successful T-cell immunotherapy is the inhibition of T-cell effector functions by the expression of checkpoint inhibitors on the surface of tumor cells. For example, the binding of such inhibitors to the PD-1 receptor on T cells is well documented to block T-cell effector function and induce apoptosis and exhaustion. PD-1 receptor inhibition thus provides a mechanism by which cancer cells successfully evade the immune system. As a strategy to overcome this, gene editing has been used to knockout PD-1 in T cells,254 leading to increased T-cell effector function.209,254 The success of this gene-editing strategy is likely extendable to other checkpoint inhibitor pathways that cancer cells exploit to circumvent immunosurveillance, and thus may be a critical technology for broadly enabling immunotherapy for diverse cancer types.
Finally, for indications such as glioblastoma, the apoptosis of the engineered T cells resulting from post-surgery anti-inflammatory glucocorticoid steroid treatment severely limits the efficacy of T-cell immunotherapy. In order to create a glucocorticoid-resistant T-cell source, gene editing was used to knockout the endogenous T-cell receptor.255 This led to successful anti-glioma T-cell therapy in mouse models255 and was the basis of a subsequent clinical trial (Table 2).
Hematologic disorders
The first gene therapy clinical trials involved the ex vivo retroviral delivery of a therapeutic adenosine deaminase (ADA) transgene to T cells to treat children with severe combined immunodeficiency (ADA-SCID),256 and later the treatment of X-linked SCID (X-SCID) by retroviral gene delivery to CD34+ hematopoietic stem cells (HSCs).257 This early focus on ex vivo gene therapy for immunodeficiency was based on the desperate need to develop treatment for these otherwise fatal disorders as well as the availability of methods for efficient retroviral gene delivery to cells in culture. The subsequent observation that this early protocol can lead to insertional mutagenesis was a primary catalyst for the gene-editing field and demonstrated the need for correction of gene mutations in these cells, in contrast to transgene delivery.63 In fact, the first example of endogenous gene correction in human cells focused on the IL2 receptor common gamma chain that is mutated in X-SCID,40 and this approach was more recently extended to gene correction in CD34+ HSCs.57 Gene-editing tools have also been developed to correct gene mutations associated with ADA-SCID258 and radiosensitive SCID, caused by impaired DNA-dependent protein kinase (DNA-PK) activity.259 Thus, the ability to efficiently alter gene sequences in T cells, CD34+ HSCs, and human pluripotent cells can provide therapeutic gene-editing strategies for a broad range of different human immunodeficiencies.
Similarly, the establishment of gene editing in CD34+ HSCs and human pluripotent cells capable of differentiating into erythroid progenitors has provided new options for treating other hematologic disorders, including sickle cell disease, caused by a specific E6V point mutation in the β-globin gene, and β-thalassemia, caused by other types of mutations to β-globin. These globin mutations have been corrected by gene editing both in human iPSCs that can be differentiated into functional erythrocytes42,48,260 and directly in CD34+ HSCs.261 Similar approaches have been developed for targeted integration of therapeutic transgenes into safe harbor sites in human iPSCs for α-thalassemia69 and Fanconi anemia.262
Sickle cell disease and β-thalassemia are unique in that deficiencies in β-globin function or expression can be compensated for by inducing upregulation of γ-globin, which is expressed during fetal development but silenced after birth. BCL11A is a transcriptional regulator that suppresses the expression of γ-globin, and thus the knockout of BCL11A has been proposed as an approach to treat both sickle cell disease and β-thalassemia. However, the absence of BCL11A in all hematopoietic lineages was observed to be detrimental in nonerythroid cells. Interestingly, an enhancer element was discovered that specifically coordinates BCL11A in erythroid cells and inactivation of this enhancer by gene editing leads to suppression of BCL11A and upregulation of γ-globin only in cells of the erythroid lineage.30,31,263 Thus, this approach provides both a mechanism of gene-editing therapy for sickle cell disease and β-thalassemia, but more broadly suggests a general strategy of therapeutic modulation of gene expression through the targeted editing of cell type-specific enhancers.
Liver-targeted gene editing
Beyond the ex vivo gene editing of blood and immune cells, there is intense interest in gene editing in vivo for gene correction and targeted gene addition to tissues for which cell transplantation is challenging or impractical. This requires the efficient delivery of gene-editing nucleases and donor vectors to target tissues. The first demonstration of highly efficient, nuclease-mediated gene editing in vivo used AAV vectors to deliver ZFNs and a factor IX cDNA, without a promoter, to the liver of a mouse model of hemophilia B.70 Cleavage of the first intron of a mutated human factor IX gene by ZFNs catalyzed the efficient integration of the factor IX cDNA into the locus, leading to correction of the hemophilic phenotype. This first study was performed in neonates in which the hepatocytes are actively dividing and thus homology-directed repair pathways are active. Notably, a subsequent study demonstrated efficacy in adult mice in which the hepatocytes have presumably exited the cell cycle, although the integrations resulted from a combination of HDR- and NHEJ-mediated events.264 Additional studies are necessary to determine the role of cell cycle and gene editing with AAV and other delivery vectors in various tissue types.
Targeted gene correction in the liver has the potential to treat many different diseases, including clotting disorders such as hemophilia A and hemophilia B, as well as lysosomal storage disorders including Fabry disease, Gaucher disease, Pompe disease, von Gierke disease, and Hurler and Hunter syndromes. However, each of these patient populations is relatively small and the types of mutations to each gene involved in these diseases are diverse. Therefore, the cost of clinical development and regulatory approval to develop safe and efficacious gene-editing tools for each of these diseases may be prohibitive. Moreover, it is unclear whether sufficient levels of targeted transgene integration or gene correction could be achieved to reach therapeutic efficacy if driven by the corresponding natural endogenous promoter for each gene. A clever approach to address each of these challenges is the targeted integration of therapeutic genes into the albumin locus downstream of the endogenous albumin promoter.71,265 Because albumin is very highly expressed, even low levels of targeted gene integration to this site are likely to lead to therapeutic levels of gene expression. Moreover, this genomic “safe harbor” can be used for diverse diseases, including those listed above, such that a single validated gene-editing reagent can be used for a significantly larger patient population. This approach has been used effectively in mouse models with AAV-based homologous donor templates to treat hemophilia without nucleases71 and with ZFNs that are likely to dramatically enhance targeting efficiency.265
The advent of the CRISPR/Cas9 system has made in vivo gene-editing tools more broadly available to the scientific community and thus many recent studies have used this approach for both developing disease models and strategies for gene therapy. The first example of in vivo gene editing with CRISPR/Cas9 involved the correction of a mouse model of hereditary tyrosinemia type I following hydrodynamic tail vein injection of plasmid DNA into mice.216 Although overall gene-editing efficiencies were relatively low (~0.4%), this model allows for selection of corrected cells to repopulate the liver and thus it was possible to demonstrate correction of the disease phenotype. Although the method of naked DNA delivery to the liver is likely not translatable to humans, this study was a landmark in demonstrating in vivo gene editing with CRISPR/Cas9 in adult tissues.
Beyond gene correction, the disruption of particular genes in the liver may also have a beneficial effect. For example, the PCSK9 gene encodes a proteinase that induces degradation of the low density lipoprotein receptor (LDLR). Decreased LDLR levels lead to lower metabolism of LDL cholesterol (LDL-C), increased LDL-C levels, and increased risk for cardiovascular disease. The discovery of natural genetic variation leading to high or low PCSK9 activity and corresponding cholesterol levels has led to intense interest in PCSK9-blocking drugs for lowering cholesterol.266,267 In contrast to continuous drug administration, two different studies have shown that a single treatment of Cas9 and a PCSK9-targeted gRNA delivered to the liver can lead to efficient gene knockout and lowered cholesterol levels.226,268
Finally, in addition to gene editing in the liver, new methods for culture and differentiation of human pluripotent cells into functional hepatocytes are providing options for ex vivo cell correction and engraftment into the liver. For example, α-1-antitrypsin mutations were seamlessly corrected by gene editing in human iPSCs and subsequently differentiated into liver cells that expressed the restored gene.45 Although this type of cell-based product may be significantly more complex than a virus-based drug, the strategy described in this study allows for a comprehensive genomic analysis of the modified cells.
Neuromuscular disorders
Advances in gene delivery and cell transplantation to the central nervous system and skeletal and cardiac muscle have created new opportunities for gene and cell therapy for many neuromuscular disorders, including DMD, the limb girdle muscular dystrophies, spinal muscular atrophy, Friedreich's ataxia, Huntington's disease, and amyotrophic lateral sclerosis (ALS). Amongst this class of diseases, genome editing has thus far advanced most prominently for DMD, although possible strategies to apply genome editing to other conditions could be envisioned. DMD is caused by mutations to the dystrophin gene, most commonly large deletions that shift the downstream gene fragment out of frame and render the protein product nonfunctional. Because the coding sequence of the dystrophin gene is exceptionally large (14 kb), it cannot be packaged into size-restricted viral delivery vectors. Although truncated minigenes have been developed that do fit into viral vectors, they are only partially functional compared to the full-length gene and their ability to reverse the human disease remains to be determined. For these reasons, and because there is currently no available approved therapy for DMD, gene editing to repair the endogenous gene is particularly compelling. Early reports suggested a mechanism to repair the dystrophin gene with gene editing,269 which was followed by proof-of-principle experiments in cultured cells from DMD patients demonstrating dystrophin gene repair by targeted integration of the deleted exons270 or restoration of dystrophin protein expression by targeted shifting of the reading frame by NHEJ-mediated indels.17 However, these two strategies suffer from addressing only a limited patient population with any particular gene-editing strategy or lacking predictable and reliable editing outcomes due to the reliance on stochastic NHEJ-mediated DNA repair, respectively. Therefore, more recent studies have focused on deleting one or more exons with a combination of nucleases to generate precisely restored protein products and address larger fractions of the DMD patient population.32,33,34 This includes a single strategy of deleting >300 kb of genomic DNA comprising exons 45–55 that could be applicable to restoring dystrophin expression in 62% of DMD patients.33 In order to develop this into an approach that could potentially be applied clinically to DMD patients, recent work has incorporated the CRISPR/Cas9 system into AAV vectors with tropism for skeletal and cardiac muscle.35,36,37 When applied locally via intramuscular injection or systemically via intravenous injection to a mouse model of DMD, gene editing by CRISPR/Cas9 restored expression of the dystrophin protein and improved muscle pathology and strength. Notably, one study showed relatively efficient in vivo gene editing of Pax7-positive muscle progenitor cells that may act as a renewable source of cells in which the dystrophin gene has been repaired.36 This translational approach builds on demonstration of in vivo gene editing in skeletal muscle with adenoviral delivery271 and correction of dystrophin mutations in single-cell mouse embryos41 to reverse disease symptoms. In the future, these efforts may be extended to cell therapies by using patient-derived cell types, such as iPS cells, that could be modified by gene correction or targeted dystrophin transgene insertion272 and expanded to large numbers and efficiently engrafted into muscle tissue.34,273
Skin disorders
The development of engineered skin grafts from autologous and allogeneic cells, including iPS cells, is creating new opportunities for treating genetic diseases that affect the skin. For example, recessive dystrophic epidermolysis bullosa is a disease caused by mutations to the gene encoding type VII collagen. This disruption of type VII collagen expression results in extensive skin blistering. This may be treatable by correcting patient cells with genome editing and using those cells to engineer autologous skin grafts.274 In one study, the mutations to the type VII collagen gene were corrected in primary patient fibroblasts that were then reprogrammed to iPS cells which could be used to form skin structures in vivo.275 Another study also corrected the disease-causing mutation in patient iPS cells, and used these cells to generate epithelial keratinocyte sheets, resulting in stratified epidermis in vitro in organotypic cultures and in vivo in mice.276
Ocular disorders
Recent successes in clinical trials for the treatment of Leber Congenital Amaurosis type 2 (LCA2) have propelled retinal disorders into the spotlight of the gene therapy field. Using a gene augmentation approach, subretinal injection of AAV encoding the full RPE65 gene was found to be both safe and efficacious in several concurrent trials.277,278,279,280 LCA is the leading cause of childhood blindness and is caused by mutations in at least 18 different genes.281 LCA10, the most common form of LCA, is caused by mutations in the approximately 7.5kb CEP290 gene, and is therefore not amenable to the standard gene therapy approach employed for LCA2 due to the large size of the disease-causing gene. While an in vitro proof-of-concept study used lentivirus to deliver the full transgene to iPSC-derived photoreceptor precursor cells,282 the proven safety of subretinal AAV delivery makes a gene-editing strategy, in which the nuclease components are delivered via AAV, particularly attractive. As proof-of-principle of gene editing for this disease, S. aureus Cas9 was used to delete an intronic region in the CEP290 gene containing a frequent mutation that creates an aberrant splice site which disrupts the gene coding sequence.283 Deletion of this intronic region restored proper CEP290 expression. Gene editing is also uniquely positioned to address autosomal dominant disorders, such as forms of primary open angle glaucoma and retinitis pigmentosa, which could potentially be treated by targeted knockout of the MYOC and RHO genes, respectively.
Respiratory disorders
Cystic fibrosis is caused by mutations to the CFTR chloride channel. Loss of function of this chloride channel results in dysregulation of epithelial fluid transport in several organs. In particular, loss of proper fluid transport in the lung results in thickening of the mucus and thus frequent infection and complications breathing. Gene editing has been used to repair the CFTR mutations in cultured patient intestinal stem cells50 and iPS cells that could be subsequently differentiated into epithelial cells.284,285 Although a long-standing challenge for gene therapy and gene editing for cystic fibrosis has been achieving efficient gene delivery to the lung epithelium, a recent study demonstrating functional correction of mice with cystic fibrosis following intranasal delivery of nanoparticles carrying triplex-forming peptide nucleic acid molecules is a very promising advance in this regard.286
Antimicrobials
Beyond altering genes in the human genome, there are a variety of ways in which genome editing can be used to address human disease and improve human health by targeting the genomes of other organisms. A primary example of this is the recent application of genome editing to attack pathogenic bacterial infections.24 For example, gene-editing nucleases can be designed to target genes conferring virulence or antibiotic resistance. Additionally, targeting genome sequences specific to pathogenic strains may facilitate their selective removal from a mixed population. Two recent studies demonstrated proof-of-principle of this approach using the CRISPR/Cas9 system to eliminate bacteria in a mouse skin colonization model287 and a moth larvae infection model,288 as well as selectively eliminating plasmids and bacterial populations. An intriguing alternative strategy to delivering complete CRISPR systems is to turn native CRISPR systems against themselves by delivery of self-targeted crRNAs, as was recently done to selectively remove bacterial strains with type I CRISPR systems.289 The choice of type I systems is notable given that the Cas3 enzyme of type I systems has exonuclease activity that may facilitate DNA disruption and removal,290 in contrast to the type II CRISPR/Cas9 system which only cuts DNA. Furthermore, the majority of native CRISPR systems are type I, with only a minor fraction belonging to the type II category.291 As with all gene therapies, a primary challenge in moving these platforms forward is the development of a suitable delivery vehicle for clinical translation, and there is promising work in the area of bacteriophage engineering for this purpose.292 Collectively, these studies suggest a possible approach to address the rising incidence of antibiotic resistance.
Conclusion and Future Directions
Tremendous progress has been made in addressing the challenges of conventional gene therapy by developing new technologies for precise modification of the human genome. This has helped to overcome some of the obstacles that have plagued the field of gene therapy for decades. Nevertheless, many challenges still remain to fully realize the potential of genome editing for gene and cell therapy. Central to these challenges are the persistent issues of safety and delivery. In this regard, rapid advances are being made both for increasing the specificity of genome-editing tools and increasing the sensitivity of methods for assessing this specificity genome-wide.189,190,195,203,204,205,206 However, it remains unclear whether all off-target effects can be accounted for in a therapy that targets one site within billions of DNA base pairs, involves modification of millions of cells, and is custom prepared for each patient. Moreover, many questions remain about how the human immune system will respond to genetically modified cells or the in vivo administration of genome-editing tools. Remarkable advances in delivery technologies are also creating many more opportunities for genome editing, including ex vivo delivery to cells with DNA-free components207,208,209,210,212 and in vivo delivery with efficient and tissue-specific vectors.220 The many successes of the preclinical studies reviewed here, as well as the current progression of genome editing in clinical trials, is a source of significant optimism for the future of this field.
The rapid progress in the field is likely to continue to lead to new technologies that will expand the scope of genome editing. Alternative genome-editing technologies, such as targetable site-specific recombinases293 that do not rely on the creation of double-strand breaks, alternative CRISPR systems with unique properties,178,179 and DNA-guided nuclease systems294 will continue to change what is possible with these tools. Epigenome editing, in which DNA-targeting platforms are used to specifically change gene regulation or chromatin structure, is also creating new ways to manipulate the genome for gene and cell therapy.295,296,297 Inducible or self-regulating systems that enable the control of the expression, activity, and/or stability of genome-editing tools may play an important role in ensuring their precision and safety. In summary, genome editing has changed the definition of gene and cell therapy and has been a key factor in the recent resurgence of this field, but there is still significant fundamental and translational work to realize the full promise of these technologies for widely treating human disease.
Acknowledgments
This work has been supported by the Muscular Dystrophy Association (MDA277360), the US Army Medical Research and Materiel Command (MD140071), a Duke-Coulter Translational Partnership Grant, a Duke/UNC-Chapel Hill CTSA Consortium Collaborative Translational Research Award, a Hartwell Foundation Individual Biomedical Research Award, a March of Dimes Foundation Basil O'Connor Starter Scholar Award, and a National Institutes of Health Director's New Innovator Award (DP2-OD008586) to C.A.G. C.A.G. has filed patent applications related to genome editing for correcting genetic diseases. C.A.G. is an advisor to Editas Medicine and M.L.M. is an employee of Editas Medicine, a company engaged in development of therapeutic genome editing.
References
- Friedmann, T and Roblin, R (1972). Gene therapy for human genetic disease? Science 175: 949–955. [DOI] [PubMed] [Google Scholar]
- Naldini, L (2015). Gene therapy returns to centre stage. Nature 526: 351–360. [DOI] [PubMed] [Google Scholar]
- Baum, C, von Kalle, C, Staal, FJ, Li, Z, Fehse, B, Schmidt, M et al. (2004). Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol Ther 9: 5–13. [DOI] [PubMed] [Google Scholar]
- Takata, M, Sasaki, MS, Sonoda, E, Morrison, C, Hashimoto, M, Utsumi, H et al. (1998). Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J 17: 5497–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak, JW, Orr-Weaver, TL, Rothstein, RJ and Stahl, FW (1983). The double-strand-break repair model for recombination. Cell 33: 25–35. [DOI] [PubMed] [Google Scholar]
- Rouet, P, Smih, F and Jasin, M (1994). Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci USA 91: 6064–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smih, F, Rouet, P, Romanienko, PJ and Jasin, M (1995). Double-strand breaks at the target locus stimulate gene targeting in embryonic stem cells. Nucleic Acids Res 23: 5012–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choulika, A, Perrin, A, Dujon, B and Nicolas, JF (1995). Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol 15: 1968–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber, MR, Ma, Y, Pannicke, U and Schwarz, K (2003). Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol 4: 712–720. [DOI] [PubMed] [Google Scholar]
- Bibikova, M, Golic, M, Golic, KG and Carroll, D (2002). Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago, Y, Chan, E, Liu, PQ, Orlando, S, Zhang, L, Urnov, FD et al. (2008). Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc Natl Acad Sci USA 105: 5809–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts, AM, Cost, GJ, Freyvert, Y, Zeitler, B, Miller, JC, Choi, VM et al. (2009). Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon, Y, McCammon, JM, Miller, JC, Faraji, F, Ngo, C, Katibah, GE et al. (2008). Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol 26: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, X, Noyes, MB, Zhu, LJ, Lawson, ND and Wolfe, SA (2008). Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol 26: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia, A and Elledge, SJ (2010). The DNA damage response: making it safe to play with knives. Mol Cell 40: 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, JR, Taylor, MR and Boulton, SJ (2012). Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47: 497–510. [DOI] [PubMed] [Google Scholar]
- Ousterout, DG, Perez-Pinera, P, Thakore, PI, Kabadi, AM, Brown, MT, Qin, X et al. (2013). Reading frame correction by targeted genome editing restores dystrophin expression in cells from Duchenne muscular dystrophy patients. Mol Ther 21: 1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronin, N and DiFiglia, M (2014). Huntingtin-lowering strategies in Huntington's disease: antisense oligonucleotides, small RNAs, and gene editing. Mov Disord 29: 1455–1461. [DOI] [PubMed] [Google Scholar]
- Ramaswamy, S and Kordower, JH (2012). Gene therapy for Huntington's disease. Neurobiol Dis 48: 243–254. [DOI] [PubMed] [Google Scholar]
- Perez, EE, Wang, J, Miller, JC, Jouvenot, Y, Kim, KA, Liu, O et al. (2008). Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol 26: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, N, Wang, J, Kim, K, Friedman, G, Wang, X, Taupin, V et al. (2010). Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol 28: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas, P, Stein, D, Tang, WW, Frank, I, Wang, SQ, Lee, G et al. (2014). Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, EM and Cullen, BR (2015). Bacterial CRISPR/Cas DNA endonucleases: A revolutionary technology that could dramatically impact viral research and treatment. Virology 479–480: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel, CL, Gomaa, AA and Barrangou, R (2014). A CRISPR design for next-generation antimicrobials. Genome Biol 15: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, HJ, Kim, E and Kim, JS (2010). Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res 20: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A, Hall, VL, Kok, FO, Shin, M, McNulty, JC, Lawson, ND et al. (2013). Targeted chromosomal deletions and inversions in zebrafish. Genome Res 23: 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, A, Wang, Z, Hu, Y, Wu, Y, Luo, Z, Yang, Z et al. (2013). Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res 41: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canver, MC, Bauer, DE, Dass, A, Yien, YY, Chung, J, Masuda, T et al. (2014). Characterization of genomic deletion efficiency mediated by clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 nuclease system in mammalian cells. J Biol Chem 289: 21312–21324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllü, C, Pars, K, Cornu, TI, Thibodeau-Beganny, S, Maeder, ML, Joung, JK et al. (2010). Autonomous zinc-finger nuclease pairs for targeted chromosomal deletion. Nucleic Acids Res 38: 8269–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, DE, Kamran, SC, Lessard, S, Xu, J, Fujiwara, Y, Lin, C et al. (2013). An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 342: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canver, MC, Smith, EC, Sher, F, Pinello, L, Sanjana, NE, Shalem, O et al. (2015). BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 527: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousterout, DG, Kabadi, AM, Thakore, PI, Perez-Pinera, P, Brown, MT, Majoros, WH et al. (2015). Correction of dystrophin expression in cells from Duchenne muscular dystrophy patients through genomic excision of exon 51 by zinc finger nucleases. Mol Ther 23: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousterout, DG, Kabadi, AM, Thakore, PI, Majoros, WH, Reddy, TE and Gersbach, CA (2015). Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat Commun 6: 6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, HL, Fujimoto, N, Sasakawa, N, Shirai, S, Ohkame, T, Sakuma, T et al. (2015). Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports 4: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, CE, Hakim, CH, Ousterout, DG, Thakore, PI, Moreb, EA, Rivera, RM et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabebordbar, M, Zhu, K, Cheng, JK, Chew, WL, Widrick, JJ, Yan, WX et al. (2016). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C, Amoasii, L, Mireault, AA, McAnally, JR, Li, H, Sanchez-Ortiz, E et al. (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351: 400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer, WD, Ehmsen, KT and Liu, J (2010). Regulation of homologous recombination in eukaryotes. Annu Rev Genet 44: 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, KR and Capecchi, MR (1987). Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51: 503–512. [DOI] [PubMed] [Google Scholar]
- Urnov, FD, Miller, JC, Lee, YL, Beausejour, CM, Rock, JM, Augustus, S et al. (2005). Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435: 646–651. [DOI] [PubMed] [Google Scholar]
- Long, C, McAnally, JR, Shelton, JM, Mireault, AA, Bassel-Duby, R and Olson, EN (2014). Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345: 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiano, V, Maeder, ML, Angstman, JF, Haddad, B, Khayter, C, Yeo, DT et al. (2011). In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells 29: 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, J, Mali, P, Huang, X, Dowey, SN and Cheng, L (2011). Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood 118: 4599–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner, F, Laganière, J, Cheng, AW, Hockemeyer, D, Gao, Q, Alagappan, R et al. (2011). Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146: 318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa, K, Rashid, ST, Strick-Marchand, H, Varela, I, Liu, PQ, Paschon, DE et al. (2011). Targeted gene correction of α1-antitrypsin deficiency in induced pluripotent stem cells. Nature 478: 391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, BE, Krebs, MP, Joung, JK, Tsai, SQ, Nishina, PM and Wiles, MV (2014). Correction of the Crb1rd8 allele and retinal phenotype in C57BL/6N mice via TALEN-mediated homology-directed repair. Invest Ophthalmol Vis Sci 55: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, LH, Laganière, J, Cooper, O, Mak, SK, Vu, BJ, Huang, YA et al. (2014). LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: reversal by gene correction. Neurobiol Dis 62: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, F, Ye, L, Chang, JC, Beyer, AI, Wang, J, Muench, MO et al. (2014). Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res 24: 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y, Liang, D, Wang, Y, Bai, M, Tang, W, Bao, S et al. (2013). Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell 13: 659–662. [DOI] [PubMed] [Google Scholar]
- Schwank, G, Koo, BK, Sasselli, V, Dekkers, JF, Heo, I, Demircan, T et al. (2013). Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13: 653–658. [DOI] [PubMed] [Google Scholar]
- Chen, F, Pruett-Miller, SM, Huang, Y, Gjoka, M, Duda, K, Taunton, J et al. (2011). High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat Methods 8: 753–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell, VM, Wang, Y, Campbell, JM, Poshusta, TL, Starker, CG, Krug, RG 2nd et al. (2012). In vivo genome editing using a high-efficiency TALEN system. Nature 491: 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Q, Lee, YK, Schaefer, EA, Peters, DT, Veres, A, Kim, K et al. (2013). A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 12: 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Händel, EM, Gellhaus, K, Khan, K, Bednarski, C, Cornu, TI, Müller-Lerch, F et al. (2012). Versatile and efficient genome editing in human cells by combining zinc-finger nucleases with adeno-associated viral vectors. Hum Gene Ther 23: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccio, A, Miselli, F, Lombardo, A, Marconi, A, Malagoli Tagliazucchi, G, Gonçalves, MA et al. (2013). Targeted gene addition in human epithelial stem cells by zinc-finger nuclease-mediated homologous recombination. Mol Ther 21: 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, ML, Green, L, Porteus, MH and Samulski, RJ (2010). Self-complementary AAV mediates gene targeting and enhances endonuclease delivery for double-strand break repair. Gene Ther 17: 1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese, P, Schiroli, G, Escobar, G, Di Tomaso, T, Firrito, C, Calabria, A et al. (2014). Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, DG, Wang, PR, Petek, LM, Hirata, RK, Sands, MS and Russell, DW (2006). Gene targeting in vivo by adeno-associated virus vectors. Nat Biotechnol 24: 1022–1026. [DOI] [PubMed] [Google Scholar]
- Grimm, D, Lee, JS, Wang, L, Desai, T, Akache, B, Storm, TA et al. (2008). In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol 82: 5887–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo, SP, Lisowski, L, Bashkirova, E, Zhen, HH, Chu, K, Keene, DR et al. (2014). Somatic correction of junctional epidermolysis bullosa by a highly recombinogenic AAV variant. Mol Ther 22: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus, MH, Cathomen, T, Weitzman, MD and Baltimore, D (2003). Efficient gene targeting mediated by adeno-associated virus and DNA double-strand breaks. Mol Cell Biol 23: 3558–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuri, P, Bartel, MA, Vazin, T, Jang, JH, Wong, TB and Schaffer, DV (2012). Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Mol Ther 20: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina, S, Von Kalle, C, Schmidt, M, McCormack, MP, Wulffraat, N, Leboulch, P et al. (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302: 415–419. [DOI] [PubMed] [Google Scholar]
- Baum, C, Kustikova, O, Modlich, U, Li, Z and Fehse, B (2006). Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther 17: 253–263. [DOI] [PubMed] [Google Scholar]
- Baum, C, Modlich, U, Göhring, G and Schlegelberger, B (2011). Concise review: managing genotoxicity in the therapeutic modification of stem cells. Stem Cells 29: 1479–1484. [DOI] [PubMed] [Google Scholar]
- Moehle, EA, Moehle, EA, Rock, JM, Rock, JM, Lee, YL, Lee, YL et al. (2007). Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc Natl Acad Sci USA 104: 3055–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo, A, Cesana, D, Genovese, P, Di Stefano, B, Provasi, E, Colombo, DF et al. (2011). Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat Methods 8: 861–869. [DOI] [PubMed] [Google Scholar]
- Zou, J, Sweeney, CL, Chou, BK, Choi, U, Pan, J, Wang, H et al. (2011). Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood 117: 5561–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, CJ and Bouhassira, EE (2012). Zinc-finger nuclease-mediated correction of α-thalassemia in iPS cells. Blood 120: 3906–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H, Haurigot, V, Doyon, Y, Li, T, Wong, SY, Bhagwat, AS et al. (2011). In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 475: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzel, A, Paulk, NK, Shi, Y, Huang, Y, Chu, K, Zhang, F et al. (2015). Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature 517: 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca, M, Lin, VG, Guo, N and Yang, Y (2013). Obligate ligation-gated recombination (ObLiGaRe): custom-designed nuclease-mediated targeted integration through nonhomologous end joining. Genome Res 23: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler, R, Perini, G and Green, MR (2001). Expressing the human genome. Nature 409: 832–833. [DOI] [PubMed] [Google Scholar]
- Pavletich, NP and Pabo, CO (1991). Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science 252: 809–817. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson, M, Rould, MA, Nekludova, L and Pabo, CO (1996). Zif268 protein-DNA complex refined at 1.6 A: a model system for understanding zinc finger-DNA interactions. Structure 4: 1171–1180. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson, M, Benson, TE and Pabo, CO (1998). High-resolution structures of variant Zif268-DNA complexes: implications for understanding zinc finger-DNA recognition. Structure 6: 451–464. [DOI] [PubMed] [Google Scholar]
- Lee, MS, Gippert, GP, Soman, KV, Case, DA and Wright, PE (1989). Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science 245: 635–637. [DOI] [PubMed] [Google Scholar]
- Pabo, CO, Peisach, E and Grant, RA (2001). Design and selection of novel Cys2His2 zinc finger proteins. Annu Rev Biochem 70: 313–340. [DOI] [PubMed] [Google Scholar]
- Gersbach, CA, Gaj, T and Barbas, CF 3rd (2014). Synthetic zinc finger proteins: the advent of targeted gene regulation and genome modification technologies. Acc Chem Res 47: 2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjarlais, JR and Berg, JM (1992). Toward rules relating zinc finger protein sequences and DNA binding site preferences. Proc Natl Acad Sci USA 89: 7345–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjarlais, JR and Berg, JM (1992). Redesigning the DNA-binding specificity of a zinc finger protein: a data base-guided approach. Proteins 12: 101–104. [DOI] [PubMed] [Google Scholar]
- Rebar, EJ and Pabo, CO (1994). Zinc finger phage: affinity selection of fingers with new DNA-binding specificities. Science 263: 671–673. [DOI] [PubMed] [Google Scholar]
- Choo, Y and Klug, A (1994). Toward a code for the interactions of zinc fingers with DNA: selection of randomized fingers displayed on phage. Proc Natl Acad Sci USA 91: 11163–11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson, AC, Kim, SH and Wells, JA (1994). In vitro selection of zinc fingers with altered DNA-binding specificity. Biochemistry 33: 5689–5695. [DOI] [PubMed] [Google Scholar]
- Wu, H, Yang, WP and Barbas, CF 3rd (1995). Building zinc fingers by selection: toward a therapeutic application. Proc Natl Acad Sci USA 92: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung, JK, Ramm, EI and Pabo, CO (2000). A bacterial two-hybrid selection system for studying protein-DNA and protein-protein interactions. Proc Natl Acad Sci USA 97: 7382–7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancafort, P, Magnenat, L and Barbas, CF 3rd (2003). Scanning the human genome with combinatorial transcription factor libraries. Nat Biotechnol 21: 269–274. [DOI] [PubMed] [Google Scholar]
- Bae, KH, Kwon, YD, Shin, HC, Hwang, MS, Ryu, EH, Park, KS et al. (2003). Human zinc fingers as building blocks in the construction of artificial transcription factors. Nat Biotechnol 21: 275–280. [DOI] [PubMed] [Google Scholar]
- Segal, DJ, Dreier, B, Beerli, RR and Barbas, CF 3rd (1999). Toward controlling gene expression at will: selection and design of zinc finger domains recognizing each of the 5'-GNN-3' DNA target sequences. Proc Natl Acad Sci USA 96: 2758–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier, B, Beerli, RR, Segal, DJ, Flippin, JD and Barbas, CF 3rd (2001). Development of zinc finger domains for recognition of the 5'-ANN-3' family of DNA sequences and their use in the construction of artificial transcription factors. J Biol Chem 276: 29466–29478. [DOI] [PubMed] [Google Scholar]
- Dreier, B, Fuller, RP, Segal, DJ, Lund, CV, Blancafort, P, Huber, A et al. (2005). Development of zinc finger domains for recognition of the 5'-CNN-3' family DNA sequences and their use in the construction of artificial transcription factors. J Biol Chem 280: 35588–35597. [DOI] [PubMed] [Google Scholar]
- Liu, Q, Xia, Z, Zhong, X and Case, CC (2002). Validated zinc finger protein designs for all 16 GNN DNA triplet targets. J Biol Chem 277: 3850–3856. [DOI] [PubMed] [Google Scholar]
- Beerli, RR, Segal, DJ, Dreier, B and Barbas, CF 3rd (1998). Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc Natl Acad Sci USA 95: 14628–14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerli, RR, Dreier, B and Barbas, CF 3rd (2000). Positive and negative regulation of endogenous genes by designed transcription factors. Proc Natl Acad Sci USA 97: 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S, Lee, MJ, Kim, H, Kang, M and Kim, JS (2011). Preassembled zinc-finger arrays for rapid construction of ZFNs. Nat Methods 8: 7. [DOI] [PubMed] [Google Scholar]
- Bhakta, MS, Henry, IM, Ousterout, DG, Das, KT, Lockwood, SH, Meckler, JF et al. (2013). Highly active zinc-finger nucleases by extended modular assembly. Genome Res 23: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, B, Schwimmer, LJ, Fuller, RP, Ye, Y, Asawapornmongkol, L and Barbas, CF 3rd (2010). Modular system for the construction of zinc-finger libraries and proteins. Nat Protoc 5: 791–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder, ML, Thibodeau-Beganny, S, Osiak, A, Wright, DA, Anthony, RM, Eichtinger, M et al. (2008). Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell 31: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, SA, Greisman, HA, Ramm, EI and Pabo, CO (1999). Analysis of zinc fingers optimized via phage display: evaluating the utility of a recognition code. J Mol Biol 285: 1917–1934. [DOI] [PubMed] [Google Scholar]
- Isalan, M, Choo, Y and Klug, A (1997). Synergy between adjacent zinc fingers in sequence-specific DNA recognition. Proc Natl Acad Sci USA 94: 5617–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isalan, M, Klug, A and Choo, Y (1998). Comprehensive DNA recognition through concerted interactions from adjacent zinc fingers. Biochemistry 37: 12026–12033. [DOI] [PubMed] [Google Scholar]
- Sander, JD, Dahlborg, EJ, Goodwin, MJ, Cade, L, Zhang, F, Cifuentes, D et al. (2011). Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat Methods 8: 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A, Christensen, RG, Rayla, AL, Lakshmanan, A, Stormo, GD and Wolfe, SA (2012). An optimized two-finger archive for ZFN-mediated gene targeting. Nat Methods 9: 588–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isalan, M, Klug, A and Choo, Y (2001). A rapid, generally applicable method to engineer zinc fingers illustrated by targeting the HIV-1 promoter. Nat Biotechnol 19: 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L, Wu, LP and Chandrasegaran, S (1992). Functional domains in Fok I restriction endonuclease. Proc Natl Acad Sci USA 89: 4275–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, YG and Chandrasegaran, S (1994). Chimeric restriction endonuclease. Proc Natl Acad Sci USA 91: 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, YG, Cha, J and Chandrasegaran, S (1996). Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA 93: 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J, Bibikova, M, Whitby, FG, Reddy, AR, Chandrasegaran, S and Carroll, D (2000). Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains. Nucleic Acids Res 28: 3361–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova, M, Carroll, D, Segal, DJ, Trautman, JK, Smith, J, Kim, YG et al. (2001). Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol 21: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus, MH and Baltimore, D (2003). Chimeric nucleases stimulate gene targeting in human cells. Science 300: 763. [DOI] [PubMed] [Google Scholar]
- Lombardo, A, Genovese, P, Beausejour, CM, Colleoni, S, Lee, YL, Kim, KA et al. (2007). Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol 25: 1298–1306. [DOI] [PubMed] [Google Scholar]
- Zou, J, Maeder, ML, Mali, P, Pruett-Miller, SM, Thibodeau-Beganny, S, Chou, BK et al. (2009). Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell 5: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer, D, Soldner, F, Beard, C, Gao, Q, Mitalipova, M, DeKelver, RC et al. (2009). Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol 27: 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch, J, Scholze, H, Schornack, S, Landgraf, A, Hahn, S, Kay, S et al. (2009). Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326: 1509–1512. [DOI] [PubMed] [Google Scholar]
- Moscou, MJ and Bogdanove, AJ (2009). A simple cipher governs DNA recognition by TAL effectors. Science 326: 1501. [DOI] [PubMed] [Google Scholar]
- Deng, D, Yan, C, Pan, X, Mahfouz, M, Wang, J, Zhu, JK et al. (2012). Structural basis for sequence-specific recognition of DNA by TAL effectors. Science 335: 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, AN, Bradley, P, Cernadas, RA, Bogdanove, AJ and Stoddard, BL (2012). The crystal structure of TAL effector PthXo1 bound to its DNA target. Science 335: 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbitzer, R, Römer, P, Boch, J and Lahaye, T (2010). Regulation of selected genome loci using de novo-engineered transcription activator-like effector (TALE)-type transcription factors. Proc Natl Acad Sci USA 107: 21617–21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, JC, Tan, S, Qiao, G, Barlow, KA, Wang, J, Xia, DF et al. (2011). A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29: 143–148. [DOI] [PubMed] [Google Scholar]
- Zhang, F, Cong, L, Lodato, S, Kosuri, S, Church, GM and Arlotta, P (2011). Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol 29: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, M, Cermak, T, Doyle, EL, Schmidt, C, Zhang, F, Hummel, A et al. (2010). Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T, Huang, S, Jiang, WZ, Wright, D, Spalding, MH, Weeks, DP et al. (2011). TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain. Nucleic Acids Res 39: 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, JD, Cade, L, Khayter, C, Reyon, D, Peterson, RT, Joung, JK et al. (2011). Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol 29: 697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, P, Xiao, A, Zhou, M, Zhu, Z, Lin, S and Zhang, B (2011). Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol 29: 699–700. [DOI] [PubMed] [Google Scholar]
- Morbitzer, R, Elsaesser, J, Hausner, J and Lahaye, T (2011). Assembly of custom TALE-type DNA binding domains by modular cloning. Nucleic Acids Res 39: 5790–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak, T, Doyle, EL, Christian, M, Wang, L, Zhang, Y, Schmidt, C et al. (2011). Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L, Piatek, MJ, Atef, A, Piatek, A, Wibowo, A, Fang, X et al. (2012). Rapid and highly efficient construction of TALE-based transcriptional regulators and nucleases for genome modification. Plant Mol Biol 78: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, E, Gruetzner, R, Werner, S, Engler, C and Marillonnet, S (2011). Assembly of designer TAL effectors by Golden Gate cloning. PLoS One 6: e19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana, NE, Cong, L, Zhou, Y, Cunniff, MM, Feng, G and Zhang, F (2012). A transcription activator-like effector toolbox for genome engineering. Nat Protoc 7: 171–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, AW, Rios, X, Chari, R, Yang, L, Zhang, F, Mali, P et al. (2012). Iterative capped assembly: rapid and scalable synthesis of repeat-module DNA such as TAL effectors from individual monomers. Nucleic Acids Res 40: e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z, Li, J, Huang, H, Wang, G, Jiang, M, Yin, S et al. (2012). An integrated chip for the high-throughput synthesis of transcription activator-like effectors. Angew Chem Int Ed Engl 51: 8505–8508. [DOI] [PubMed] [Google Scholar]
- Reyon, D, Tsai, SQ, Khayter, C, Foden, JA, Sander, JD and Joung, JK (2012). FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol 30: 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Burgk, JL, Schmidt, T, Kaiser, V, Höning, K and Hornung, V (2013). A ligation-independent cloning technique for high-throughput assembly of transcription activator–like effector genes. Nat Biotechnol 31: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino, C, Morbitzer, R, Lütge, F, Dannemann, N, Lahaye, T and Cathomen, T (2011). A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res 39: 9283–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T, Huang, S, Zhao, X, Wright, DA, Carpenter, S, Spalding, MH et al. (2011). Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res 39: 6315–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer, D, Wang, H, Kiani, S, Lai, CS, Gao, Q, Cassady, JP et al. (2011). Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol 29: 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holkers, M, Maggio, I, Liu, J, Janssen, JM, Miselli, F, Mussolino, C et al. (2013). Differential integrity of TALE nuclease genes following adenoviral and lentiviral vector gene transfer into human cells. Nucleic Acids Res 41: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L, Guell, M, Byrne, S, Yang, JL, De Los Angeles, A, Mali, P et al. (2013). Optimization of scarless human stem cell genome editing. Nucleic Acids Res 41: 9049–9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holkers, M, Maggio, I, Henriques, SF, Janssen, JM, Cathomen, T and Gonçalves, MA (2014). Adenoviral vector DNA for accurate genome editing with engineered nucleases. Nat Methods 11: 1051–1057. [DOI] [PubMed] [Google Scholar]
- Chevalier, BS and Stoddard, BL (2001). Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility. Nucleic Acids Res 29: 3757–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo, P, Prieto, J, Muñoz, IG, Alibés, A, Stricher, F, Serrano, L et al. (2008). Molecular basis of xeroderma pigmentosum group C DNA recognition by engineered meganucleases. Nature 456: 107–111. [DOI] [PubMed] [Google Scholar]
- Grizot, S, Smith, J, Daboussi, F, Prieto, J, Redondo, P, Merino, N et al. (2009). Efficient targeting of a SCID gene by an engineered single-chain homing endonuclease. Nucleic Acids Res 37: 5405–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman, LM, Chisholm, KM, Chevalier, BS, Chadsey, MS, Edwards, ST, Savage, JH et al. (2002). Mutations altering the cleavage specificity of a homing endonuclease. Nucleic Acids Res 30: 3870–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman, D, Chadsey, M, Fauce, S, Engel, A, Bruett, A, Monnat, R Jr et al. (2004). Isolation and characterization of new homing endonuclease specificities at individual target site positions. J Mol Biol 342: 31–41. [DOI] [PubMed] [Google Scholar]
- Rosen, LE, Morrison, HA, Masri, S, Brown, MJ, Springstubb, B, Sussman, D et al. (2006). Homing endonuclease I-CreI derivatives with novel DNA target specificities. Nucleic Acids Res 34: 4791–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J, Grizot, S, Arnould, S, Duclert, A, Epinat, JC, Chames, P et al. (2006). A combinatorial approach to create artificial homing endonucleases cleaving chosen sequences. Nucleic Acids Res 34: e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier, BS, Kortemme, T, Chadsey, MS, Baker, D, Monnat, RJ and Stoddard, BL (2002). Design, activity, and structure of a highly specific artificial endonuclease. Mol Cell 10: 895–905. [DOI] [PubMed] [Google Scholar]
- Arnould, S, Perez, C, Cabaniols, JP, Smith, J, Gouble, A, Grizot, S et al. (2007). Engineered I-CreI derivatives cleaving sequences from the human XPC gene can induce highly efficient gene correction in mammalian cells. J Mol Biol 371: 49–65. [DOI] [PubMed] [Google Scholar]
- Gürlevik, E, Schache, P, Goez, A, Kloos, A, Woller, N, Armbrecht, N et al. (2013). Meganuclease-mediated virus self-cleavage facilitates tumor-specific virus replication. Mol Ther 21: 1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy, A, Valton, J, Leduc, S, Armier, J, Galetto, R, Gouble, A et al. (2013). Targeted gene therapy of xeroderma pigmentosum cells using meganuclease and TALEN™. PLoS One 8: e78678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière, J, Hauer, J, Poirot, L, Brochet, J, Souque, P, Mollier, K et al. (2014). Variable correction of Artemis deficiency by I-Sce1-meganuclease-assisted homologous recombination in murine hematopoietic stem cells. Gene Ther 21: 529–532. [DOI] [PubMed] [Google Scholar]
- Gao, H, Smith, J, Yang, M, Jones, S, Djukanovic, V, Nicholson, MG et al. (2010). Heritable targeted mutagenesis in maize using a designed endonuclease. Plant J 61: 176–187. [DOI] [PubMed] [Google Scholar]
- Kleinstiver, BP, Wolfs, JM, Kolaczyk, T, Roberts, AK, Hu, SX and Edgell, DR (2012). Monomeric site-specific nucleases for genome editing. Proc Natl Acad Sci USA 109: 8061–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel, S, Jarjour, J, Astrakhan, A, Adey, A, Gouble, A, Duchateau, P et al. (2014). megaTALs: a rare-cleaving nuclease architecture for therapeutic genome engineering. Nucleic Acids Res 42: 2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs, JM, DaSilva, M, Meister, SE, Wang, X, Schild-Poulter, C and Edgell, DR (2014). MegaTevs: single-chain dual nucleases for efficient gene disruption. Nucleic Acids Res 42: 8816–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurdeley, M, Bietz, F, Li, J, Thomas, S, Stoddard, T, Juillerat, A et al. (2013). Compact designer TALENs for efficient genome engineering. Nat Commun 4: 1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou, R, Fremaux, C, Deveau, H, Richards, M, Boyaval, P, Moineau, S et al. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science 315: 1709–1712. [DOI] [PubMed] [Google Scholar]
- Wiedenheft, B, Sternberg, SH and Doudna, JA (2012). RNA-guided genetic silencing systems in bacteria and archaea. Nature 482: 331–338. [DOI] [PubMed] [Google Scholar]
- Horvath, P and Barrangou, R (2010). CRISPR/Cas, the immune system of bacteria and archaea. Science 327: 167–170. [DOI] [PubMed] [Google Scholar]
- Fineran, PC and Charpentier, E (2012). Memory of viral infections by CRISPR-Cas adaptive immune systems: acquisition of new information. Virology 434: 202–209. [DOI] [PubMed] [Google Scholar]
- Hsu, PD, Lander, ES and Zhang, F (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau, JE, Dupuis, MÈ, Villion, M, Romero, DA, Barrangou, R, Boyaval, P et al. (2010). The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468: 67–71. [DOI] [PubMed] [Google Scholar]
- Deltcheva, E, Chylinski, K, Sharma, CM, Gonzales, K, Chao, Y, Pirzada, ZA et al. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek, M, Chylinski, K, Fonfara, I, Hauer, M, Doudna, JA and Charpentier, E (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali, P, Yang, L, Esvelt, KM, Aach, J, Guell, M, DiCarlo, JE et al. (2013). RNA-guided human genome engineering via Cas9. Science 339: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong, L, Ran, FA, Cox, D, Lin, S, Barretto, R, Habib, N et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek, M, East, A, Cheng, A, Lin, S, Ma, E and Doudna, J (2013). RNA-programmed genome editing in human cells. Elife 2: e00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, SW, Kim, S, Kim, JM and Kim, JS (2013). Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol 31: 230–232. [DOI] [PubMed] [Google Scholar]
- Fonfara, I, Le Rhun, A, Chylinski, K, Makarova, KS, Lécrivain, AL, Bzdrenga, J et al. (2014). Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res 42: 2577–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chylinski, K, Makarova, KS, Charpentier, E and Koonin, EV (2014). Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res 42: 6091–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg, SH, Redding, S, Jinek, M, Greene, EC and Doudna, JA (2014). DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstiver, BP, Prew, MS, Tsai, SQ, Topkar, VV, Nguyen, NT, Zheng, Z et al. (2015). Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstiver, BP, Prew, MS, Tsai, SQ, Nguyen, NT, Topkar, VV, Zheng, Z et al. (2015). Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat Biotechnol 33: 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek, M, Jiang, F, Taylor, DW, Sternberg, SH, Kaya, E, Ma, E et al. (2014). Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343: 1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders, C, Niewoehner, O, Duerst, A and Jinek, M (2014). Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 513: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimasu, H, Ran, FA, Hsu, PD, Konermann, S, Shehata, SI, Dohmae, N et al. (2014). Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156: 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, EA, Kocak, DD, Fitzgibbon, CJ, McMenemy, J, Gersbach, CA and Marszalek, PE (2015). Structure and specificity of the RNA-guided endonuclease Cas9 during DNA interrogation, target binding and cleavage. Nucleic Acids Res 43: 8924–8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche, B, Gootenberg, JS, Abudayyeh, OO, Slaymaker, IM, Makarova, KS, Essletzbichler, P et al. (2015). Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 163: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmakov, S, Abudayyeh, OO, Makarova, KS, Wolf, YI, Gootenberg, JS, Semenova, E et al. (2015). Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol Cell 60: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepek, M, Brondani, V, Büchel, J, Serrano, L, Segal, DJ and Cathomen, T (2007). Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat Biotechnol 25: 786–793. [DOI] [PubMed] [Google Scholar]
- Miller, JC, Holmes, MC, Wang, J, Guschin, DY, Lee, YL, Rupniewski, I et al. (2007). An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol 25: 778–785. [DOI] [PubMed] [Google Scholar]
- Doyon, JB, Zeitler, B, Cheng, J, Cheng, AT, Cherone, JM, Santiago, Y et al. (2011). Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat Cell Biol 13: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J, Gaj, T and Barbas, CF 3rd (2010). Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. J Mol Biol 400: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran, FA, Hsu, PD, Lin, CY, Gootenberg, JS, Konermann, S, Trevino, AE et al. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154: 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali, P, Aach, J, Stranges, PB, Esvelt, KM, Moosburner, M, Kosuri, S et al. (2013). CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, SQ, Wyvekens, N, Khayter, C, Foden, JA, Thapar, V, Reyon, D et al. (2014). Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32: 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger, JP, Thompson, DB and Liu, DR (2014). Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol 32: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y, Sander, JD, Reyon, D, Cascio, VM and Joung, JK (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaymaker, IM, Gao, L, Zetsche, B, Scott, DA, Yan, WX and Zhang, F (2016). Rationally engineered Cas9 nucleases with improved specificity. Science 351: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstiver, BP, Pattanayak, V, Prew, MS, Tsai, SQ, Nguyen, NT, Zheng, Z et al. (2016). High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529(7587): 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y, Foden, JA, Khayter, C, Maeder, ML, Reyon, D, Joung, JK et al. (2013). High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31: 822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, PD, Scott, DA, Weinstein, JA, Ran, FA, Konermann, S, Agarwala, V et al. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31: 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak, V, Lin, S, Guilinger, JP, Ma, E, Doudna, JA and Liu, DR (2013). High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol 31: 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak, V, Ramirez, CL, Joung, JK and Liu, DR (2011). Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods 8: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, R, Lombardo, A, Arens, A, Miller, JC, Genovese, P, Kaeppel, C et al. (2011). An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat Biotechnol 29: 816–823. [DOI] [PubMed] [Google Scholar]
- Sander, JD, Ramirez, CL, Linder, SJ, Pattanayak, V, Shoresh, N, Ku, M et al. (2013). In silico abstraction of zinc finger nuclease cleavage profiles reveals an expanded landscape of off-target sites. Nucleic Acids Res 41: e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres, A, Gosis, BS, Ding, Q, Collins, R, Ragavendran, A, Brand, H et al. (2014). Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell 15: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C, Gore, A, Yan, W, Abalde-Atristain, L, Li, Z, He, C et al. (2014). Whole-genome sequencing analysis reveals high specificity of CRISPR/Cas9 and TALEN-based genome editing in human iPSCs. Cell Stem Cell 15: 12–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis, E, Sandoe, J, Williams, LA, Boulting, GL, Moccia, R, Wainger, BJ et al. (2014). Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 14: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X, Scott, DA, Kriz, AJ, Chiu, AC, Hsu, PD, Dadon, DB et al. (2014). Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat Biotechnol 32: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuscu, C, Arslan, S, Singh, R, Thorpe, J and Adli, M (2014). Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat Biotechnol 32: 677–683. [DOI] [PubMed] [Google Scholar]
- O'Geen, H, Henry, IM, Bhakta, MS, Meckler, JF and Segal, DJ (2015). A genome-wide analysis of Cas9 binding specificity using ChIP-seq and targeted sequence capture. Nucleic Acids Res 43: 3389–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, SQ, Zheng, Z, Nguyen, NT, Liebers, M, Topkar, VV, Thapar, V et al. (2015). GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol 33: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frock, RL, Hu, J, Meyers, RM, Ho, YJ, Kii, E and Alt, FW (2015). Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat Biotechnol 33: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D, Bae, S, Park, J, Kim, E, Kim, S, Yu, HR et al. (2015). Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods 12: 237–43, 1 p following 243. [DOI] [PubMed] [Google Scholar]
- Wang, X, Wang, Y, Wu, X, Wang, J, Wang, Y, Qiu, Z et al. (2015). Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat Biotechnol 33: 175–178. [DOI] [PubMed] [Google Scholar]
- Hendel, A, Bak, RO, Clark, JT, Kennedy, AB, Ryan, DE, Roy, S et al. (2015). Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol 33: 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S, Kim, D, Cho, SW, Kim, J and Kim, JS (2014). Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res 24: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann, K, Lin, S, Boyer, E, Simeonov, DR, Subramaniam, M, Gate, RE et al. (2015). Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci USA 112: 10437–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna, S, Kwaku Dad, AB, Beloor, J, Gopalappa, R, Lee, SK and Kim, H (2014). Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res 24: 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J, Gaj, T, Patterson, JT, Sirk, SJ and Barbas, CF 3rd (2014). Cell-penetrating peptide-mediated delivery of TALEN proteins via bioconjugation for genome engineering. PLoS One 9: e85755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj, T, Guo, J, Kato, Y, Sirk, SJ and Barbas, CF 3rd (2012). Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat Methods 9: 805–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorden, ER, Levinson, HM and Leong, KW (2015). Integration of drug, protein, and gene delivery systems with regenerative medicine. Drug Deliv Transl Res 5: 168–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X and Gonçalves, MA (2015). Engineered viruses as genome editing devices. Mol Ther 24: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabadi, AM, Ousterout, DG, Hilton, IB and Gersbach, CA (2014). Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res 42: e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H, Xue, W, Chen, S, Bogorad, RL, Benedetti, E, Grompe, M et al. (2014). Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol 32: 551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, K, Ely, A, Mussolino, C, Cathomen, T and Arbuthnot, P (2013). Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol Ther 21: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J, Zhang, W, Lin, J, Wang, F, Wu, M, Chen, C et al. (2014). An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther 22: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan, A, Schaffer, DV and Samulski, RJ (2012). The AAV vector toolkit: poised at the clinical crossroads. Mol Ther 20: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj, T, Epstein, BE and Schaffer, DV (2015). Genome engineering using adeno-associated virus: basic and clinical research applications. Mol Ther 24: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, DW and Hirata, RK (1998). Human gene targeting by viral vectors. Nat Genet 18: 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, DG, Petek, LM and Russell, DW (2003). Human gene targeting by adeno-associated virus vectors is enhanced by DNA double-strand breaks. Mol Cell Biol 23: 3550–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellhaus, K, Cornu, TI, Heilbronn, R and Cathomen, T (2010). Fate of recombinant adeno-associated viral vector genomes during DNA double-strand break-induced gene targeting in human cells. Hum Gene Ther 21: 543–553. [DOI] [PubMed] [Google Scholar]
- Deyle, DR and Russell, DW (2009). Adeno-associated virus vector integration. Curr Opin Mol Ther 11: 442–447. [PMC free article] [PubMed] [Google Scholar]
- Lai, Y, Yue, Y, Liu, M, Ghosh, A, Engelhardt, JF, Chamberlain, JS et al. (2005). Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol 23: 1435–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran, FA, Cong, L, Yan, WX, Scott, DA, Gootenberg, JS, Kriz, AJ et al. (2015). In vivo genome editing using Staphylococcus aureus Cas9. Nature 520: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland, AE, Baral, R, Singhal, P, Loveluck, K, Shen, S, Sanchez, M et al. (2015). Characterization of Staphylococcus aureus Cas9: a smaller Cas9 for all-in-one adeno-associated virus delivery and paired nickase applications. Genome Biol 16: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L, Krymskaya, L, Wang, J, Henley, J, Rao, A, Cao, LF et al. (2013). Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol Ther 21: 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, L, Wang, J, Beyer, AI, Teque, F, Cradick, TJ, Qi, Z et al. (2014). Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Delta32 mutation confers resistance to HIV infection. Proc Natl Acad Sci USA 111: 9591–9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal, PK, Ferreira, LM, Collins, R, Meissner, TB, Boutwell, CL, Friesen, M et al. (2014). Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15: 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather, BD, Romano Ibarra, GS, Sommer, K, Curinga, G, Hale, M, Khan, IF et al. (2015). Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci Transl Med 7: 307ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen, CB, Wang, J, Tilton, JC, Miller, JC, Kim, KA, Rebar, EJ et al. (2011). Engineering HIV-resistant human CD4+ T cells with CXCR4-specific zinc-finger nucleases. PLoS Pathog 7: e1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia, R, Pauls, E, Riveira-Munoz, E, Clotet, B, Esté, JA and Ballana, E (2014). Zinc finger endonuclease targeting PSIP1 inhibits HIV-1 integration. Antimicrob Agents Chemother 58: 4318–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel, HJ, Morrison, JH, Saenz, DT, Fuchs, JR, Kvaratskhelia, M, Ekker, SC et al. (2014). TALEN knockout of the PSIP1 gene in human cells: analyses of HIV-1 replication and allosteric integrase inhibitor mechanism. J Virol 88: 9704–9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voit, RA, McMahon, MA, Sawyer, SL and Porteus, MH (2013). Generation of an HIV resistant T-cell line by targeted “stacking” of restriction factors. Mol Ther 21: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W, Kaminski, R, Yang, F, Zhang, Y, Cosentino, L, Li, F et al. (2014). RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci USA 111: 11461–11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cradick, TJ, Keck, K, Bradshaw, S, Jamieson, AC and McCaffrey, AP (2010). Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Mol Ther 18: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, ND, Stone, D, Sedlak, RH, De Silva Feelixge, HS, Roychoudhury, P, Schiffer, JT et al. (2014). AAV-mediated delivery of zinc finger nucleases targeting hepatitis B virus inhibits active replication. PLoS One 9: e97579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, SR, Yang, HC, Kuo, YT, Liu, CJ, Yang, TY, Sung, KC et al. (2014). The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Mol Ther Nucleic Acids 3: e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C, Qu, L, Wang, H, Wei, L, Dong, Y and Xiong, S (2015). Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res 118: 110–117. [DOI] [PubMed] [Google Scholar]
- Kennedy, EM, Bassit, LC, Mueller, H, Kornepati, AV, Bogerd, HP, Nie, T et al. (2015). Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology 476: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X, Hao, R, Chen, S, Guo, D and Chen, Y (2015). Inhibition of hepatitis B virus by the CRISPR/Cas9 system via targeting the conserved regions of the viral genome. J Gen Virol 96: 2252–2261. [DOI] [PubMed] [Google Scholar]
- Grosse, S, Huot, N, Mahiet, C, Arnould, S, Barradeau, S, Clerre, DL et al. (2011). Meganuclease-mediated inhibition of HSV1 infection in cultured cells. Mol Ther 19: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, Y, Sun, L, Gao, D, Ding, C, Li, Z, Li, Y et al. (2014). High-efficiency targeted editing of large viral genomes by RNA-guided nucleases. PLoS Pathog 10: e1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert, M, Boyle, NM, Stone, D, Stensland, L, Huang, ML, Magaret, AS et al. (2014). In vitro inactivation of latent HSV by targeted mutagenesis using an HSV-specific homing endonuclease. Mol Ther Nucleic Acids 3: e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, EM, Kornepati, AV, Goldstein, M, Bogerd, HP, Poling, BC, Whisnant, AW et al. (2014). Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J Virol 88: 11965–11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel, J (2013). Breakthrough of the year 2013. Cancer immunotherapy. Science 342: 1432–1433. [DOI] [PubMed] [Google Scholar]
- Miller, JF and Sadelain, M (2015). The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell 27: 439–449. [DOI] [PubMed] [Google Scholar]
- Provasi, E, Genovese, P, Lombardo, A, Magnani, Z, Liu, PQ, Reik, A et al. (2012). Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med 18: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torikai, H, Reik, A, Liu, PQ, Zhou, Y, Zhang, L, Maiti, S et al. (2012). A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119: 5697–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdien, B, Mock, U, Atanackovic, D and Fehse, B (2014). TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther 21: 539–548. [DOI] [PubMed] [Google Scholar]
- Torikai, H, Reik, A, Soldner, F, Warren, EH, Yuen, C, Zhou, Y et al. (2013). Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood 122: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahimi, P, Chang, WG, Kluger, MS, Qyang, Y, Tellides, G, Saltzman, WM et al. (2015). Efficient gene disruption in cultured primary human endothelial cells by CRISPR/Cas9. Circ Res 117: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane, JD, Lee, G, Zheng, Z, Mendel, M, Abate-Daga, D, Bharathan, M et al. (2015). Clinical scale zinc finger nuclease-mediated gene editing of PD-1 in tumor infiltrating lymphocytes for the treatment of metastatic melanoma. Mol Ther 23: 1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik, A., Holmes, MC, Zhou, Y, Mendel, M, Liu, P-Q, Lee, G et al. (2007). Targeted killing of glioblastoma multiforme in vivo by IL-13 zetakine redirected CTLs made glucocorticoid resistant with zinc finger nucleases. Blood 110: 765a. [Google Scholar]
- Blaese, RM, Culver, KW, Miller, AD, Carter, CS, Fleisher, T, Clerici, M et al. (1995). T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science 270: 475–480. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo, M, Hacein-Bey, S, de Saint Basile, G, Gross, F, Yvon, E, Nusbaum, P et al. (2000). Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288: 669–672. [DOI] [PubMed] [Google Scholar]
- Joglekar, AV, Hollis, RP, Kuftinec, G, Senadheera, S, Chan, R and Kohn, DB (2013). Integrase-defective lentiviral vectors as a delivery platform for targeted modification of adenosine deaminase locus. Mol Ther 21: 1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, SH, Kuehle, J, Reimann, C, Mlambo, T, Alzubi, J, Maeder, ML et al. (2015). Rescue of DNA-PK signaling and T-cell differentiation by targeted genome editing in a PRKDC deficient iPSC disease model. PLoS Genet 11: e1005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X, Wang, Y, Yan, W, Smith, C, Ye, Z, Wang, J et al. (2015). Production of gene-corrected adult beta globin protein in human erythrocytes differentiated from patient iPSCs after genome editing of the sickle point mutation. Stem Cells 33: 1470–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban, MD, Cost, GJ, Mendel, MC, Romero, Z, Kaufman, ML, Joglekar, AV et al. (2015). Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood 125: 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio, P, Baños, R, Lombardo, A, Quintana-Bustamante, O, Alvarez, L, Garate, Z et al. (2014). Targeted gene therapy and cell reprogramming in Fanconi anemia. EMBO Mol Med 6: 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra, J, Reik, A, Chang, KH, Stehling-Sun, S, Zhou, Y, Hinkley, SJ et al. (2015). Functional footprinting of regulatory DNA. Nat Methods 12: 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguela, XM, Sharma, R, Doyon, Y, Miller, JC, Li, H, Haurigot, V et al. (2013). Robust ZFN-mediated genome editing in adult hemophilic mice. Blood 122: 3283–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, R, Anguela, XM, Doyon, Y, Wechsler, T, DeKelver, RC, Sproul, S et al. (2015). In vivo genome editing of the albumin locus as a platform for protein replacement therapy. Blood 126: 1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abifadel, M, Varret, M, Rabès, JP, Allard, D, Ouguerram, K, Devillers, M et al. (2003). Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34: 154–156. [DOI] [PubMed] [Google Scholar]
- Abifadel, M, Elbitar, S, El Khoury, P, Ghaleb, Y, Chémaly, M, Moussalli, ML et al. (2014). Living the PCSK9 adventure: from the identification of a new gene in familial hypercholesterolemia towards a potential new class of anticholesterol drugs. Curr Atheroscler Rep 16: 439. [DOI] [PubMed] [Google Scholar]
- Ding, Q, Strong, A, Patel, KM, Ng, SL, Gosis, BS, Regan, SN et al. (2014). Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res 115: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau, J, Chapdelaine, P, Boisvert, S, Almeida, LP, Corbeil, J, Montpetit, A et al. (2011). Endonucleases: tools to correct the dystrophin gene. J Gene Med 13: 522–537. [DOI] [PubMed] [Google Scholar]
- Popplewell, L, Koo, T, Leclerc, X, Duclert, A, Mamchaoui, K, Gouble, A et al. (2013). Gene correction of a duchenne muscular dystrophy mutation by meganuclease-enhanced exon knock-in. Hum Gene Ther 24: 692–701. [DOI] [PubMed] [Google Scholar]
- Xu, L, Park, KH, Zhao, L, Xu, J, El Refaey, M, Gao, Y et al. (2015). CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol Ther 24: 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabdallah, BF, Duval, A, Rousseau, J, Chapdelaine, P, Holmes, MC, Haddad, E et al. (2013). Targeted gene addition of microdystrophin in mice skeletal muscle via human myoblast transplantation. Mol Ther Nucleic Acids 2: e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi, R, Arpke, RW, Irion, S, Dimos, JT, Grskovic, M, Kyba, M et al. (2012). Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdoni, C, Osborn, MJ and Tolar, J (2016). Gene editing toward the use of autologous therapies in recessive dystrophic epidermolysis bullosa. Transl Res 168: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn, MJ, Starker, CG, McElroy, AN, Webber, BR, Riddle, MJ, Xia, L et al. (2013). TALEN-based gene correction for epidermolysis bullosa. Mol Ther 21: 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiano, V, Zhen, HH, Haddad, B, Derafshi, BH, Bashkirova, E, Melo, SP et al. (2014). Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med 6: 264ra163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge, JW, Smith, AJ, Barker, SS, Robbie, S, Henderson, R, Balaggan, K et al. (2008). Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 358: 2231–2239. [DOI] [PubMed] [Google Scholar]
- Maguire, AM, Simonelli, F, Pierce, EA, Pugh, EN Jr, Mingozzi, F, Bennicelli, J et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 358: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan, AV, Aleman, TS, Boye, SL, Schwartz, SB, Kaushal, S, Roman, AJ et al. (2008). Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA 105: 15112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth, WW, Aleman, TS, Kaushal, S, Cideciyan, AV, Schwartz, SB, Wang, L et al. (2008). Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 19: 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Camacho, OF and Zenteno, JC (2015). Review and update on the molecular basis of Leber congenital amaurosis. World J Clin Cases 3: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnight, ER, Wiley, LA, Drack, AV, Braun, TA, Anfinson, KR, Kaalberg, EE et al. (2014). CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther 21: 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder, M.L. et al. Therapeutic Correction of an LCA-Causing Splice Defect in the CEP290 Gene by CRISPR/Cas-Mediated Genome Editing. Mol Ther 23, S273–S274 (2015).
- Crane, AM, Kramer, P, Bui, JH, Chung, WJ, Li, XS, Gonzalez-Garay, ML et al. (2015). Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports 4: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth, AL, Menon, T, Parker, GS, Qualls, SJ, Lewis, BM, Ke, E et al. (2015). Functional Gene Correction for Cystic Fibrosis in Lung Epithelial Cells Generated from Patient iPSCs. Cell Rep 12: 1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeer, NA, Anandalingam, K, Fields, RJ, Caputo, C, Kopic, S, Gupta, A et al. (2015). Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat Commun 6: 6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikard, D, Euler, CW, Jiang, W, Nussenzweig, PM, Goldberg, GW, Duportet, X et al. (2014). Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol 32: 1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citorik, RJ, Mimee, M and Lu, TK (2014). Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat Biotechnol 32: 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa, AA, Klumpe, HE, Luo, ML, Selle, K, Barrangou, R and Beisel, CL (2014). Programmable removal of bacterial strains by use of genome-targeting CRISPR-Cas systems. MBio 5: e00928–e00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkunas, T, Gasiunas, G, Fremaux, C, Barrangou, R, Horvath, P and Siksnys, V (2011). Cas3 is a single-stranded DNA nuclease and ATP-dependent helicase in the CRISPR/Cas immune system. EMBO J 30: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova, KS, Wolf, YI, Alkhnbashi, OS, Costa, F, Shah, SA, Saunders, SJ et al. (2015). An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 13: 722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, TK and Koeris, MS (2011). The next generation of bacteriophage therapy. Curr Opin Microbiol 14: 524–531. [DOI] [PubMed] [Google Scholar]
- Gaj, T, Sirk, SJ and Barbas, CF 3rd (2014). Expanding the scope of site-specific recombinases for genetic and metabolic engineering. Biotechnol Bioeng 111: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts, DC, Jore, MM, Westra, ER, Zhu, Y, Janssen, JH, Snijders, AP et al. (2014). DNA-guided DNA interference by a prokaryotic Argonaute. Nature 507: 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton, IB and Gersbach, CA (2015). Enabling functional genomics with genome engineering. Genome Res 25: 1442–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung, AJ, Joung, JK, Khalil, AS and Collins, JJ (2015). Chromatin regulation at the frontier of synthetic biology. Nat Rev Genet 16: 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore, PI, Black, JB, Hilton, IB and Gersbach, CA (2016). Editing the epigenome: technologies for programmable transcription and epigenetic modulation. Nat Methods 13(2): 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]