New insights into the regulation of T cells by γc family cytokines (original) (raw)
Key Points
- The common cytokine receptor γ-chain (γc) family of cytokines consists of interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15 and IL-21, each of which is a short-chain four α-helical bundle type I cytokine. Mutations in the gene encoding γc (IL2RG) in humans result in X-linked severe combined immunodeficiency, which is characterized by a marked defect in the development of T and natural killer (NK) cells and functional defects of B cells; in mice, deletion of this gene is characterized by the absence of B, T and NK cells.
- γc family cytokines and the related cytokine thymic stromal lymphopoietin (TSLP) have distinct effects on the regulation of survival and proliferation of T cells. IL-2 and TSLP increase the proliferation and/or survival of effector T cells, whereas IL-7, IL-15 and TSLP are survival factors for naive and memory αβ T cells, as well as γδ T cells. In addition, the combination of IL-15 and IL-21 increases the proliferation and decreases the apoptosis of CD8+ T cells.
- As well as the direct effects of γc family cytokines and TSLP on the homeostasis of T cells, they also have indirect effects on T cells through their regulation of dendritic cell (DC) functions. IL-15 and TSLP induce the up-regulation of expression of co-stimulatory molecules and increased presentation of antigen on DCs, whereas IL-7 and IL-21 suppress the maturation of DCs.
- Although IL-2 is an important factor for the development and function of regulatory T (TReg) cells, the lack of IL-2, IL-2Rα or IL-2Rβ does not alter the expression of forkhead box P3 (FOXP3) or result in a complete loss of TReg cells. By contrast, STAT5 activation is sufficient to increase the number of CD4+CD25+ TReg cells even when IL-2-induced signalling is defective, which shows that STAT5A and STAT5B are crucial factors downstream of IL-2R and indicates that other factors that activate the STAT5 pathway might also contribute to TReg cell development and could partially compensate when IL-2-induced signalling is defective. Indeed, IL-7, IL-15 and TSLP also contribute to TReg cell development and function.
- In the primary response to antigen, naive CD4+ T cells differentiate to distinct polarized subsets, including T helper 1 (TH1), TH2, TH17 and T follicular helper (TFH) cells. Recent studies show that IL-2 and IL-4 are both required for the efficient induction of TH2 cells and that IL-21 can promote the differentiation of TH17 cells and TFH cells. In addition to their contributions to TH cell differentiation, γc cytokines also contribute to the generation and activity of cytotoxic CD8+ T cells, with IL-2, IL-15 and IL-21 increasing the cytolytic activity of CD8+ T cells during priming and increasing their antitumour immunity.
- The actions of γc cytokines have clinical relevance, and modulation of their effects has implications for the treatment of cancer, autoimmunity, allergy and immunodeficiency. Administration of IL-2, IL-15 and IL-21 has antitumour effects; treatment with IL-2, IL-7 and IL-15 could be used in immunodeficiency disorders; blocking of IL-4, IL-9 and TSLP can decrease allergic symptoms; and neutralization of IL-21 could prevent and/or ameliorate several autoimmune diseases.
Abstract
Common cytokine receptor γ-chain (γc) family cytokines have crucial roles in the development, proliferation, survival and differentiation of multiple cell lineages of both the innate and adaptive immune systems. In this Review, we focus on our current understanding of the distinct and overlapping effects of interleukin-2 (IL-2), IL-7, IL-9, IL-15 and IL-21, as well as the IL-7-related cytokine thymic stromal lymphopoietin (TSLP), on the survival and proliferation of conventional αβ T cells, γδ T cells and regulatory T cells. This knowledge potentially allows for the therapeutic manipulation of immune responses for the treatment of cancer, autoimmunity, allergic diseases and immunodeficiency, as well as for vaccine development.
Similar content being viewed by others
T cells in health and disease
Article Open access 19 June 2023
Main
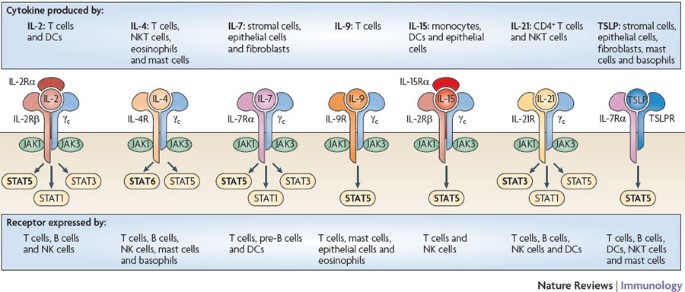
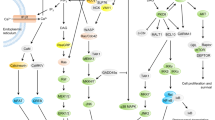
Cytokines are hormones of the immune system that have important functions related to cellular proliferation, differentiation and survival. Type I cytokines have a common structure that contains four α-helical bundles and they include many interleukins, as well as some growth and haematopoietic factors. One important family of type I cytokines is the common cytokine receptor γ-chain (γc) family, which consists of interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15 and IL-21 (Fig. 1), and is so named because the receptors for these cytokines share γc (also known as IL-2Rγ and CD132)1,2.
Figure 1: Receptors for γc family cytokines and TSLP.
Shown are the receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, IL-21 and thymic stromal lymphopoietin (TSLP). IL-2 and IL-15 are the only two of these cytokines to have three receptor chains. The receptors for these two cytokines share the common cytokine receptor γ-chain (γc; also known as IL-2Rγ) and IL-2Rβ, and the receptors for IL-7 and TSLP share IL-7Rα. Of the cytokines shown, only TSLP does not signal through a receptor containing γc. There are three classes of IL-2 receptor that bind IL-2 with low affinity (IL-2Rα alone), intermediate affinity (IL-2Rβ and γc) and high affinity (IL-2Rα, IL-2Rβ and γc); only the high-affinity IL-2 receptor is shown. The receptor for each γc family cytokine activates Janus kinase 1 (JAK1) and JAK3, whereas the receptor for TSLP has been reported to not activate any JAK25,26. The main signal transducer and activator of transcription (STAT) proteins that are activated by these cytokine receptors are shown in bold. STAT5 refers to both STAT5A and STAT5B. DC, dendritic cell; NK cell, natural killer cell; NKT cell, natural killer T cell.
γc was first discovered as a component of the receptor for IL-2 (Ref. 3), which is the prototypical member of this family. The IL-2 receptor (IL-2R) consists of three chains (IL-2Rα, IL-2Rβ and γc), which together form the high-affinity IL-2R (Fig. 1), but which in other combinations can bind IL-2 with low affinity (IL-2Rα alone) or intermediate affinity (IL-2Rβ and γc). The structures of some of the receptors for members of the γc family are known, providing insight into how different cytokines can each interact with γc4.
The gene encoding γc (IL2RG) is mutated in humans with X-linked severe combined immunodeficiency (XSCID)5, and these patients lack T cells and natural killer (NK) cells, which indicates that γc is crucial for the development of these cells. The finding that the immune defects in patients with XSCID are much more severe than those of humans or mice lacking IL-2, in which the development of T and NK cells is normal, led to the hypothesis and subsequent confirmation that γc is shared by the receptors for multiple cytokines1.
IL-2 is a T cell growth factor, can augment NK cell cytolytic activity and promotes immunoglobulin production by B cells6. In addition, it contributes to the development of regulatory T (TReg) cells and therefore peripheral T cell tolerance7, as well as regulating the proliferation and apoptosis of activated T cells8,9. IL-4 is required for the development and function of T helper 2 (TH2) cells and is therefore regarded as the classical TH2 cell cytokine. IL-4 also has an important role in allergy and immunoglobulin class switching10. IL-7 has a central role in the development of T cells in both humans and mice. Indeed, defective IL-7-induced signalling is responsible for the effects on T cell development that are observed in patients with XSCID5, as well as in patients with SCID caused by mutations in Janus kinase 3 (JAK3)11,12, which encodes a signalling molecule downstream of γc, or by mutations in IL7RA (also known as CD127)13 (Table 1). Interestingly, IL-7 is also required for the development of B cells in mice but it is not necessary for B cell development in humans; B cells develop normally in patients with XSCID (IL2RG mutation), JAK3-deficient SCID and IL-7RA-deficient SCID1. However, in an in vitro model, IL-7 can promote the development of human B cells from adult bone marrow haematopoietic stem cells (HSCs), although not from cord blood HSCs14. In addition, IL-7 is well known for its potent role as a lymphocyte survival factor15,16. IL-9 is produced by a subset of activated CD4+ T cells17,18 and it induces the activation of epithelial cells, B cells, eosinophils and mast cells19 (Fig. 1). Although IL-9 has been shown to function as a T cell growth factor during the late phase of an immune response20, its role in T cell biology remains unclear. IL-15 is essential for the development of NK cells, and it is the defective IL-15-induced signalling that results in the failure of NK cell development in patients with both XSCID and JAK3-deficient SCID1. IL-15 also has an essential role in CD8+ T cell homeostasis16. IL-21 is the most recently described member of the γc family2 and it has broad actions that include promoting the terminal differentiation of B cells to plasma cells, cooperating with IL-7 or IL-15 to drive the expansion of CD8+ T cell populations and acting as a pro-apoptotic factor for NK cells and incompletely activated B cells2. IL-21 has also been shown to be an essential mediator of the development of type 1 diabetes mellitus21,22 and systemic lupus erythematosus (SLE)23 in animal models, and to have potent antitumour actions2.
Table 1 The basis of defects in severe combined immunodeficiency (SCID)
γc family cytokines all signal through the JAK–STAT (signal transducer and activator of transcription) pathway. Interestingly, IL-2, IL-7, IL-9 and IL-15 mainly activate STAT5A and STAT5B (together referred to here as STAT5), whereas IL-4 generally activates STAT6 and IL-21 mainly activates STAT3 (Ref. 24) (Fig. 1). The activation of different STAT proteins could help to explain the different effects of these cytokines.
As mentioned above, some of the γc family cytokines broadly contribute to lymphocyte homeostasis, which is the main focus of this Review. We also discuss another cytokine that is not a member of the γc family but that has overlapping functions with IL-7, known as thymic stromal lymphopoietin (TSLP)25. Whereas the IL-7 receptor contains IL-7Rα and γc, the TSLP receptor consists of IL-7Rα and TSLPR (also known as CRLF2), which is closely related to γc26,27 (Fig. 1).
Direct effects of γ c family cytokines on T cells
Regulation of naive and memory αβ T cell homeostasis. γc-deficient mice have a low thymic output of T cells and lymphopaenia, and those T cells that do develop have impaired survival28. IL-7 seems to be the most important of the γc family cytokines for regulating the homeostasis of naive and memory T cells29,30,31,32 (Fig. 2). In contrast to other γc family cytokines, the levels of which increase after immune cell activation, IL-7 is constitutively produced by stromal and epithelial cells in the bone marrow and thymus and by fibroblastic reticular cells in the T cell zones of secondary lymphoid organs16,33. The availability of IL-7 is regulated by both its production and its consumption by CD4+ T cells16,34. So, decreased numbers of CD4+ T cells in humans are associated with increased levels of IL-7 (Ref. 34).
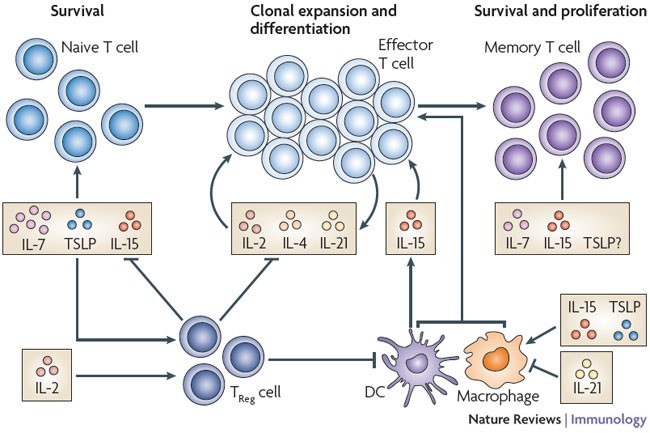
Figure 2: Direct and indirect effects of γc family cytokines and TSLP on T cell proliferation, homeostasis and differentiation.
Cytokines of the common cytokine receptor γ-chain (γc) family can directly influence the survival, activation, proliferation and differentiation of T cells (top half of the figure), as well as indirectly affecting these processes through effects on dendritic cells (DCs), macrophages and regulatory T (TReg) cells (bottom half of the figure). Although interleukin-15 (IL-15) is a crucial factor for memory CD8+ T cell homeostasis, it is also responsible for the recovery of naive CD8+ T cells in lymphopaenic conditions. In the absence of IL-7, IL-15 has important effects on the homeostasis of memory CD4+ T cells. Both IL-7 and thymic stromal lymphopoietin (TSLP) are important for the survival of naive T cells, with IL-7 having the greater role. The effect of TSLP on memory T cells has not been evaluated. IL-2, IL-4 and IL-21 are produced by activated T cells and have essential roles in T cell differentiation. In addition, IL-2 and IL-15 can increase the proliferation of T cells after antigen stimulation.
A distinctive feature of IL-7 compared with the other γc family cytokines relates to the expression of its receptor. Whereas expression of most of the cognate receptor chains for γc family cytokines is upregulated after T cell receptor (TCR) activation, IL-7Rα is expressed by naive and memory resting T cells but its expression is downregulated after TCR activation29,35 (Table 2). This indicates that IL-7 mainly mediates its effects on naive and memory T cells rather than on activated T cells (see below). IL-2, IL-7 and other pro-survival cytokines can transiently decrease the expression of IL-7Rα on T cells35,36,37, decreasing their responsiveness to IL-7 and also decreasing IL-7 consumption, thereby increasing the availability of IL-7 for other cells that express high levels of IL-7Rα and are poised to receive survival signals in vivo37. Maintaining IL-7Rα expression depends, at least in part, on the transcription factor GABP (GA-binding protein)38, and GABP and the transcription repressor GFI1 (growth factor independent 1) control the up- and downregulation of IL-7Rα expression, respectively39.
IL-7 uses at least two different mechanisms to support T cell homeostasis. First, it promotes T cell survival by activating the pro-survival phosphoinositide 3-kinase (PI3K)–AKT signalling pathway and by increasing the expression of survival factors such as B cell lymphoma 2 (BCL-2) and myeloid cell leukaemia sequence 1 (MCL1), whereas it inhibits the expression of the pro-apoptotic factors BAX and BAD15,16. Second, IL-7 induces the proliferation of naive and memory T cells in lymphopaenic conditions and of memory T cells, but not naive T cells, under normal physiological conditions29,30,40,41 (Fig. 2).
Table 2 Expression of receptors for γc family cytokines and TSLP
Although Il7ra −/− and Il7 −/− mice each have markedly decreased numbers of T cells, the absence of IL-7 in Il7 −/− mice can be partially compensated for by increasing levels of TSLP, which can result in the partial restoration of normal T and B cell numbers42,43. These observations indicated that TSLP might have a role in T cell homeostasis. Indeed, administration of recombinant TSLP to Il2rg −/− mice (that is, γc-deficient mice) results in a partial increase in the number of CD4+ and CD8+ T cells. Consistent with this, restoration of CD4+ and CD8+ T cell numbers after irradiation is less efficient in Tslpr −/− mice than in irradiated wild-type mice42. Moreover, TSLP promotes the survival of CD8+ T cells in both normal and lymphopaenic conditions44. Interestingly, whereas IL-7 induces the proliferation (as well as survival) of naive CD8+ T cells in lymphopaenic mice, TSLP does not affect the proliferation of these cells44. A possible explanation for this observation is that IL-7, but not TSLP, can activate JAK3 and is a more potent activator of STAT5 (Refs 25,26).
IL-15 is another important homeostatic cytokine and memory CD8+ T cells that express high levels of IL-2Rβ (also known as CD122)30,45,46,47, particularly the IL-2RβhiLY49+ subset of memory CD8+ T cells48, are the most sensitive to IL-15. Although IL-15 does not have an essential role in the homeostatic proliferation of memory CD4+ T cells, IL-15 was reported to affect the homeostasis of these cells in the absence of IL-7 (Ref. 49). Furthermore, depleting CD8+ T cells and NK cells, which are the main consumers of IL-15, results in a greater availability of IL-15 and increases the homeostatic proliferation of memory CD4+ T cells49. IL-15 is not crucial for the development of naive T cells, but Il15 −/− mice have decreased numbers of naive CD8+ T cells and these cells have decreased proliferative rates, which probably explains the slower recovery rate of adoptively transferred naive CD8+ T cells in Il15 −/− mice compared with wild-type mice40,47,50. Although memory CD8+ T cells express high levels of IL-15Rα46 (Table 2), their proliferation is greater in response to administration of an IL-15–IL-15Rα complex than of purified IL-15 alone51,52; in this system, IL-15 that is bound to IL-15Rα is presented to cells expressing the other IL-15R subunits, IL-2Rβ and γc53. Indeed, under physiological conditions, _trans_-presentation of IL-15 by IL-15Rα on the surface of other non-T cells is required54, underscoring the importance of this mode of signalling for IL-15.
IL-7 and IL-15 can also function cooperatively with IL-21 to expand CD8+ T cell populations in vitro55. Whereas IL-21 alone induces the survival of naive but not memory mouse CD8+ T cells, in the presence of IL-15 and IL-21 combined, the rate of apoptosis in both populations of CD8+ T cells is markedly decreased and cell proliferation is increased55. Similarly, the combination of IL-15 and IL-21 increases antigen-independent proliferation of human naive CD8+ T cells in vitro56, the number of CD8+ T cells producing IL-2 and interferon-γ (IFNγ) and the cytotoxic activity of these cells after TCR activation55,56. Although Il21r −/− mice have normal numbers of peripheral CD8+ T cells57, overexpression of IL-21 increases the number of memory CD8+ T cells58; it is unclear whether this is an effect of IL-21 alone or the result of synergistic actions of IL-21 with constitutively produced IL-7 or IL-15. Together, these data underscore the wide range of actions of various γc family cytokines, in particular IL-7 and IL-15, in naive and memory T cell homeostasis.
Proliferation and survival of effector T cells. IL-2 is perhaps the earliest cytokine to be secreted by T cells after TCR stimulation59 and it is important for the initiation of TH2 cell differentiation60,61. It is well known that IL-2 can induce the proliferation and survival of TCR-activated human and mouse T cells6,62 and is required for sustained expansion of T cell populations8. Although administration of supra-physiological levels of IL-2 to mice infected with lymphocytic choriomeningitis virus during the expansion phase of the antiviral T cell response unexpectedly decreases the number of antigen-specific CD4+ T cells63, this might reflect the ability of IL-2 to induce apoptosis of T cells that have been recently activated through their TCR (known as activation-induced cell death (AICD))9. Conversely, IL-2 treatment during the contraction phase of the T cell response results in increased survival and accumulation of T cells8,63,64. Overall, the role of IL-2 is multi-faceted owing to its complex effects on driving T cell proliferation, promoting the clonal expansion of TReg cells (see later) and mediating AICD.
The role of IL-7 and IL-15 in the expansion of effector and memory T cell populations has been widely studied. Immediately after TCR activation, most T cells downregulate IL-7Rα and upregulate IL-2Rα, IL-15Rα and IL-2Rβ expression (Table 2). It was therefore predicted that IL-7 is not required for the function of activated T cells29, and that IL-2 and/or IL-15, the production of which is also increased by activated dendritic cells (DCs)65,66,67, could increase the proliferative rate and/or decrease the contraction of effector T cell populations64,68,69. Although a selective population of primed T cells re-expresses IL-7Rα70, IL-7 signalling is not essential for the formation of functional memory CD4+ and CD8+ T cells71,72,73,74, which could indicate potentially redundant functions of TSLP and IL-7 or instead the important actions of other cytokines. Several findings support a possible role for TSLP in the expansion of effector and memory T cell populations: TSLPR expression is increased after TCR activation44,75 (Table 2), TSLP increases the proliferation of TCR-activated CD4+ T cells in vitro42,75 and TSLP production is increased during the acute phase of an immune response to pathogens and allergens76.
Regulation of γδ T cell homeostasis. γδ T cells are a population of T cells that arise from the same precursors as αβ T cells in the thymus. They migrate to the periphery, mostly to epithelial tissues, and have broad immunological actions, which include the production of cytokines and chemokines, cytolytic activity in response to pathogens, regulation of the viability and proliferation of keratinocytes, induction of macrophage and DC responses and presentation of antigen to T cells77,78. The expression of both γc and IL-7Rα is essential for normal γδ T cell development78. Under lymphopaenic conditions, γδ T cells undergo MHC-independent homeostatic proliferation that requires IL-7 or IL-15 (Refs 79,80). Although αβ and γδ T cells express comparable levels of IL-7Rα and IL-2Rβ79, partial depletion of αβ T cells, NK cells or γδ T cells themselves significantly increases the homeostatic proliferation of γδ T cells, which shows that these cells compete for limited quantities of IL-7 and IL-15 (Refs 79,80,81). So, the maintenance of γδ T cells as well as αβ T cells is regulated by IL-7 and IL-15.
Maintenance of T Reg cells. TReg cells are a subset of CD4+ T cells that were defined in part by their constitutive expression of IL-2Rα (also known as CD25) and the TReg cell-specific transcription factor forkhead box P3 (FOXP3), which controls the development and function of these cells7. Although IL-2 induces the proliferation and clonal expansion of conventional T cells6,62, IL-2 also mediates immune tolerance at least in part through its regulation of TReg cells. IL-2 deficiency is characterized by a decrease in the number and function of TReg cells and, accordingly, leads to lymphoproliferation and autoimmunity7. However, the lack of IL-2, IL-2Rα or IL-2Rβ does not alter FOXP3 expression or result in a complete loss of TReg cells82,83,84,85. By contrast, γc-deficient (Il2rg −/−) mice and Jak3 −/− mice, in addition to having very low numbers of T cells, are devoid of FOXP3+ TReg cells83,86.
STAT5 seems to be crucial for the development of TReg cells and its activation is sufficient to increase the number of CD4+CD25+ TReg cells even in the absence of IL-2 production87 or when there is defective IL-2-induced signalling88. In addition, deletion of Stat5a and Stat5b genes in mice is characterized by a marked decrease in the number of TReg cells in the thymus and in the periphery86. Correspondingly, a patient with a missense mutation in the STAT5B gene had a decreased number of CD4+CD25hi T cells and these cells had a marked decrease in the level of expression of FOXP3 and impaired suppressive activity89. These findings confirm earlier observations indicating that STAT5A and STAT5B are crucial factors for signal transduction downstream of IL-2R90,91,92,93 and suggest that other γc family cytokines, such as IL-7 and IL-15, that also activate STAT5 might contribute to TReg cell development and maintenance.
Although deficiency of IL-7 or IL-15 (which both activate STAT5) does not alter the number of FOXP3+ TReg cells88,94, the absence of IL-7- or IL-15-induced signalling in combination with disrupted IL-2R signalling results in a greater decrease in the number of TReg cells than is observed in mice lacking either IL-2 or IL-2Rα alone88,95. Interestingly, mouse TReg cells express low levels of IL-7Rα94,95, and in contrast to other subsets of T cells that can re-express IL-7Rα after culture in vitro, peripheral TReg cells from mice do not upregulate IL-7Rα expression after in vitro culture94. Nevertheless, the low level of IL-7Rα that is expressed by mouse TReg cells seems to be functional, as IL-7, even in the absence of IL-2-induced signalling, can mediate the survival, although not the proliferation, of mouse TReg cells95. Similarly, most human CD4+CD25+FOXP3+ TReg cells have lower levels of IL-7Rα expression than do CD4+CD25+FOXP3− T cells96,97. Il7ra −/− mice have a marked decrease in the number of TReg cells in lymphoid tissues and decreased suppressive activity compared with Il7 −/− mice94. These differences can be explained by the ability of TSLP (which signals through a receptor that contains IL-7Rα) to also mediate the induction of TReg cells94. In conclusion, IL-2, IL-7, IL-15 and TSLP all contribute to the development and function of TReg cells.
Although IL-2, IL-4, IL-7, IL-15 and IL-21 can promote the survival of TReg cells and rescue them from apoptosis in vitro, only IL-2 induces their proliferation and clonal expansion98. Consistent with this, the peripheral homeostasis of TReg cells in vivo is more dependent on IL-2 than on the other γc family cytokines7, and neutralizing IL-2 in mice not only decreases the number of TReg cells in the thymus but also prevents their clonal expansion in lymph nodes99. Correspondingly, IL-2 therapy during immune reconstitution after chemotherapy markedly increases the size of the TReg cell compartment100.
Indirect effects of γ c family cytokines on T cells
T cell survival and proliferation through DCs. It is well known that γc family cytokines have pleiotropic effects on the immune system and that they can stimulate various populations of cells in addition to T cells, which in turn affect T cell homeostasis. For example, DCs are key players in the activation of an adaptive immune response, and γc family and related cytokines, the expression of which is induced by pathogens, can activate (for example, IL-15 or TSLP) or inhibit (for example, IL-7 or IL-21) the function of DCs (Fig. 2).
DCs constitutively express IL-2Rβ and γc66,101,102 and upregulate the expression of IL-15Rα in response to type I and II IFNs and inducers of nuclear factor-κB (NF-κB) activation, such as ligands for Toll-like receptors (TLRs)65,66. Similar signals promote the production of IL-15 by DCs and epithelial cells65,66. Therefore, IL-15 has both paracrine and autocrine actions on DCs that result in the increased survival of mature DCs, the upregulation of expression of co-stimulatory molecules and the increased presentation of antigen by DCs to CD4+ and CD8+ T cells65,66,67,103. DCs from aged mice produce less IL-15 than do those from young mice, and the functional defects of DCs from aged mice can be reversed by IL-15 treatment104. So, the decrease in IL-15 production by DCs with age might be a factor that contributes to decreased immunity to pathogens in the elderly. Moreover, infection of humans with hepatitis C virus decreases IFNα-mediated IL-15 production by DCs and therefore decreases the maturation of functional DCs, which has been suggested to be a possible mechanism for the poor T cell-mediated immunity against this virus105.
Both IL-4 and TSLP are involved in TH2 cell responses and have essential roles in allergic diseases10,25,76; however, their effects on DCs differ. IL-4 is a survival factor for DCs and, in combination with granulocyte/macrophage colony-stimulating factor, promotes the differentiation of DCs from mouse bone marrow progenitor cells and from human monocytes in vitro148,149. DCs pre-cultured in the presence of IL-4 express a relatively low level of MHC and co-stimulatory molecules, which is indicative of an immature phenotype, and these cells respond poorly to IFNα106. Unlike IL-4, TSLP is not required for DC differentiation, but (as shown by in vitro experiments) it promotes the activation of DCs and their upregulation of expression of MHC class II and co-stimulatory molecules, including CD80, CD86 and OX40L25. Human DCs that are stimulated with TSLP support naive CD4+ T cell homeostasis and induce robust proliferation and differentiation of human CD4+ T cells into inflammatory TH2 cells25. In mice, TSLP has an important role in intestinal immunity by inhibiting the lipopolysaccharide-induced production of IL-12 by DCs and thereby decreasing the number of IFNγ+CD4+ T cells that are generated107. Interestingly, IL-7 maintains the immature phenotype of DCs and can downregulate MHC class II expression by mature mouse DCs, which correlates with decreased homeostatic proliferation of CD4+ T cells108. IL-21 is not required for DC differentiation, but pre-treatment of DCs with IL-21 inhibits their maturation in response to TLR stimuli, thereby suppressing DC functions, such as antigen presentation and cytokine and chemokine secretion109,110. Because IL-21 is produced by CD4+ T cells after antigen stimulation2 and IL-21-primed DCs have inhibitory effects on T cell responses, the production of IL-21 by T cells could activate a DC-mediated negative feedback loop.
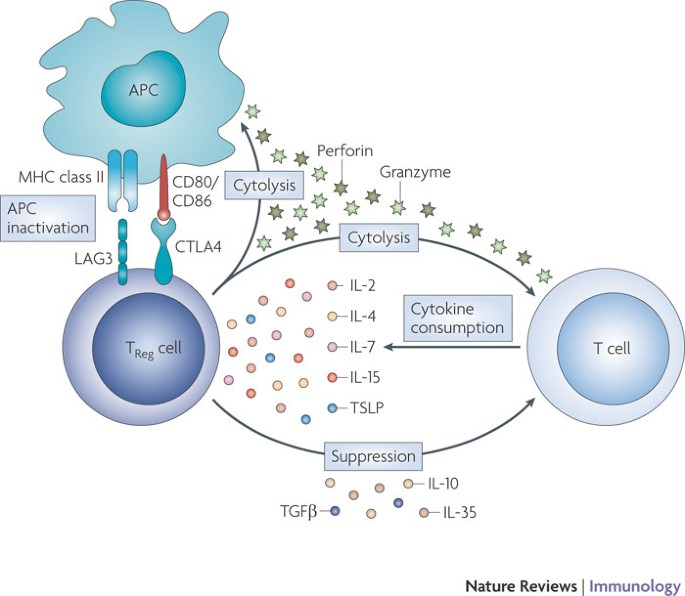
Conventional T cell homeostasis through T Reg cells. As discussed above, γc family cytokines have an important role in the development and maintenance of TReg cells. In turn, TReg cells inhibit the proliferation of autoreactive T cells, thereby preventing autoimmunity, and suppress the response of conventional T cells to foreign and self-antigens. Several mechanisms have been proposed to explain how TReg cells mediate this suppression (Fig. 3), which include: the inhibition of responder T cells by producing suppressive cytokines such as transforming growth factor-β (TGFβ), IL-10 and IL-35; the inactivation of antigen-presenting cells (APCs) through expression of the inhibitory molecules cytotoxic T lymphocyte antigen 4 (CTLA4) and lymphocyte activation gene 3 (LAG3); the killing of target cells through cytolytic activity; and the consumption of pro-survival γc family cytokines, thereby resulting in the apoptosis of conventional T cells in vitro98,111. Although it is not yet clear whether the cytokine-deprivation mechanism occurs in vivo, TReg cells can induce cytokine-dependent apoptosis of conventional CD4+ T cells in mice98. Note that the mechanisms listed above are not mutually exclusive and that more than one mechanism might be used.
Figure 3: Mechanisms of T cell regulation by TReg cells.
Regulatory T (TReg) cells use several mechanisms to suppress the activation and proliferation of conventional T cells. TReg cells modulate the functions of antigen-presenting cells (APCs) by inhibiting their maturation and blocking the cell surface expression of MHC molecules and co-stimulatory molecules (CD80 and CD86), thereby attenuating interactions between APCs and T cells. TReg cells might have cytolytic effects on target T cells, as well as on APCs, through the secretion of granzymes and perforin. TReg cells suppress the activation and proliferation of T cells through the secretion of inhibitory cytokines, such as transforming growth factor-β (TGFβ), interleukin-10 (IL-10) and IL-35 and by the consumption of cytokines of the common cytokine receptor γ-chain (γc) family. Deprivation of γc family cytokines induces the expression of pro-apoptotic proteins by conventional T cells and increases their apoptotic rate. CTLA4, cytotoxic T lymphocyte antigen 4; LAG3, lymphocyte activation gene 3; TSLP, thymic stromal lymphopoietin.
γ c family cytokines and T cell differentiation
Naive T cells can differentiate during a primary antigen response into several distinct polarized subsets, such as TH1, TH2, TH17 and T follicular helper (TFH) cells. These subsets produce discrete sets of cytokines and chemokines to allow responses to different classes of pathogen. Four γc family cytokines are among the main cytokines that are produced by these polarized cells: TH1 cells produce IL-2, TH2 cells produce IL-4 and IL-9, and TH17 and TFH cells, as well as TH1 and TH2 cells, produce IL-21. These cytokines act on other target cells to direct immune responses, but IL-2, IL-4 and IL-21 also have important roles in the early development of CD4+ T cell subsets.
T H 1 cell differentiation. TH1 cell differentiation depends mainly on APC-derived IL-12, which leads to IFNγ production and increased IL-12Rβ2 expression by APCs112. Although IL-2 is the earliest detected cytokine to be produced by naive CD4+ T cells after TCR stimulation59, and this cytokine is one of the main products of TH1 cells113, it is not clear whether IL-2-induced signalling contributes to early commitment to the TH1 cell lineage. IL-21 was reported to be a TH2-type cytokine that had inhibitory effects on TH1 cells114, but IL-21 does not affect expression of the TH1 cell-associated transcription factor T-bet (also known as TBX21) or of IL-12Rβ2 by mouse CD4+ T cells114. Instead, IL-21 can inhibit IFNγ production by naive CD4+ T cells that are undergoing TH1 cell differentiation by repressing expression of the T-box transcription factor Eomesodermin115. It is unclear whether this inhibition of IFNγ production by IL-21 has a role in modulating TH1 cell responses in vivo as opposed to promoting the differentiation of TH17 cells (see below). Interestingly, in human peripheral blood T cells stimulated through the TCR, IL-21 actually induces IFNγ, T-bet and IL-12Rβ2 expression116, which indicates that, under certain circumstances, IL-21 might promote TH1 cell differentiation.
T H 2 cell differentiation. In vitro studies have shown that IL-2 and IL-4 are both required for the efficient induction of TH2 cells. IL-2 is produced early after the activation of naive CD4+ T cells and activates STAT5A and STAT5B to promote increased transcription of the Il4ra gene, leading to the increased cell surface expression of IL-4Rα (also known as CD124) and subsequent increased responsiveness to IL-4 (Ref. 61). IL-2 also induces binding of STAT5 to consensus binding sites located within DNase I hypersensitivity sites in the Il4 locus, thereby promoting increased accessibility of this locus to the formation of transcriptional complexes60. A genome-wide in vivo analysis showed that IL-2, through its effects on STAT5, activates not only the Il4ra locus but also the entire TH2 cytokine locus, which includes Sept8, Kif3a, Il4, Il13, Rad50, Il15 and Irf1. Interestingly, this analysis showed that STAT5A and STAT5B bind first at the Il4ra locus and then at the TH2 cytokine locus in vivo, which is consistent with the observation that IL-4 is produced by TH2 cells after they express IL-4Rα61. So, IL-2-induced signalling during TH2 cell differentiation results in both increased production of IL-4 and increased responsiveness to IL-4, leading to stabilization of this lineage. Other cytokines that can activate STAT5, including IL-7 and IL-15, were also shown to induce IL-4Rα expression, which indicates that multiple STAT5 activators might be able to prime T cells for TH2 cell differentiation61. Although IL-21 can also promote STAT5 activation, IL-21-induced signalling does not affect the efficiency of TH2 cell differentiation in vitro57, which is consistent with the fact that IL-21 mainly activates STAT3 rather than STAT5. IL-21R-deficient mice have decreased responses to TH2 cell-inducing pathogens; however, it is possible that this results from decreased effects of IL-21 on macrophage activation117 rather than from direct effects on TH2 cells.
T H 17 cell differentiation. The differentiation of TH17 cells depends in part on TGFβ, an immunosuppressive cytokine that also has a role in TReg cell differentiation. The presence of either IL-6 or IL-21 during priming with TGFβ subverts T cell differentiation from the FOXP3-directed TReg cell pathway to the TH17 cell pathway through the induction of expression of the orphan nuclear receptor retinoic acid receptor-related orphan receptor-γt (RORγt; also known as RORC)118,119,120. TH17 versus TReg cell differentiation is therefore determined by the presence of IL-6 or IL-21.
IL-2 can promote the development of TReg cells, and it inhibits the differentiation of naive CD4+ T cells into TH17 cells121,122. Accordingly, administration of IL-2 to tumour-bearing mice can decrease the number of IL-17-producing cells and increase the number of TReg cells123. Correspondingly, Il2 −/− mice have a decrease in the number of TReg cells84 and an increase in the production of IL-17 (Ref. 121), which indicates that IL-17-producing cells might contribute to the autoimmune disease that develops in Il2 −/− mice7. However, Il17 −/− Il2 −/− mice develop systemic autoimmune haemolytic anaemia to the same extent as Il2 −/− mice, which indicates that IL-17-producing cells are not absolutely required for this disease process and that other potentially redundant cytokines also contribute124. Although IL-2 inhibits TH17 cell differentiation, it can provide proliferative signals to human TH17 cells, as shown by the IL-2-induced in vitro proliferation of TH17 cells from normal donors and from patients with uveitis or scleritis125. Interestingly, the inhibitory effects of IL-2 on the TH17 cell lineage can be prevented by IL-1, which indicates that the local cytokine profile controls the IL-17+ T cell pool126.
The role of IL-21 in the differentiation of TH17 cells is controversial. In vitro experiments have shown that IL-21 is crucial for upregulating IL-23R expression by TH17 cells120. IL-23, which is produced by APCs, is an important factor in the differentiation and proliferation of TH17 cells and therefore in inflammatory diseases, but IL-23R is not expressed by naive CD4+ T cells. IL-21 therefore promotes the expansion of TH17 cell populations by increasing their responsiveness to IL-23. Although TH17 cell differentiation is decreased in the absence of IL-21-induced signalling in vitro118,119,120, the role of IL-21 in TH17 cell development in vivo and in TH17 cell-mediated autoimmune disease is less clear. Specifically, TH17 cell development in the lamina propria of the small intestine can occur in the absence of IL-21-induced signalling127. Moreover, although one study reported that the development of experimental autoimmune encephalomyelitis (EAE) was significantly decreased in IL-21-deficient mice119, two other studies found no difference in the development of EAE in either IL-21- or IL-21R-deficient mice128,129. So, although IL-21 can promote the differentiation of TH17 cells, its effects can apparently be compensated for by other cytokines, at least in certain circumstances.
T FH cell differentiation. TFH cells are a distinct subset of CD4+ T cells that provide help to B cells in germinal centres during the generation of T cell-dependent antibody responses. TFH cells are characterized by the expression of high levels of CXC-chemokine receptor 5 (CXCR5) and the co-stimulatory molecules ICOS and CD40L. TFH cells produce high levels of IL-21 (Ref. 130), which can act on B cells in germinal centres and as an autocrine factor for TFH cells. Il21 −/− mice have defective germinal centre formation as well as decreased numbers of TFH cells131. Unlike TH17 cells, which can also produce high levels of IL-21 (Ref. 2), TFH cells develop independently of the transcription factor RORγt and do not produce IL-17 (Ref. 132). Although the differentiation of TFH cells during a normal T cell-dependent antibody response requires IL-21 production, the excessive differentiation of TFH cells that accompanies systemic autoimmunity in sanroque mice is independent of IL-21, which indicates that there are alternative mechanisms for the maintenance and/or proliferation of TFH cells in germinal centres in some systemic autoimmune diseases133.
CD8+ T cell differentiation. CD8+ T cells also undergo differentiation into polarized T cytotoxic 1 (TC1), TC2 and TC17 cell populations, which parallel the CD4+ TH1, TH2 and TH17 cell populations. One distinction is that naive CD8+ T cells produce only minimal levels of IL-2 and no IL-21, so that the source of these cytokines during an immune response must be from either activated CD4+ T cells or other cells, such as natural killer T cells (which can produce IL-21 (Ref. 134)). In addition to a role for γc family cytokines in the expansion of CD8+ T cell populations, IL-2 and IL-21 have distinct effects on CD8+ T cell differentiation when they are present during TCR priming. The presence of IL-21 during priming leads to the generation of CD28hiCD8+ T cells that can produce IL-2, potentially overcoming the requirement for IL-2 from CD4+ TH cells135. Moreover, whereas priming of tumour-specific CD8+ T cells in vitro in the presence of IL-2 can potently promote their proliferation and increase their cytolytic activity, priming in the presence of IL-21 was shown to inhibit these processes136. However, when these two populations of CD8+ T cells primed under different conditions were transferred into tumour-bearing mice, the IL-21-primed CD8+ T cells had greater antitumour immunity and greater secondary clonal expansion and persistence than did the IL-2-primed CD8+ T cells. These differences, which persisted in vivo in the absence of further cytokine stimulation, were associated with distinctive and persistent gene expression profiles in IL-2- versus IL-21-primed CD8+ T cells136, which indicates that these cytokines might induce epigenetic changes at the time of priming. So, distinct γc family cytokines have different effects on CD8+ T cell differentiation, with particularly marked diversity between IL-2 and IL-21 in priming for antitumour effects.
Therapeutic implications
As is evident from the information presented above, γc family cytokines and TSLP have crucial roles in regulating numerous activities of immune cells, which have been harnessed to modulate immune responses for therapeutic purposes (Table 3). IL-2 is already used in the clinic to expand and maintain CD4+ T cell populations in patients infected with HIV137,138 and as an anticancer agent, with efficacy in the treatment of some patients with melanoma and renal cell carcinoma139. The related cytokine IL-15 holds promise as an adjuvant for vaccines139; IL-15 preferentially induces the proliferation of CD8+ T cells rather than TReg cells and therefore, in contrast to IL-2, is not expected to induce increased tolerance, and IL-15 has stronger effects than IL-2 on the activity of NK cells and cytotoxic T lymphocytes139. IL-7 and TSLP are other potential agents that might be used to increase the number of T cells in individuals with inherited or acquired immunodeficiency (Table 3). Indeed, the treatment of SIV-infected primates with IL-7 was shown to increase the number of circulating naive and memory T cells140 and, similarly, the administration of IL-7 to humans induces a selective increase in the number of CD4+ and CD8+ T cells but does not affect the number of TReg cells32,141.
Table 3 Effects of decreased versus increased signalling induced by γc family cytokines and TSLP
IL-21 could also have substantial clinical potential (Table 3); its potent antitumour effects have been described in animal models with large established tumours, and it is now in Phase II clinical trials for the treatment of humans with cancer2,142. In addition, blocking IL-21 might prove valuable in treating autoimmune diseases. In this regard, diabetes does not develop in the non-obese diabetic (NOD) mouse model of type 1 diabetes mellitus when the animals are crossed to the Il21r −/− background21, and similarly, manifestations of SLE no longer develop when BXSB-Yaa mice are crossed to the Il21r −/− background23. These studies underscore the potential role of IL-21 in autoimmunity and indicate that interfering with the action of IL-21 might have therapeutic potential for several autoimmune disorders. Finally, the IL-7-related cytokine TSLP seems to have a role in the development of atopic dermatitis and asthma25,76 and perhaps also other allergic diseases. Blocking TSLP with a soluble TSLPR-specific antibody has been shown to protect against the development of pulmonary allergic inflammation in a mouse model143,144,145. These studies collectively underscore a range of potential therapeutic uses for γc family cytokines and TSLP.
Concluding remarks and future directions
The γc family cytokines have central roles in the regulation of a range of immunological processes. The sharing of γc between the receptors for members of this family could be a mechanism for inducing overlapping actions but it could also be a basis for the ability of this family of cytokines to compete with each other for the recruitment of γc. In addition, these cytokines can affect signalling by other members of the family by altering the expression of their receptors, creating a system of intricate cross-regulation (for example, IL-2 increases the expression of its own receptor and IL-4Rα, but decreases the expression of IL-7Rα). The actions of these cytokines have clear clinical relevance, and increasing or decreasing their effects has implications for the treatment of cancer, autoimmunity, allergy and immunodeficiency. Future efforts will be directed not only towards further elucidation of the basic biology of these cytokines, including aspects of gene regulation and signalling, but also towards achieving therapeutic benefits in a range of pathological states.
References
- Leonard, W. J. Cytokines and immunodeficiency diseases. Nature Rev. Immunol. 1, 200–208 (2001).
Article CAS Google Scholar - Spolski, R. & Leonard, W. J. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26, 57–79 (2008).
Article CAS PubMed Google Scholar - Takeshita, T. et al. Cloning of the γ chain of the human IL-2 receptor. Science 257, 379–382 (1992).
Article CAS PubMed Google Scholar - Wang, X., Lupardus, P., Laporte, S. L. & Garcia, K. C. Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 27, 29–60 (2009). A comprehensive review that details the structures of cytokine receptor families that contain gp130, γ c or β c , highlights structural similarities and differences between these families, and discusses their abilities to bind ligands and mediate signalling.
Article CAS PubMed PubMed Central Google Scholar - Noguchi, M. et al. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73, 147–157 (1993). This paper showed that mutations in IL2RG result in XSCID in humans and therefore revealed crucial roles for γ c in the development of T cells and NK cells. The authors correctly speculated that γ c has important roles beyond the action of IL-2.
Article CAS PubMed Google Scholar - Kim, H. P., Imbert, J. & Leonard, W. J. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 17, 349–366 (2006).
Article CAS PubMed Google Scholar - Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Article CAS PubMed Google Scholar - D'Souza, W. N. & Lefrancois, L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J. Immunol. 171, 5727–5735 (2003).
Article CAS PubMed Google Scholar - Lenardo, M. J. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature 353, 858–861 (1991).
Article CAS PubMed Google Scholar - Holgate, S. T. & Polosa, R. Treatment strategies for allergy and asthma. Nature Rev. Immunol. 8, 218–230 (2008).
Article CAS Google Scholar - Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Article CAS PubMed Google Scholar - Russell, S. M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Article CAS PubMed Google Scholar - Puel, A., Ziegler, S. F., Buckley, R. H. & Leonard, W. J. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nature Genet. 20, 394–397 (1998).
Article CAS PubMed Google Scholar - Parrish, Y. K. et al. IL-7 dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J. Immunol. 182, 4255–4266 (2009).
Article CAS PubMed Google Scholar - Mazzucchelli, R. & Durum, S. K. Interleukin-7 receptor expression: intelligent design. Nature Rev. Immunol. 7, 144–154 (2007).
Article CAS Google Scholar - Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
Article CAS PubMed Google Scholar - Veldhoen, M. et al. Transforming growth factor-β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature Immunol. 9, 1341–1346 (2008).
Article CAS Google Scholar - Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+IL-10+Foxp3− effector T cells. Nature Immunol. 9, 1347–1355 (2008).
Article CAS Google Scholar - Hauber, H. P., Bergeron, C. & Hamid, Q. IL-9 in allergic inflammation. Int. Arch. Allergy Immunol. 134, 79–87 (2004).
Article CAS PubMed Google Scholar - Uyttenhove, C., Simpson, R. J. & Van Snick, J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc. Natl Acad. Sci. USA 85, 6934–6938 (1988).
Article CAS PubMed PubMed Central Google Scholar - Spolski, R., Kashyap, M., Robinson, C., Yu, Z. & Leonard, W. J. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc. Natl Acad. Sci. USA 105, 14028–14033 (2008).
Article CAS PubMed PubMed Central Google Scholar - Datta, S. & Sarvetnick, N. E. IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS ONE 3, e3118 (2008).
Article CAS PubMed PubMed Central Google Scholar - Bubier, J. A. et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl Acad. Sci. USA 106, 1518–1523 (2009). References 21–23 show that IL-21 has a crucial role in the pathogenesis of both organ-specific and systemic autoimmune diseases.
Article CAS PubMed PubMed Central Google Scholar - Leonard, W. J. & Spolski, R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nature Rev. Immunol. 5, 688–698 (2005).
Article CAS Google Scholar - Liu, Y. J. et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 25, 193–219 (2007).
Article CAS PubMed Google Scholar - Pandey, A. et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nature Immunol. 1, 59–64 (2000).
Article CAS Google Scholar - Park, L. S. et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192, 659–670 (2000).
Article CAS PubMed PubMed Central Google Scholar - Nakajima, H., Shores, E. W., Noguchi, M. & Leonard, W. J. The common cytokine receptor γ chain plays an essential role in regulating lymphoid homeostasis. J. Exp. Med. 185, 189–195 (1997). This was the first paper to show a crucial role for γ c in lymphocyte homeostasis.
Article CAS PubMed PubMed Central Google Scholar - Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature Immunol. 1, 426–432 (2000).
Article CAS Google Scholar - Goldrath, A. W. et al. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195, 1515–1522 (2002).
Article CAS PubMed PubMed Central Google Scholar - Seddon, B., Tomlinson, P. & Zamoyska, R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nature Immunol. 4, 680–686 (2003).
Article CAS Google Scholar - Sportes, C. et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205, 1701–1714 (2008).
Article CAS PubMed PubMed Central Google Scholar - Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nature Immunol. 8, 1255–1265 (2007).
Article CAS Google Scholar - Fry, T. J. & Mackall, C. L. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 174, 6571–6576 (2005).
Article CAS PubMed Google Scholar - Alves, N. L., van Leeuwen, E. M., Derks, I. A. & van Lier, R. A. Differential regulation of human IL-7 receptor α expression by IL-7 and TCR signaling. J. Immunol. 180, 5201–5210 (2008).
Article CAS PubMed Google Scholar - Xue, H. H. et al. IL-2 negatively regulates IL-7 receptor α chain expression in activated T lymphocytes. Proc. Natl Acad. Sci. USA 99, 13759–13764 (2002).
Article CAS PubMed PubMed Central Google Scholar - Park, J. H. et al. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21, 289–302 (2004). References 36 and 37 show that IL-2- and IL-7-induced signals negatively regulate the expression of IL-7Rα.
Article CAS PubMed Google Scholar - Xue, H. H. et al. GA binding protein regulates interleukin 7 receptor α-chain gene expression in T cells. Nature Immunol. 5, 1036–1044 (2004).
Article CAS Google Scholar - Chandele, A. et al. Formation of IL-7Rαhigh and IL-7Rαlow CD8 T cells during infection is regulated by the opposing functions of GABPα and Gfi-1. J. Immunol. 180, 5309–5319 (2008).
Article CAS PubMed Google Scholar - Tan, J. T. et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl Acad. Sci. USA 98, 8732–8737 (2001).
Article CAS PubMed PubMed Central Google Scholar - Min, B., Yamane, H., Hu-Li, J. & Paul, W. E. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J. Immunol. 174, 6039–6044 (2005).
Article CAS PubMed Google Scholar - Al-Shami, A. et al. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med. 200, 159–168 (2004).
Article CAS PubMed PubMed Central Google Scholar - Chappaz, S., Flueck, L., Farr, A. G., Rolink, A. G. & Finke, D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood 110, 3862–3870 (2007).
Article CAS PubMed Google Scholar - Rochman, Y. & Leonard, W. J. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J. Immunol. 181, 7699–7705 (2008).
Article CAS PubMed Google Scholar - Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Article CAS PubMed Google Scholar - Schluns, K. S., Williams, K., Ma, A., Zheng, X. X. & Lefrancois, L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168, 4827–4831 (2002).
Article CAS PubMed Google Scholar - Berard, M., Brandt, K., Bulfone-Paus, S. & Tough, D. F. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J. Immunol. 170, 5018–5026 (2003).
Article CAS PubMed Google Scholar - Judge, A. D., Zhang, X., Fujii, H., Surh, C. D. & Sprent, J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196, 935–946 (2002).
Article CAS PubMed PubMed Central Google Scholar - Purton, J. F. et al. Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 204, 951–961 (2007).
Article CAS PubMed PubMed Central Google Scholar - Sandau, M. M., Winstead, C. J. & Jameson, S. C. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J. Immunol. 179, 120–125 (2007).
Article CAS PubMed Google Scholar - Rubinstein, M. P. et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc. Natl Acad. Sci. USA 103, 9166–9171 (2006).
Article CAS PubMed PubMed Central Google Scholar - Stoklasek, T. A., Schluns, K. S. & Lefrancois, L. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177, 6072–6080 (2006).
Article CAS PubMed Google Scholar - Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 (2002). This paper describes the formation of stable IL-15–IL-15Rα complexes on the cell surface that mediate trans -presentation of IL-15 and provide survival signals for target cells.
Article CAS PubMed Google Scholar - Burkett, P. R. et al. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl Acad. Sci. USA 100, 4724–4729 (2003).
Article CAS PubMed PubMed Central Google Scholar - Zeng, R. et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 201, 139–148 (2005). This paper describes the ability of IL-21 to act synergistically with other γ c family cytokines as a proliferative agent for CD8+ T cells in vitro and in vivo during an antitumour response.
Article CAS PubMed PubMed Central Google Scholar - Alves, N. L., Arosa, F. A. & van Lier, R. A. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J. Immunol. 175, 755–762 (2005).
Article CAS PubMed Google Scholar - Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002).
Article CAS PubMed Google Scholar - Allard, E. L. et al. Overexpression of IL-21 promotes massive CD8+ memory T cell accumulation. Eur. J. Immunol. 37, 3069–3077 (2007).
Article CAS PubMed Google Scholar - Sojka, D. K., Bruniquel, D., Schwartz, R. H. & Singh, N. J. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J. Immunol. 172, 6136–6143 (2004).
Article CAS PubMed Google Scholar - Cote-Sierra, J. et al. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl Acad. Sci. USA 101, 3880–3885 (2004).
Article CAS PubMed PubMed Central Google Scholar - Liao, W. et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nature Immunol. 9, 1288–1296 (2008). References 60 and 61 show a central role of IL-2 in T H 2 cell differentiation. IL-2 activates STAT5, which binds to the Il4 and Il4ra loci and promotes their transcription.
Article CAS Google Scholar - Morgan, D. A., Ruscetti, F. W. & Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193, 1007–1008 (1976).
Article CAS PubMed Google Scholar - Blattman, J. N. et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nature Med. 9, 540–547 (2003).
Article CAS PubMed Google Scholar - Rubinstein, M. P. et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood 112, 3704–3712 (2008).
Article CAS PubMed PubMed Central Google Scholar - Mattei, F., Schiavoni, G., Belardelli, F. & Tough, D. F. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 167, 1179–1187 (2001).
Article CAS PubMed Google Scholar - Dubois, S. P., Waldmann, T. A. & Muller, J. R. Survival adjustment of mature dendritic cells by IL-15. Proc. Natl Acad. Sci. USA 102, 8662–8667 (2005).
Article CAS PubMed PubMed Central Google Scholar - Ohteki, T. et al. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J. Exp. Med. 203, 2329–2338 (2006).
Article CAS PubMed PubMed Central Google Scholar - Yajima, T. et al. IL-15 regulates CD8+ T cell contraction during primary infection. J. Immunol. 176, 507–515 (2006).
Article CAS PubMed Google Scholar - Oh, S. et al. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc. Natl Acad. Sci. USA 105, 5201–5206 (2008).
Article CAS PubMed PubMed Central Google Scholar - Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 4, 1191–1198 (2003).
Article CAS Google Scholar - Hand, T. W., Morre, M. & Kaech, S. M. Expression of IL-7 receptor α is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc. Natl Acad. Sci. USA 104, 11730–11735 (2007).
Article CAS PubMed PubMed Central Google Scholar - Haring, J. S. et al. Constitutive expression of IL-7 receptor α does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J. Immunol. 180, 2855–2862 (2008).
Article CAS PubMed Google Scholar - Klonowski, K. D., Williams, K. J., Marzo, A. L. & Lefrancois, L. Cutting edge: IL-7-independent regulation of IL-7 receptor α expression and memory CD8 T cell development. J. Immunol. 177, 4247–4251 (2006).
Article CAS PubMed Google Scholar - Lacombe, M. H., Hardy, M. P., Rooney, J. & Labrecque, N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J. Immunol. 175, 4400–4407 (2005).
Article CAS PubMed Google Scholar - Rochman, I., Watanabe, N., Arima, K., Liu, Y. J. & Leonard, W. J. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J. Immunol. 178, 6720–6724 (2007). This was the first demonstration of a direct effect of TSLP on human T cells. TSLPR expression is increased by activated CD4+ T cells. TSLP binding to TSLPR promotes the activation of STAT5, which induces the upregulation of IL-2Rα expression and thereby increases the sensitivity of CD4+ T cells to IL-2.
Article CAS PubMed Google Scholar - Rochman, Y. & Leonard, W. J. Thymic stromal lymphopoietin: a new cytokine in asthma. Curr. Opin. Pharmacol. 8, 249–254 (2008).
Article CAS PubMed PubMed Central Google Scholar - Born, W. K., Reardon, C. L. & O'Brien, R. L. The function of γδ T cells in innate immunity. Curr. Opin. Immunol. 18, 31–38 (2006).
Article CAS PubMed Google Scholar - Jameson, J. & Havran, W. L. Skin γδ T-cell functions in homeostasis and wound healing. Immunol. Rev. 215, 114–122 (2007).
Article CAS PubMed Google Scholar - Baccala, R. et al. γδ T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J. Immunol. 174, 4606–4612 (2005).
Article CAS PubMed Google Scholar - French, J. D., Roark, C. L., Born, W. K. & O'Brien, R. L. γδ T cell homeostasis is established in competition with αβ T cells and NK cells. Proc. Natl Acad. Sci. USA 102, 14741–14746 (2005).
Article CAS PubMed PubMed Central Google Scholar - Laky, K., Lewis, J. M., Tigelaar, R. E. & Puddington, L. Distinct requirements for IL-7 in development of TCR γδ cells during fetal and adult life. J. Immunol. 170, 4087–4094 (2003).
Article CAS PubMed Google Scholar - Furtado, G. C., Curotto de Lafaille, M. A., Kutchukhidze, N. & Lafaille, J. J. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J. Exp. Med. 196, 851–857 (2002).
Article CAS PubMed PubMed Central Google Scholar - Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature Immunol. 6, 1142–1151 (2005). Using Il2 −/− or Il2ra −/− mice, the authors show that IL-2 is important for maintaining the homeostasis of T Reg cells. Although IL-2 is not required for T Reg cell development, γ c -deficient mice are devoid of FOXP3+ T Reg cells. These data are consistent with the idea that more than one cytokine contributes to T Reg cell development and it is now clear that IL-2, IL-7 and TSLP are three such cytokines (see Refs 94,95).
CAS Google Scholar - Antony, P. A. et al. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J. Immunol. 176, 5255–5266 (2006).
Article CAS PubMed Google Scholar - Bayer, A. L., Yu, A. & Malek, T. R. Function of the IL-2R for thymic and peripheral CD4+CD25+Foxp3+ T regulatory cells. J. Immunol. 178, 4062–4071 (2007).
Article CAS PubMed Google Scholar - Yao, Z. et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109, 4368–4375 (2007).
Article CAS PubMed PubMed Central Google Scholar - Antov, A., Yang, L., Vig, M., Baltimore, D. & Van Parijs, L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J. Immunol. 171, 3435–3441 (2003).
Article CAS PubMed Google Scholar - Burchill, M. A., Yang, J., Vogtenhuber, C., Blazar, B. R. & Farrar, M. A. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178, 280–290 (2007).
Article CAS PubMed Google Scholar - Cohen, A. C. et al. Cutting edge: decreased accumulation and regulatory function of CD4+CD25high T cells in human STAT5b deficiency. J. Immunol. 177, 2770–2774 (2006).
Article CAS PubMed Google Scholar - Lin, J. X. et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 2, 331–339 (1995).
Article CAS PubMed Google Scholar - Lin, J. X., Mietz, J., Modi, W. S., John, S. & Leonard, W. J. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J. Biol. Chem. 271, 10738–10744 (1996).
Article CAS PubMed Google Scholar - Nakajima, H. et al. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor α chain induction. Immunity 7, 691–701 (1997).
Article CAS PubMed Google Scholar - Imada, K. et al. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J. Exp. Med. 188, 2067–2074 (1998).
Article CAS PubMed PubMed Central Google Scholar - Mazzucchelli, R. et al. Development of regulatory T cells requires IL-7Rα stimulation by IL-7 or TSLP. Blood 112, 3283–3292 (2008). Whereas mice deficient in IL-7 or TSLPR have relatively normal numbers of T Reg cells, combined deficiency of IL-7 and TSLPR greatly decreases the number of T Reg cells, indicating that both IL-7 and TSLP contribute to T Reg cell development.
Article CAS PubMed PubMed Central Google Scholar - Bayer, A. L., Lee, J. Y., de la Barrera, A., Surh, C. D. & Malek, T. R. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J. Immunol. 181, 225–234 (2008).
Article CAS PubMed Google Scholar - Liu, W. et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ TReg cells. J. Exp. Med. 203, 1701–1711 (2006).
Article CAS PubMed PubMed Central Google Scholar - Seddiki, N. et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 (2006).
Article CAS PubMed PubMed Central Google Scholar - Pandiyan, P. & Lenardo, M. J. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol. Direct 3, 6 (2008).
Article CAS PubMed PubMed Central Google Scholar - Bayer, A. L., Yu, A., Adeegbe, D. & Malek, T. R. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J. Exp. Med. 201, 769–777 (2005).
Article CAS PubMed PubMed Central Google Scholar - Zhang, H. et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nature Med. 11, 1238–1243 (2005).
Article CAS PubMed Google Scholar - Fukao, T. & Koyasu, S. Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur. J. Immunol. 30, 1453–1457 (2000).
Article CAS PubMed Google Scholar - Mnasria, K. et al. Anti-CD25 antibodies affect cytokine synthesis pattern of human dendritic cells and decrease their ability to prime allogeneic CD4+ T cells. J. Leukocyte Biol. 84, 460–467 (2008).
Article CAS PubMed Google Scholar - Combe, C. L. et al. Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc. Natl Acad. Sci. USA 103, 6635–6640 (2006).
Article CAS PubMed PubMed Central Google Scholar - Moretto, M. M., Lawlor, E. M. & Khan, I. A. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J. Immunol. 181, 7977–7984 (2008).
Article CAS PubMed Google Scholar - Jinushi, M. et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J. Immunol. 171, 5423–5429 (2003).
Article CAS PubMed Google Scholar - Sriram, U. et al. IL-4 suppresses dendritic cell response to type I interferons. J. Immunol. 179, 6446–6455 (2007).
Article CAS PubMed Google Scholar - Taylor, B. C. et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206, 655–667 (2009).
Article CAS PubMed PubMed Central Google Scholar - Guimond, M. et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nature Immunol. 10, 149–157 (2009). This article describes a new role for IL-7 in regulating CD4+ T cell proliferation. Increased accessibility to IL-7 in lymphopaenic conditions decreases the homeostatic proliferation of CD4+ T cells by decreasing the expression of MHC class II molecules by IL-7Rα-expressing DCs.
Article CAS Google Scholar - Brandt, K., Bulfone-Paus, S., Foster, D. C. & Ruckert, R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood 102, 4090–4098 (2003).
Article CAS PubMed Google Scholar - Strengell, M., Lehtonen, A., Matikainen, S. & Julkunen, I. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J. Leukoc. Biol. 79, 1279–1285 (2006).
Article CAS PubMed Google Scholar - Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nature Rev. Immunol. 8, 523–532 (2008).
Article CAS Google Scholar - Szabo, S. J., Sullivan, B. M., Peng, S. L. & Glimcher, L. H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 21, 713–758 (2003).
Article CAS PubMed Google Scholar - Gor, D. O., Rose, N. R. & Greenspan, N. S. TH1–TH2: a procrustean paradigm. Nature Immunol. 4, 503–505 (2003).
Article CAS Google Scholar - Wurster, A. L. et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon γ-producing Th1 cells. J. Exp. Med. 196, 969–977 (2002).
Article CAS PubMed PubMed Central Google Scholar - Suto, A., Wurster, A. L., Reiner, S. L. & Grusby, M. J. IL-21 inhibits IFN-γ production in developing Th1 cells through the repression of Eomesodermin expression. J. Immunol. 177, 3721–3727 (2006).
Article CAS PubMed Google Scholar - Strengell, M., Sareneva, T., Foster, D., Julkunen, I. & Matikainen, S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J. Immunol. 169, 3600–3605 (2002).
Article PubMed Google Scholar - Pesce, J. et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J. Clin. Invest. 116, 2044–2055 (2006).
Article CAS PubMed PubMed Central Google Scholar - Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Article CAS PubMed PubMed Central Google Scholar - Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Article CAS PubMed Google Scholar - Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 8, 967–974 (2007). References 118–120 describe the role of IL-21 in the development of the T H 17 cell lineage and in the inflammatory response.
Article CAS Google Scholar - Laurence, A. et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007).
Article CAS PubMed Google Scholar - Veldhoen, M., Hirota, K., Christensen, J., O'Garra, A. & Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 206, 43–49 (2009).
Article CAS PubMed PubMed Central Google Scholar - Kryczek, I. et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 178, 6730–6733 (2007).
Article CAS PubMed Google Scholar - Hoyer, K. K., Kuswanto, W. F., Gallo, E. & Abbas, A. K. Distinct roles of helper T-cell subsets in a systemic autoimmune disease. Blood 113, 389–395 (2009).
Article CAS PubMed PubMed Central Google Scholar - Amadi-Obi, A. et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature Med. 13, 711–718 (2007).
Article CAS PubMed Google Scholar - Kryczek, I. et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool: IL-1 subverts IL-2-mediated suppression. J. Immunol. 179, 1423–1426 (2007).
Article CAS PubMed Google Scholar - Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Article CAS PubMed PubMed Central Google Scholar - Coquet, J. M., Chakravarti, S., Smyth, M. J. & Godfrey, D. I. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J. Immunol. 180, 7097–7101 (2008).
Article CAS PubMed Google Scholar - Sonderegger, I., Kisielow, J., Meier, R., King, C. & Kopf, M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur. J. Immunol. 38, 1833–1838 (2008).
Article CAS PubMed Google Scholar - Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Article CAS PubMed Google Scholar - Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008).
Article CAS PubMed Google Scholar - Nurieva, R. I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Article CAS PubMed PubMed Central Google Scholar - Linterman, M. A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Article CAS PubMed PubMed Central Google Scholar - Coquet, J. M. et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 178, 2827–2834 (2007).
Article CAS PubMed Google Scholar - Li, Y., Bleakley, M. & Yee, C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J. Immunol. 175, 2261–2269 (2005).
Article CAS PubMed Google Scholar - Hinrichs, C. S. et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111, 5326–5333 (2008). This study showed the contrasting effects of IL-2 and IL-21 during the priming of CD8+ T cells in an antitumour immune response, indicating that the presence of IL-21 during CD8+ T cell priming results in persistence of the cells in vivo and potent antitumour activity.
Article CAS PubMed PubMed Central Google Scholar - Kovacs, J. A. et al. Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. J. Clin. Invest. 115, 2139–2148 (2005).
Article CAS PubMed PubMed Central Google Scholar - Porter, B. O. et al. Inferiority of IL-2 alone versus IL-2 with HAART in maintaining CD4 T cell counts during HAART interruption: a randomized controlled trial. AIDS 23, 203–212 (2009).
Article CAS PubMed Google Scholar - Waldmann, T. A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature Rev. Immunol. 6, 595–601 (2006).
Article CAS Google Scholar - Beq, S. et al. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J. Immunol. 176, 914–922 (2006).
Article CAS PubMed Google Scholar - Rosenberg, S. A. et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 29, 313–319 (2006).
Article CAS PubMed PubMed Central Google Scholar - Andorsky, D. J. & Timmerman, J. M. Interleukin-21: biology and application to cancer therapy. Expert Opin. Biol. Ther. 8, 1295–1307 (2008).
Article CAS PubMed Google Scholar - Al-Shami, A., Spolski, R., Kelly, J., Keane-Myers, A. & Leonard, W. J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202, 829–839 (2005).
Article CAS PubMed PubMed Central Google Scholar - Zhou, B. et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nature Immunol. 6, 1047–1053 (2005).
Article CAS Google Scholar - Ying, S. et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 174, 8183–8190 (2005).
Article CAS PubMed Google Scholar - Gilmour, K. C. et al. Defective expression of the interleukin-2/interleukin-15 receptor β subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood 98, 877–879 (2001).
Article CAS PubMed Google Scholar - Waldmann, T. A. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J. Clin. Immunol. 27, 1–18 (2007).
Article CAS PubMed Google Scholar - Mayordomo, J. I. et al. Bone marrow-derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells 15, 94–103 (1997).
Article CAS PubMed Google Scholar - Thurner, B. et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 223, 1–15 (1999).
Article CAS PubMed Google Scholar
Acknowledgements
We thank J.-X. Lin for critical comments. This work was supported by the Division of Intramural Research, National Heart, Lung and Blood Institute, National Institutes of Health, USA.
Author information
Authors and Affiliations
- National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, 20892-1674, Maryland, USA
Yrina Rochman, Rosanne Spolski & Warren J. Leonard
Authors
- Yrina Rochman
You can also search for this author inPubMed Google Scholar - Rosanne Spolski
You can also search for this author inPubMed Google Scholar - Warren J. Leonard
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toWarren J. Leonard.
Ethics declarations
Competing interests
Yrina Rochman, Rosanne Spolski and Warren J. Leonard
New insights into the regulation of T cells by γc family cytokines.
Nature Reviews Immunology 9, 480–490 (2009); doi:10.1038/nri2580
Warren Leonard and Rosanne Spolski are inventors on patents and patent applications related to the γc family cytokines and TSLP.
Related links
Glossary
X-linked severe combined immunodeficiency
(XSCID). A recessive, inherited disease in which the gene encoding the common cytokine receptor γ-chain (γc) on the X chromosome is mutated. γc is an essential component of six cytokine receptors and its mutation results in a profound immunodeficiency that accounts for approximately half of all cases of SCID and is characterized by an absence of T cells and natural killer cells. Patients with XSCID have a normal number of B cells but these are non-functional.
Regulatory T cell
(TReg cell). A specialized type of CD4+ T cell that can suppress the responses of other T cells. TReg cells provide a crucial mechanism for the maintenance of peripheral T cell tolerance. They are characterized by the expression of the α-chain of the interleukin-2 receptor (IL-2Rα; also known as CD25) and the transcription factor forkhead box P3 (FOXP3).
Plasma cell
A non-dividing, terminally differentiated, immunoglobulin-secreting cell of the B cell lineage.
Systemic lupus erythematosus
(SLE). An autoimmune disease in which autoantibodies that are specific for DNA, RNA or proteins associated with nucleic acids form immune complexes that damage small blood vessels, especially in the kidneys. Patients with SLE generally have abnormal B and T cell function.
_Trans_-presentation
A process by which the α-chain of the interleukin-15 receptor (IL-15Rα) presents IL-15 in trans to other cells expressing a complex (with an intermediate affinity for IL-15) that contains IL-2Rβ and the common cytokine receptor γ-chain (γc), which then transduce a signal.
Activation-induced cell death
(AICD). A process in which activated T cells re-stimulated through their T cell receptor undergo cell death after engagement of cell death receptors, such as CD95 or the tumour necrosis factor receptor, or after exposure to reactive oxygen species.
Type I and type II IFNs
Interferons (IFNs) are proteins with potent antiviral activity that are of particular importance during the early response to pathogens. Type I (or viral) IFNs comprise families (α, β and ω) of homologous proteins that interact with a common two-chain receptor (consisting of IFNAR1 and IFNAR2). Type II (or immune) IFN is represented by a single protein (IFNγ) that interacts with a different two-chain receptor (consisting of IFNGR1 and IFNGR2).
DNAse I hypersensitivity sites
Sites of nuclease sensitivity when nuclei from cells are exposed to limiting concentrations of DNase I. The digested regions of DNA correspond to sites of open DNA, which might be transcription factor-binding sites or areas of altered nucleosome conformation.
Lamina propria
The layer of mucosal tissue directly under the mucosal epithelial cell surface of the gastrointestinal tract, in which effector immune cells for mucosal immunity reside.
Experimental autoimmune encephalomyelitis
(EAE). An experimental model of multiple sclerosis that is induced by immunization of susceptible animals with myelin-derived antigens, such as myelin basic protein, proteolipid protein or myelin oligodendrocyte glycoprotein.
Germinal centres
These structures, which are found in peripheral lymphoid tissues (for example, the spleen or lymph nodes), are sites of B cell proliferation and selection for clones that produce antigen-specific antibodies of higher affinity.
Sanroque mice
An autoimmune strain of mice that carries a loss-of-function mutation in the gene roquin (also known as Rc3h1). These mice have a T cell-mediated systemic lupus erythematosus-like syndrome and severe autoimmune diabetes when on a susceptible genetic background.
Non-obese diabetic (NOD) mice
NOD mice spontaneously develop type 1 diabetes mellitus as a result of autoreactive T cell-mediated destruction of pancreatic β-islet cells.
Rights and permissions
About this article
Cite this article
Rochman, Y., Spolski, R. & Leonard, W. New insights into the regulation of T cells by γc family cytokines.Nat Rev Immunol 9, 480–490 (2009). https://doi.org/10.1038/nri2580
- Issue Date: July 2009
- DOI: https://doi.org/10.1038/nri2580