Hydrogen - Thermophysical Properties (original) (raw)
Hydrogen, H2, is a colorless, odorless gas.
Hydrogen is easily ignited. Once ignited it burns with a pale blue, almost invisible flame. The vapors are lighter than air. It is flammable over a wide range of vapor/air concentrations. Hydrogen is not toxic but is a simple asphyxiate by the displacement of oxygen in the air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.
Hydrogen is used to make other chemicals, in petroleum refining and in oxyhydrogen welding and cutting.
The phase diagram of hydrogen is shown below the table.
Chemical, physical and thermal properties of hydrogen:
Values at 25 oC (77 oF, 298 K) and atmospheric pressure
Hydrogen - Thermophysical Properties
| Molecular Weight | 2.016 |
|---|---|
| Specific Gravity , air = 1 | 0.070 |
| Specific Volume ( ft3 /lb, m3 /kg ) | 194, 12.1 |
| Density of liquid at atmospheric pressure ( lb/ft3, kg/m3 ) | 4.43, 71.0 |
| Absolute Viscosity ( lbm /ft s, centipoises ) | 6.05 10-6, 0.009 |
| Sound velocity in gas ( m/s ) | 1315 |
| Specific Heat - cp - ( Btu/lb oF or cal/g oC, J/kgK ) | 3.42, 14310 |
| Specific Heat Ratio - cp /cv | 1.405 |
| Gas constant - R - ( ft lb/lb o R, J/kg oC ) | 767, 4126 |
| Thermal Conductivity ( Btu/hr ft oF, W/m oC ) | 0.105, 0.182 |
| Boiling Point - saturation pressure 14.7 psia and 760 mm Hg - ( oF, o K ) | -423, 20.4 |
| Latent Heat of Evaporation at boiling point ( Btu/lb, J/kg ) | 192, 447000 |
| Freezing or Melting Point at 1 atm ( oF, oC ) | -434.6, -259.1 |
| Latent Heat of Fusion ( Btu/lb, J/kg ) | 25.0, 58000 |
| Critical Temperature ( oF, oC ) | -399.8, -240.0 |
| Critical Pressure ( psia, MN/m2 ) | 189, 1.30 |
| Critical Volume ( ft3 /lb, m3 /kg ) | 0.53, 0.033 |
| Flammable | yes |
| Heat of combustion ( Btu/ft3, Btu/lb, kJ/kg ) | 320, 62050, 144000 |
Follow the links below to get values for the listed properties of hydrogen at varying pressure and temperature :
See also more about atmospheric pressure , and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure ,
as well as Thermophysical properties of: Acetone , Acetylene , Air , Ammonia , Argon , Benzene , Butane , Carbon dioxide , Carbon monoxide , Ethane , Ethanol , Ethylene , Helium , Hydrogen sulfide , Methane , Methanol , Nitrogen , Oxygen , Pentane , Propane , Toluene , Water and Heavy water, D2O .
Hydrogen is a gas at standard conditions. However, at very low temperature and/or high pressures the gas becomes a liquid or a solid.
The hydrogen phase diagram shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the hydrogen boiling point with changes in pressure. It also shows the saturation pressure with changes in temperature.
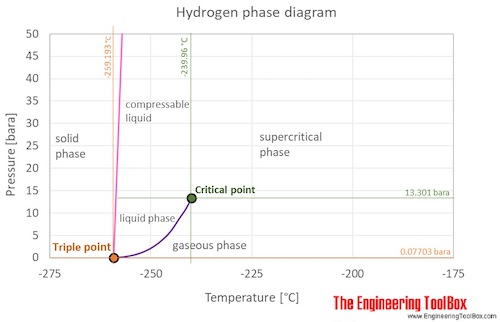
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.