CD8+ T-cell memory in tumor immunology and immunotherapy (original) (raw)
. Author manuscript; available in PMC: 2006 Jul 13.
Summary
The cellular and molecular mechanisms underlying the formation of distinct central, effector, and exhausted CD8+ T-cell memory subsets were first described in the setting of acute and chronic viral diseases. The role of these T-cell memory subsets are now being illuminated as relevant to the tumor-bearing state. The generation and persistence of productive CD8+ T-cell memory subsets is determined, in part, by antigen clearance, costimulation, responsiveness to homeostatic cytokines, and CD4+ T-helper cells. By contrast, chronic exposure to antigen, negative costimulation, and immunomodulation by CD4+ T regulatory cells corrupt productive CD8+ T memory formation. It has become clear from human and mouse studies that the mere generation of CD8+ T-cell memory is not a ‘surrogate marker’ for cancer vaccine efficacy. Some current cancer vaccine strategies may fail because they amplify, rather than correct or reset, the corrupted CD8+ memory population. Thus, much of the present effort in the development of vaccines for cancer and chronic infectious diseases is aimed at creating effective memory responses. Therapeutic vaccines for cancer and chronic infectious diseases may achieve consistent efficacy by ablation of the dysfunctional immune state and the provision of newly generated, non-corrupted memory cells by adoptive cell transfer.
Introduction
‘All happy families resemble one another, each unhappy family is unhappy in its own way.’ – Leo Tolstoy
For over a century, the prospect of employing a patient's immune system to treat cancer has been the source of much hope and promise, yet at the same time frustration and dismay (1). There has been a significant investment in both time and resources towards developing T-cell cancer vaccines, driven largely by the success of vaccination strategies against some viral and bacterial pathogens (2, 3) and the ability to molecularly define major histocompatibility complex class I- and class II-restricted epitopes expressed on the surface of cancer cells (4-6). Despite much effort, vaccination approaches to date can at best induce objective cancer regressions consistent with standard oncologic criteria (7) in only a small minority of patients with solid cancers (5, 8). This outcome appears to be in contrast to what can occur in vaccination strategies against certain hematologic malignancies, such as B-cell lymphoma (9). In some instances, failure of consistent clinical responses has occurred despite impressive immunologic responses, particularly those elicited using anchor-modified epitopes as immunogens (10-16). When combined with results from recent animal studies (17, 18), these findings offer the somewhat surprising conclusion that generation of a large in vivo population of tumor-reactive CD8+ T cells alone is insufficient to mediate clinically significant tumor regression. Is there any hope, then, that cancer vaccines can be made to work?
Adoptive cell transfer (ACT) (19, 20), the infusion of ex vivo expanded tumor-reactive immune cells to patients with cancer, offers a powerful proof of principle that self/tumor-reactive CD8+ T cells can mediate objective cancer regression (21, 22). Significantly, the reactivity contained in the tumor infiltrating lymphocyte population expanded and transferred to patients in these protocols is entirely autologous; thus, there is a latent pool of tumor-reactive T cells present in patients with cancer capable of mediating tumor regression if properly activated (23, 24). Why have modern cancer vaccines failed to live up to their expectations thus far? Borrowing from Tolstoy's analogy, does each vaccine have its own idiosyncratic reason for failure? Alternatively, are there a limited number of overarching immunological principles governing why cancer vaccines are inconsistent, by any measure, in their ability to benefit patients with cancer? In this review, we address the failure of cancer vaccines, as currently formulated and employed, to mediate reproducible, objective clinical responses against solid cancers. We begin by briefly outlining the state of the art regarding the formation and function of T-cell memory in the settings of acute and chronic viral infections. Chronic viral infections, in particular, may model many of the immunologic impasses that have limited the success of cancer vaccines to date as both settings induce corrupted CD8+ T-cell memory populations. We next assess what specific factors may limit modern cancer vaccines and conclude by suggesting how immunotherapists might employ these factors to improve future vaccine efforts.
Generation and character of CD8+ memory T cells
Much of our current knowledge on the development and character of CD8+ T-cell memory has come from the study of acute viral infections. The dynamic response of antigen specific CD8+ T cells to an acute viral infection has now been resolved at cellular, molecular, and gene-expression levels in mice (25-27) and to a more limited extent in humans (28-30). While it is possible – and even likely – that a productive immune response against cancer will have unique attributes given the self nature of many tumor-associated antigens targeted in cancer vaccine protocols (4, 8, 31), it is instructive to analyze key aspects of an antiviral T-cell response.
In brief, a productive encounter of naïve CD8+ T cells to antigen stimulation follows a prototypical, tri-phasic response: (i) an initial activation phase characterized by a multi-log clonal expansion of antigen-specific cells and concurrent acquisition of peripheral tissue-homing capabilities, effector cytokine release, and cytolytic activity; (ii) a death phase characterized by a rapid, apoptosis-induced contraction of antigen-specific effector T cells; and (iii) formation of a persistent population of antigen-experienced cells that represent immunologic memory (Fig. 1A). An analogous response has also been observed among self/tumor-reactive CD8+ T cells activated in vivo with a recombinant viral vaccine and exogenous common g chain (gc) cytokine support (18, 32). This finding demonstrates that the study of responses to an acute viral infection may be predictive of some aspects of the biology of self/tumor-reactive CD8+ T cells.
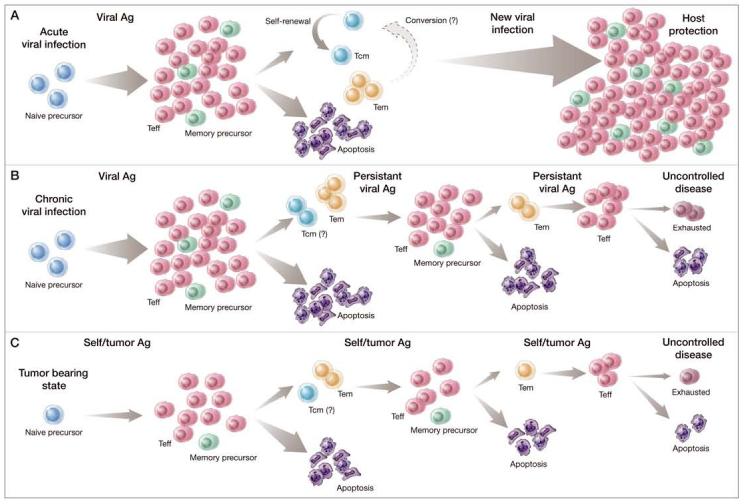
Figure 1.
Comparison of normal and corrupted CD8+ T-cell memory formation. CD8+ T-cell memory formation in the settings of an acute viral infection (A), chronic viral infection (B), and the tumor-bearing state (C) begins when a population of naive, antigen (Ag)-specific CD8+ T cells (blue cells) are stimulated to divide by exposure to cognate Ag. A productive encounter with cognate Ag induces a multi-log clonal expansion of this naïve precursor pool. As these cells divide, they acquire the phenotypic and functional attributes of terminally differentiated effector T cells (TEFF) (red cells) cells that allow these cells to clear the stimulating Ag. At the conclusion of the 1o response, the majority (∼90%) of responding CD8+ T cells die by apoptosis; a limited subset of TEFF cells (∼10%, green cells) advance to form a stable pool of memory CD8+ T cells. The memory pool is heterogeneous and can be divided into self-renewing central memory T cells (TCM) (aquamarine cells) and effector memory T cells (TEM) cells (yellow cells). Whether TEM convert to TCM with time is the current subject of controversy. Under normal conditions, both pools of memory T cells contribute to host protection from future pathogen challenge. In the settings of a chronic viral infection or the tumor-bearing state, the normal pattern of CD8 + memory T-cell production can be altered. With time, memory cells are driven to a highly differentiated or ‘exhausted’ phenotypic and functional state (purple cells), culminating in some cases in the deletion of responding cells. Ultimately, the memory CD8+ T cells generated under these conditions are unable to efficiently clear the challenging Ag, resulting in uncontrolled disease.
Several key features distinguish the persistent antigen specific cells of the third phase of the immune response described above. These features include an increased precursor frequency compared with naive hosts (33, 34), the capacity for antigen-independent self-renewal (35) through homeostatic proliferation in response to interleukin-7 (IL-7), IL-15, and possibly IL-21 (36-42), and the rapid acquisition of effector functions and clonal proliferation upon antigen re-challenge (43, 44). These attributes, which collectively constitute the hallmarks of immunologic memory, provide the host with long-lived protection from future pathogen encounters.
Several groups have recently demonstrated the critical importance of CD4+ T-cell help (Th) in the induction and maintenance of antigen-specific memory CD8+ T cells. In the absence of Th, memory CD8+ T cells exhibit impaired functionality, persistence, and most importantly ability to efficiently control a secondary pathogen or tumor challenge (45-47). Whether Th initiates a programming of antigen specific CD8+ T cells during priming (46), contributes to the maintenance of memory CD8+ T cells after the primary response (48, 49), or both is an active area of exploration. Conversely, another subset of CD4+ T cells, the CD4+ CD25highFOXP3+ T-regulatory cells (Treg) (50), have been shown to negatively regulate both the quality and quantity of memory CD8+ Th cells (51, 52).
CD8+ memory T cells are heterogeneous in phenotype, function, and protective capacity
Analogous to memory B lymphocytes (53), memory CD8+ T cells are heterogeneous with respect to phenotypic markers, effector functions, and tissue-homing capabilities. One mode of classifying memory T cells is to divide the populations into two broad categories, termed central memory T cells (TCM) and effector memory T cells (TEM). In this classification schema, first proposed by Sallusto et al. (54), TCM are antigen-experienced cells that constitutively express CD62L and CCR7, two surface molecules necessary for cellular extravasation in high endothelial venules and migration to T-cell zones of peripheral lymph nodes (Fig. 2). By contrast, TEM are antigen-experienced T cells that have significantly downregulated these markers and hence have a propensity to populate peripheral tissues, such as the liver and lung, as well as inflammatory sites (44, 55). Based on these findings, a division of labor among memory T cells was proposed: TEM function as sentinels for immediate protection from a peripheral challenge, while TCM provide protection from a systemic challenge and can generate a second wave of effector cells (54). In addition to the ability of TCM to preferentially migrate to secondary lymph nodes, the capacity to secrete IL-2 has been associated with CD8+ TCM but not with TEM cells (44, 54, 56, 57). Whether TCM and TEM differ with respect to other important effector functions, such as immediate cytolytic activity, has been debated (44, 54, 56-60).
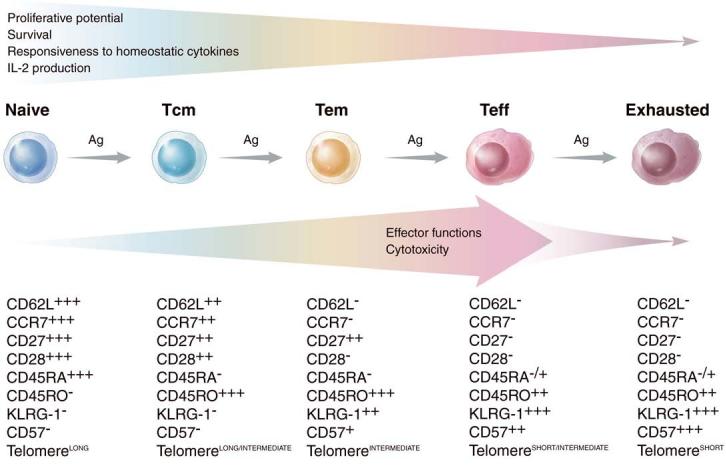
Figure 2.
Phenotypic and functional changes in CD8+ T cells induced by chronic antigen stimulation. Chronic or repetitive antigen stimulation, such as that induced by chronic viral infections, the tumor-bearing state, and aggressive prime-boost regimens, drives naive CD8+ T cells to a terminally differentiated effector state (red cells) and ultimately to exhaustion (purple cells). The phenotypic changes characterizing this process are illustrated as low expression (+), intermediate expression (++), and high expression (+++) of various cell surface markers. Together with a shortening of the telomere length, CD8+ T cells progressively lose their proliferative potential and become exhausted and/or undergo apoptosis. In addition, CD8+ T cells lose the ability to secrete IL-2 and to respond to homeostatic cytokines such as IL-7 and IL-15. By contrast, effector functions and lytic capability are progressively gained with stimulation, peaking at the effector state but later becoming impaired in exhausted cells. Whether effector memory T cells (TEM) may revert to central memory T cells (TCM) following antigen clearance (not shown) is a current subject of controversy.
The question of which of these two T-cell memory populations, if either, should be targeted in future vaccine trials is a subject of considerable interest, and it is now beginning to be addressed in vivo in animal models. In both mice and non-human primates, CD8+ TCM have been shown to be superior mediators of host protection against viral and bacterial challenge compared to TEM cells (44, 61, 62). Wherry et al. (44) reached similar conclusions on the superiority of antigen-specific TCM cells whether the antigen challenge came from peripheral or systemic sites, thereby challenging the division of labor paradigm. Consistent with these findings, our group (63) has recently shown that adoptively transferred self/tumor-reactive CD8+ TCM are superior mediators of therapeutic antitumor immunity to an established cancer compared with TEM cells when given in combination with a systemically administered tumor-antigen vaccine.
The enhanced ability of TCM cells to confer host protection and tumor treatment has been correlated with their greater proliferative capacity upon antigen-reencounter compared with TEM cells (44, 63, 64). Ultimately, this potential allows for generation of a larger absolute number of terminally differentiated effector T cells (TEFF) that can infiltrate peripheral sites to mediate antigen clearance. In addition to enhanced proliferative capacity, we have also shown that TCM engage in more effective interactions with antigen-presenting cells (APCs) expressing cognate antigen compared with TEM because of their preferential homing to secondary lymphoid tissues (63). The superiority of CD8+ TCM to TEM has not been uniformly observed in all models (57, 65, 66). However, these results may be due to a relative early antigen re-challenge; it has recently been shown that the proliferative capacity of memory CD8+ T cells following antigen clearance progressively increases with time both in the TCM and TEM subpopulations, but is most prominent among TCM cells (64).
These data suggest that TCM may be more potent on a per cell basis in mediating antigen clearance compared with TEM cells. Therefore, the generation of TCM should be an important immunologic end-point to consider in future preventative and therapeutic vaccine trials. Future studies are needed to confirm results directly comparing the therapeutic efficacy of TCM to TEM, particularly in settings of an established disease such as chronic viral hepatitis, human immunodeficiency virus (HIV), tuberculosis, and cancer. Likewise, additional correlations in non-human primate models and humans will also be necessary to elucidate which T cell memory subset is most advantageous for conferring protection or treatment against different pathogens and cancers.
Chronic antigen stimulation induces corrupted memory CD8+ T cells
Key aspects of CD8+ T-cell memory formation and function can either be altered or impaired in settings of persistent antigen stimulation, such as chronic viral infections (67) and the tumor-bearing state (23, 68-70). These changes affect the cell's acquisition and magnitude of effector functions, capacity for antigen-independent cytokine-mediated homeostatic proliferation, intrinsic proliferative capacity, and ultimately the ability to confer host protection. In mice, it has been clearly demonstrated that chronic viral infection can convert an otherwise effective antiviral CD8+ T cell response to an ineffective or inefficient response (71-75) (Fig. 1B). This conversion is associated with a step-wise impairment, or ‘exhaustion’, in the effector functions and proliferative capacity of responding antigen-specific CD8+ T-cells (Fig. 2). In general, this loss occurs in a predictable and hierarchical manner.
These findings are not restricted solely to chronic viral infections. We have recently shown that repeated in vitro stimulation of CD8+ T cells that recognize a class I-restricted epitope derived from the shared self/melanoma antigen, gp100 (17), was also associated with a progressive alteration in effector functions and proliferative capacity (76). Significantly, these alterations correlated with the conversion of a curative in vivo antitumor response to a non-curative response. Similar findings have been observed in other murine tumor models (68, 77). Collectively, these findings may help explain two seeming paradoxes in modern tumor immunotherapy: the failure of vigorous vaccination regimens to mediate cancer regression despite significant increases in the tumor-reactive precursor frequency (10) and the inability of highly in vitro reactive and cytolytic tumor-specific CD8+ T-cell clones to mediate cancer regression when adoptively transferred (78-80).
Chronically stimulated memory CD8+ T cells are functionally corrupted
In mice, the inability to secrete IL-2 represents a sentinel event associated with the impairment in effector functions of chronically stimulated viral- and tumor-reactive CD8+ T cells (72-74, 76). Similarly, impairment in the ability to secrete tumor necrosis factor-a (72-74) and loss of cytolytic activity (72-74, 81) have also been shown to be comparatively early events. By contrast, the ability to secrete interferon-g remains intact until late effector cells are generated (72-74, 76, 77, 82). Analogous progressive impairments in CD8+ function have been observed in humans during chronic viral infections, such as HIV, Epstein–Barr virus, and cytomegalovirus (81, 83-87).
In addition to impairments in cytokine release and cytolytic function, memory CD8+ T cells generated in the settings of chronic or repetitive antigen stimulation can enter into a state of replicative senescence. This state is characterized by a decreased ability to dilute carboxyfluorescein diacetate succinamidyl ester upon antigen or anti-CD3/anti-CD28 stimulation (76, 88, 89), a decrease in telomere length (87), and an increase in surface expression for the natural killer-like receptors CD57 and KLRG-1 (Fig. 2) (83, 86). As discussed previously, the enhanced proliferative capacity of TCM over TEM is a major contributing factor in the superior ability of TCM to confer host protection. It is therefore likely that an impaired proliferative response of CD8+ T cells will compromise their ability to protect the host from a pathogen challenge or cancer. This impairment has recently been demonstrated in mice, where the reduced proliferative capacity of antigen-specific CD8+ T cells was associated with the reduced efficacy of therapeutic vaccination against a chronic viral infection (88).
Finally, chronic antigen exposure can skew the normal pattern of immunodominance, the hierarchy of the CD8+ T-cell response to a highly restricted number of possible epitopes (71, 72, 82, 90). As a result, formerly minor reactivities come to dominate the CD8+ T-cell response, while responses that are dominant in the acute setting may be minimized or deleted altogether. Changes in immunodominance may have implications for the efficiency of pathogen clearance (72, 82, 90). Ultimately, changes in immunodominance can culminate with the physical deletion of chronically stimulated antigen-specific CD8+ T cells (72-74).
Cells that have entered a terminally differentiated state of exhaustion during a chronic viral infection are likely to have counterparts in tumor immunotherapy. First, in adoptive immunotherapies in mice and humans, we have found that cells capable of undergoing vigorous in vivo expansion confer superior antitumor treatment compared with cells that expand less robustly (21, 63, 76). Second, it has recently been shown that telomere length positively correlates with long-term persistence and ultimately tumor regression induced by adoptively transferred tumor-reactive CD8+ T cells (91, 92). Thus, the proliferative capacity of tumor-reactive CD8+ T cells is critical to their ability to mount an optimal in vivo antitumor response. Whether the tumor-bearing state can alter patterns of immunodominance through the chronic stimulation of responding CD8+ T cells has yet to be determined.
Phenotypic alterations in chronically stimulated memory CD8+ T cells
Beyond functional changes, repetitively stimulated CD8+ T cells exhibit progressive changes in surface phenotype (72, 74, 76, 82, 89, 93, 94) (Fig. 2). For example, whereas memory CD8+ T cells produced from an acute viral infection will become enriched in an antigen-experienced CD62LhighCCR7+TCM population with time (44, 64), these markers are progressively downregulated during a chronic viral infection or repetitive vaccination (72, 76, 82, 89, 94). Thus, chronic antigen exposure can drive the selective production of TEM and TEFF cells over TCM cells (Fig. 1A,B). This results in the re-partitioning of memory CD8+ T cells to peripheral tissues, away from central lymphoid organs (72, 74). As previously noted, TEM cells may confer less protective and therapeutic immunity compared with TCM cells, regardless of whether the antigen challenge is to a peripheral or systemic site (44).
In addition to differences in tissue homing phenotype, chronically stimulated CD8+ T cells downregulate other surface molecules, such as IL-7Ra and the IL-2/IL-15Rb chain (76, 85, 93). This downregulation impairs the responsiveness of memory CD8+ T cells to pro-survival/homeostatic signals delivered by IL-7 and IL-15 (Fig. 2).
Chronic exposure to antigen also induces the progressive downregulation of CD27 and CD28, two costimulatory molecule receptors that serve to augment T-cell receptor (TCR)-induced activation, proliferation, and memory formation (87, 94, 95). At the same time, upregulation of the inhibitory costimulatory molecule PD_1 (programmed death-1) occurs (75). Emerging findings from Barber et al. (75) suggest that the expression of PD_1 but not cytotoxic T-lymphocyte antigen-4 (CTLA-4) on exhausted T cells contributes to the functional impairments that characterize T cells in this state. In a model of chronic viral disease, the blockade of the PD-1 / PDL-1 inhibitory signals promoted improvements in both proliferation and effector functions of exhausted T cells. It is unclear, however, whether exhausted T cells restored with PDL-1 blockade are as functionally ‘fit’ as memory cells generated in response to an acute viral infection.
Current therapeutic cancer vaccines amplify corruptedand senescent CD8+ memory
Current regimens for the administration of T-cell cancer vaccines have been selected because of their ability to induce strong T-cell expansion in vivo. However, it is now clear that the generation of large quantities of memory T cells alone is not sufficient to consistently induce objective tumor responses in patients (10, 11, 13-16). Current cancer vaccines can have pleiotropic effects on CD8+ T cells depending on the T cell's avidity and state of differentiation. Naturally occurring selftumor-reactive CD8+ T cells with low avidity are generally found in a naive or low differentiated state, because the intensity of antigen experience in the tumor-bearing host is low or absent (antigenic ‘ignorance’) (70, 96). Early and reiterative boosting with high doses of immunogen induce this relatively naý[unk]ve CD8+ T-cell population to strongly proliferate and differentiate into TEM, TEFF, and ultimately exhausted T cells (94). By contrast, highly avid T cells that have experienced chronic stimulation become senescent in the tumor-bearing host (Fig. 1C) might undergo activation-induced death due to over-stimulation (70, 97). Thus, current therapeutic cancer vaccines may fail because they amplify rather than correct the corrupted CD8+ T-cell memory population formed during states of chronic antigen exposure.
Selective generation of TCM, which has been shown in both viral and tumor models to confer superior protective and therapeutic immunity compared to TEM/EFF, might be a critical key for improving cancer immunotherapies and vaccines. We and others (55, 56, 58, 98-101) have now begun to elucidate the conditions that enable the differentiation of TCM. The transcription factors that dictate the memory program are being identified (102-105). Although the selective generation of TCM in vivo may be desirable, vaccinologists have not yet achieved this goal. Initial antigen signal strength (98), strength and quality of costimulation (99, 101), the presence of cytokines, such as IL-15 (55, 56, 58), and the precursor frequency of naive antigen-specific cells (100) each may influence the relative ratio of TCM to TEM cells generated in response to infection or vaccination. Questions remain whether immunotherapists can generate highly avid TCM in vivo by modifying vaccine regimens in the setting of chronic antigen stimulation, where highly avid self/tumor-reactive CD8+ T cells might become senescent or exhausted (23, 69, 70) (Fig. 1B). The stimuli required for the reversion of senescent cells into less differentiated/central memory state in vivo or in vitro have not yet been identified. Indeed, such dedifferentiation may be non-physiologic. At the same time, less differentiated T cells, characterized by low avidity TCR, might not be the optimal target for vaccination. A more plausible approach might be the transduction of high affinity self/tumor-specific TCRs into naive CD8+ T cells followed by their expansion and selective differentiation into TCM in vitro prior to adoptive transfer.
Induction of corrupted memory T cells might also be the consequence of immunization in the absence of T-cell help typical of class I epitope vaccines. Viral studies have revealed the requirement of Th for the generation and maintenance of functional CD8+ T-cell memory (45-47). Consistent with the viral findings, cotransfer of Th in a ACT-based immunotherapy model resulted in long-term maintenance of self/tumor-reactive CD8+ T-cell effector function and tumor regression (49). In patients, co-immunization with class II-restricted epitopes has been utilized with the aim of helping CD8+ T-cell antitumor responses (106). However, this strategy has been unsuccessful to date; coimmunization with class II epitopes impaired self/tumor-specific CD8+ T-cell responses (106). These surprising findings may indicate that Treg rather than Th are preferentially activated by immunization with self/tumor class II peptides or that the influence of Treg is dominant over Th. Alternatively, provision of exogenous c cytokines, such as IL-2, IL-7, IL-15, and IL-21, may supplant the requirement for Th to expand antigen-specific CD8+ T cells following a primary response (17, 46, 56, 107-109). While exogenous IL-2 induces Treg activation and expansion in vivo (110, 111), IL-7, IL-15, and possibly IL-21 are emerging as possible vaccine adjuvants with the potential to selectively drive CD8+ T-cell responses over Treg responses (112). Finally, the functionality of helpless CD8+ memory T cells might be enhanced by PDL-1 blockade (75).
Generation of functional memory T cells is impaired in the tumor-bearing host
To support its own proliferation and ability to metastasize, tumor cells can negatively affect the immune environment (113-115). A full elucidation of the mechanisms employed by tumors to corrupt host immunity is beyond the scope of the present article, but significant new studies indicate that the tumor's role in evading host immunity is more active than passive (113-115, 129). One mechanism, particularly relevant to the formation of T-cell memory, involves the indirect induction of Treg through conversion of immature myeloid dendritic cells (DCs) by tumor cells into transforming growth factor-b (TGF-b)-secreting APCs (116). Treg have been shown to negatively regulate the numbers and the functionality of memory CD8+ T cells (50-52). In addition, Treg are capable of suppressing the antitumor activity of self/tumor-reactive T cells (49, 117-120). Furthermore, the presence of Treg in the tumor bed has been associated with poor survival in patients with ovarian cancer (121, 122). Selective depletion of Treg may offer a way of resetting the dysfunctional immunity characterizing the tumor-bearing state and augmenting the generation and function of vaccine-elicited CD8+ memory T cells to cancer (49, 108, 123,124). Alternatively, Toll-like receptor (TLR) agonists might be used to reverse T-cell inhibition by Treg (125, 126).
Removal of Treg cells alone may be not sufficient for optimal antitumor T-cell memory response. The magnitude, duration, and persistence of T-cell responses might be also be negatively influenced by the presence of cellular competitors acting as ‘sinks’ for activating and homeostatic cytokines (108, 124, 127). In particular, reduced access to IL-7 and IL-15, two critical cytokines for memory formation and maintenance, has been recently linked to an impairment of antitumor effector function and persistence of self/tumor-reactive CD8+ T cells (108, 124). Elimination of both cellular cytokine sinks and Treg by nonspecific modalities such as systemic chemotherapy and/or total body irradiation followed by immunization of adoptively transferred T cells could be an effective strategy to reprogram T-cell memory in the absence of the negative regulatory effects of the host immune cells (108, 128, 130).
Conclusion
CD8+ T-cell memory is heterogeneous and is characterized by a collection of cells with unique functions, phenotypes, and anatomic localization. It may be instructive to compare the memory T-cell subsets found in the tumor-bearing state with those existing during chronic infectious disease states. Chronic exposure to antigen drives T cells to a state of senescence and exhaustion without the formation of TCM. Dysfunctional immunity, enforced by Treg and alterations of the cytokine environment, further characterize both the tumor-bearing and chronic inflammatory states. Together, these factors may contribute to the relative ineffectiveness of therapeutic vaccines for solid tumors and chronic infectious diseases and represent an enormous hurdle for active immunotherapies. Therapeutic vaccines for cancer and chronic infectious diseases may achieve more consistent efficacy by resetting the dysfunctional and corrupted immune state. Effective immunotherapies may be accomplished through a reprogramming of the CD8+ T cell memory compartment by blockade of negative signals, such as those delivered by PD_1, or through the provision of newly generated, non-corrupted effector and memory T cells into an ablated host with ACT.
Acknowledgements
CAK dedicates this manuscript to Grandma Anne Anderson and her personal battle with cancer. The authors thank Alan Hoofring for his excellent artistic contributions to the artwork in this manuscript.
References
- 1.Starnes CO. Coley's toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 2.Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med. 2005;11:S54–S62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 3.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11:S25–S32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 5.Boon T, Coulie PG, Van den Eynde BJ, Van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006 doi: 10.1146/annurev.immunol.24.021605.090733. doi: 10.1146/annurev.immunol.24.021605. 090733. [DOI] [PubMed] [Google Scholar]
- 6.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–276. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 7.Restifo NP, Rosenberg SA. Use of standard criteria for assessment of cancer vaccines. Lancet Oncol. 2005;6:3–4. doi: 10.1016/S1470-2045(04)01693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmerman JM, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, et al. Tumor progression can occur despite the Induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res. 2003;9:2973–2980. [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall JL, et al. Phase I study of sequential vaccinations with fowlpox-CEA (6D) -TRICOM alone and sequentially with vaccinia-CEA (6D) -TRICOM, with and without granulocyte-macrophage colonystimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 13.Speiser DE, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayyoub M, et al. Activation of human melanoma reactive CD8+ T cells by vaccination with an immunogenic peptide analog derived from Melan-A/melanoma antigen recognized by T cells-1. Clin Cancer Res. 2003;9:669–677. [PubMed] [Google Scholar]
- 15.Smith JW, et al. Adjuvant immunization of HLA-A2-positive melanoma patients with a modified gp100 peptide induces peptidespecific CD8+ T-cell responses. J Clin Oncol. 2003;21:1562–1573. doi: 10.1200/JCO.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer DC, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–7216. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 20.Dudley ME, Rosenberg SA. Adoptive-cell transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 24.Khong HT, Rosenberg SA. Pre-existing immunity to tyrosinase-related protein (TRP) -2, a new TRP-2 isoform, and the NY-ESO-1 melanoma antigen in a patient with a dramatic response to immunotherapy. J Immunol. 2002;168:951–956. doi: 10.4049/jimmunol.168.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 26.Badovinac VP, Harty JT. CD8(+) T-cell homeostasis after infection: setting the ‘curve’. Microbes Infect. 2002;4:441–447. doi: 10.1016/s1286-4579(02)01558-7. [DOI] [PubMed] [Google Scholar]
- 27.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechner F, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Epps HL, et al. Long-lived memory T lymphocyte responses after hantavirus infection. J Exp Med. 2002;196:579–588. doi: 10.1084/jem.20011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mongkolsapaya J, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 31.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 32.Hwang L, Yu Z, Palmer DC, Restifo NP. The in vivo expansion rate of properly stimulated transferred CD8+ T cells exceeds that of an aggressively growing mouse tumor. Cancer Res. 2006;66:1132–1138. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 34.Murali-Krishna K, et al. Counting antigenspecific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 35.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells inMHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 36.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 37.Goldrath AW, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL) -15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kieper WC, et al. Overexpression of interleukin (IL) -7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker TC, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schluns KS, Ký`eper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 42.King C, Ilý`c A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 43.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 44.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 45.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 47.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 48.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antony PA, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 51.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci USA. 2002;99:8832–8837. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 54.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 55.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8 (+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klebanoff CA, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 58.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 60.Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 61.Castý`glioný` P, Gerloni M, Zanettý` M. Genetically programmed B lymphocytes are highly efficient in inducing anti-virus protective immunity mediated by central memory CD8 T cells. Vaccine. 2004;23:699–708. doi: 10.1016/j.vaccine.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 62.Vaccarý` M, Trindade CJ, Venzon D, Zanettý` M, Franchý`ný` G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J Immunol. 2005;175:3502–3507. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- 63.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Wý`llý`ams J, Krzych U. Protracted protection to Plasmodium berghei malaria is linked to functionally and henotypically heterogeneous liver memory CD8+ T cells. J Immunol. 2003;171:2024–2034. doi: 10.4049/jimmunol.171.4.2024. [DOI] [PubMed] [Google Scholar]
- 66.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 67.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 68.den Boer AT, vanMierlo GJ, Fransen MF, Melief CJ, Offringa R, Toes RE. The tumoricidal activity of memory CD8+ T cells is hampered by persistent systemic antigen but full functional capacity is regained in an antigen-free environment. J Immunol. 2004;172:6074–6079. doi: 10.4049/jimmunol.172.10.6074. [DOI] [PubMed] [Google Scholar]
- 69.Zý`ppelý`us A, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 70.Anichini A, Vegettý` C, Mortarý`ni R. The paradox of T-cell-mediated antitumor immunity in spite of poor clinical outcome in human melanoma. Cancer Immunol Immunother. 2004;53:855–864. doi: 10.1007/s00262-004-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 74.Zhou S, Ou R, Huang L, Price GE, Moskophidis D. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J Virol. 2004;78:3578–3600. doi: 10.1128/JVI.78.7.3578-3600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 76.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sussman JJ, Parihar R, Winstead K, Finkelman FD. Prolonged culture of vaccine primed lymphocytes results in decreased antitumor killing and change in cytokine secretion. Cancer Res. 2004;64:9124–9130. doi: 10.1158/0008-5472.CAN-03-0376. [DOI] [PubMed] [Google Scholar]
- 78.Dudley ME, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 79.Dudley ME, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Appay V, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tewari K, Sacha J, Gao X, Suresh M. Effect of chronic viral infection on epitope selection, cytokine production, and surface phenotype of CD8 T cells and the role of IFN-gamma receptor in immune regulation. J Immunol. 2004;172:1491–1500. doi: 10.4049/jimmunol.172.3.1491. [DOI] [PubMed] [Google Scholar]
- 83.Brenchley JM, et al. Expression of CD57 defines replicative senescence and antigen induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 84.van Leeuwen EM, et al. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 85.Boutboul F, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19:1981–1986. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 86.Thimme R, et al. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Papagno L, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Champagne P, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 90.Bergmann CC, Altman JD, Hinton D, Stohlman SA. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- 91.Robbins PF, et al. Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Powell DJ, Jr, Rosenberg SA. Phenotypic and functional maturation of tumor antigen reactive CD8+ T lymphocytes in patients undergoing multiple course peptide vaccination. J Immunother. 2004;27:36–47. doi: 10.1097/00002371-200401000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Appay V, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 96.Dutoit V, et al. Degeneracy of antigen recognition as the molecular basis for the high frequency of naive A2/Melan-a peptide multimer (+) CD8 (+) T cells in humans. J Exp Med. 2002;196:207–216. doi: 10.1084/jem.20020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mortarini R, et al. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535–2545. [PubMed] [Google Scholar]
- 98.van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- 99.Gett AV, Sallusto F, Lanzavecchý`a A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 100.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancoý`s L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bondanza A, et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- 102.Ichii H, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 103.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 104.Manders PM, et al. Inaugural Article: BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc Natl Acad Sci USA. 2005;102:7418–7425. doi: 10.1073/pnas.0501585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 106.Phan GQ, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 108.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 111.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+CD25hiFoxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang T, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 114.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 116.Ghiringhelli F, et al. Tumor cells convert immature myeloid dendritic cells into TGFbeta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and nonself. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 118.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Viguier M, et al. Foxp3 expressing CD4+CD25 (high) regulatory T cells are over-represented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 120.Woo EY, et al. Regulatory CD4 (+) CD25 (+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 121.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 122.Sato E, et al. Intraepithelial CD8+ tumor infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dannull J, et al. Enhancement of vaccinemediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang LX, et al. Interleukin-7-dependent expansion and persistence of melanoma specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 126.Peng G, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 127.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cellmediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rapoport AP, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 129.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wrzesinki C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17:195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]