Ethanol - Thermophysical properties (original) (raw)
Ethanol (Ethyl Alcohol), C2H5 OH, is a volatile, flammable, colorless liquid with a slight characteristic odor. It is produced via petrochemical processes or naturally by the fermentation of sugars by yeasts.
Ethanol is most commonly consumed as a popular recreational drug . It is a psychoactive substance and is the principal type of alcohol found in alcoholic drinks. It also has medical applications as an antiseptic and disinfectant. The compound is widely used as a chemical solvent, either for scientific chemical testing or in synthesis of other organic compounds. Ethanol is also used as a clean-burning fuel source.
The phase diagram of ethanol is shown below the table.
Chemical, physical and thermal properties of ethanol :
Values are given for liquid at 25 oC /77 oF / 298 K and 1 bara, if not other phase, temperature or pressure given.
For full table with Imperial Units - rotate the screen!
Ethanol - Thermophysical properties
| Property | Value | Unit | Value | Unit | Value | Unit | Value | Unit |
|---|---|---|---|---|---|---|---|---|
| Acidity (pKa1 ) | 15.9 | |||||||
| Autoignition temperature | 636 | K | 363 | °C | 685 | °F | ||
| Boiling Point | 351.39 | K | 78.2 | °C | 172.8 | °F | ||
| Critical density | 5.91 | mol/dm3 | 272 | kg/m3 | 0.528 | slug/ft3 | 17.0 | lb/ft3 |
| Critical Pressure | 6.25 | MPa=MN/m2 | 62.5 | bara | 61.7 | atm | 906 | psia=lbf /in2 |
| Critical temperature | 513.9 | K | 240.8 | °C | 465.4 | °F | ||
| Critical Volume | 169 | cm3 /mol | 0.00367 | m3 /kg | 1.89 | ft3 /slug | 0.0588 | ft3 /lb |
| Density (gas) at 0.08 bar | 3.15 | mol/m3 | 0.145 | kg/m3 | 0.00028 | slug/ft3 | 0.0091 | lb/ft3 |
| Density (liquid) | 17046 | mol/m3 | 785.3 | kg/m3 | 1.524 | slug/ft3 | 49.02 | lb/ft3 |
| Flammable (gas and liquid) | yes | |||||||
| Flash point | 286 | K | 13 | °C | 55 | °F | ||
| Gas constant (individual) - R | 180.5 | J/kg K | 0.05013 | Wh/(kg K) | 1079 | [ft lbf/slug °R] | 33.54 | [ft lbf/lb °R] |
| Gibbs free energy of formation (gas) | -168 | kJ/mol | -3647 | kJ/kg | -1568 | Btu/lb | ||
| Specific heat capacity, Cp (isobaric) (gas) | 74 | J/mol K | 1.60 | kJ/kg K | 0.383 | Btu/lb°F or cal/g K | ||
| Specific heat capacity, Cp (liquid) | 118 | J/mol K | 2.57 | kJ/kg K | 0.614 | Btu/lb°F or cal/g K | ||
| Specific heat capacity, Cv (isochoric) (gas) | 65 | J/mol K | 1.42 | kJ/kg K | 0.339 | Btu/lb°F or cal/g K | ||
| Specific heat capacity, Cv (liquid) | 100 | J/mol K | 2.18 | kJ/kg K | 0.520 | Btu/lb°F or cal/g K | ||
| Heat (enthalpy) of combustion (gas) | 1336.8 | kJ/mol | 29017 | kJ/kg | 12.5 | Btu/lb | ||
| Heat (enthalpy) of formation (gas) | -234 | kJ/mol | -5079 | kJ/kg | -2184 | Btu/lb | ||
| Heat (enthalpy) of fusion at -173°F/-114°C | 4.9 | kJ/mol | 106 | kJ/kg | 45.73 | Btu/lb | ||
| Heat (enthalpy) of evaporation | 42.32 | kJ/mol | 919 | kJ/kg | 394.94 | Btu/lb | ||
| Ionization potential | 10.47 | eV | ||||||
| log KOW (Octanol/Water Partition Coefficient) | -0.31 | |||||||
| Melting point | 159.01 | K | -114.1 | °C | -173.5 | °F | ||
| Molecular Weight | 46.069 | g/mol | 0.10156 | lb/mol | ||||
| Solubility in water | 1000 | mg/ml | ||||||
| Sound velocity in liquid | 1139 | m/s | 3736 | ft/s | 2551 | mi/h | ||
| Sound velocity in gas, at 0.08 bara | 246 | m/s | 807 | ft/s | 551 | mi/h | ||
| Specific Gravity (gas) (relativ to air) | 1.59 | |||||||
| Specific Gravity (liquid) (relativ to water) | 0.79 | |||||||
| Specific Heat Ratio (gas) - Cp/Cv | 1.13 | |||||||
| Specific Heat Ratio (liquid) - Cp/Cv | 1.18 | |||||||
| Specific Volume (gas), at 0.08 bar | 0.318 | m3 /mol | 6.90 | m3 /kg | 3554 | ft3 /slug | 110 | ft3 /lb |
| Specific Volume, (liquid) | 0.0000587 | m3 /mol | 0.00127 | m3 /kg | 0.656 | ft3 /slug | 0.0204 | ft3 /lb |
| Standard molar entropy , S° (gas) | 283 | J/mol K | 6.14 | kJ/kg K | 1.47 | Btu/lb °F | ||
| Standard molar entropy, S° (liquid) | 160 | J/mol K | 3.47 | kJ/kg K | 0.83 | Btu/lb °F | ||
| Surface tension | 21.97 | dynes/cm | 0.02197 | N/m | ||||
| Thermal Conductivity | 0.167 | W/m K | 0.0965 | Btu/hr ft °F | ||||
| Triple point pressure | 4.3x10 -10 | MPa=MN/m2 | 4.3x10 -9 | bara | 4.24x10 -9 | atm | 6.24x10-8 | psia=lbf /in2 |
| Triple point temperature | 150.00 | K | -123.15 | °C | -189.67 | °F | ||
| Vapor (saturation) pressure | 0.008 | MPa=MN/m2 | 60.0 | mm Hg | 0.0790 | atm | 1.16 | psi=lbf /in2 |
| Viscosity , dynamic (absolute) | 1.074 | cP | 721.7 | [lbm /ft s*10-6 ] | 22.43 | [lbf s/ft2*10-6 ] | ||
| Viscosity , kinematic | 1.36 | cSt | 14.6 | [ft2/s*10-6 ] |
Follow the links below to get values for the listed properties of ethanol at varying pressure and temperature :
- Density and specific weight
- Dynamic and kinematic viscosity
- Specific Heat (Heat Capacity), Cp and Cv
See also more about atmospheric pressure , and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure ,
as well as Thermophysical properties of: Acetone , Acetylene , Air , Ammonia , Argon , Benzene , Butane , Carbon dioxide , Carbon monoxide , Ethane , Ethylene , Helium , Hydrogen , Hydrogen sulfide , Methane , Methanol , Nitrogen , Oxygen , Pentane , Propane , Toluene , Water and Heavy water, D2O .
Ethanol is a liquid at standard conditions. However, at low temperature and/or very high pressures it becomes a solid.
The phase diagram for ethanol shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the ethanol boiling point with changes in pressure. It also shows the saturation pressure with changes in temperature.
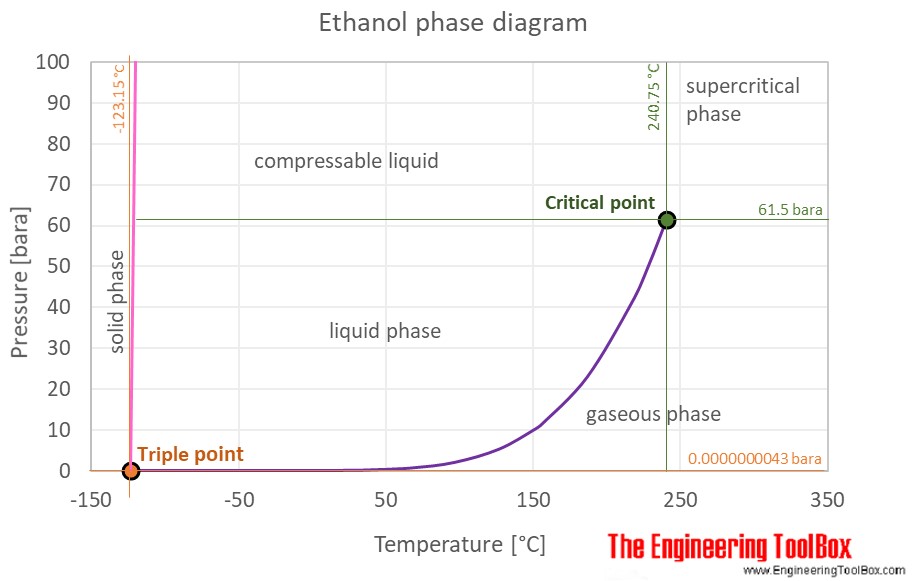
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.