Ethylene - Thermophysical Properties (original) (raw)
Ethylene, C2H4 , is a highly flammable, colorless and noncorrosive gas with a sweet odor. It is easily ignited and a flame can easily flash back to the source of the leak. Under prolonged exposure to fire or heat the containers may rupture violently and rocket. Can cause explosion. Vapors arising from the boiling liquid are lighter than air. Ethylene is not toxic, but is a simple asphyxiant.
Ethylene is used as an anesthetic, a refrigerant, and to make other chemicals as polymers and plastics.
Ethylene is produced in petrochemical processes, as steam cracking where hydrocarbons and steam are heated to 750–950 °C. This process converts large hydrocarbons into smaller ones and introduces unsaturated products.
The phase diagram of ethylene is shown below the table.
Chemical, physical and thermal properties of ethylene:
Values are given for gas phase at 25 oC /77 oF / 298 K and 1 bara, if not other phase, temperature or pressure given.
For full table with Imperial Units - rotate the screen!
Ethylene - Thermophysical Properties
| Property | Value | Unit | Value | Unit | Value | Unit | Value | Unit |
|---|---|---|---|---|---|---|---|---|
| Autoignition temperature | 723 | K | 450 | °C | 842 | °F | ||
| Boiling Point | 169 | K | -104 | °C | -155 | °F | ||
| Critical density | 7.63 | mol/dm3 | 214 | kg/m3 | 0.415 | slug/ft3 | 13.4 | lb/ft3 |
| Critical pressure | 5.06 | MPa=MN/m2 | 50.6 | bar | 49.9 | atm | 734 | psi=lbf /in2 |
| Critical temperature | 282.4 | K | 9.2 | °C | 48.6 | °F | ||
| Critical volume | 131 | cm3 /mol | 0.00467 | m3 /kg | 2.41 | ft3 /slug | 0.0748 | ft3 /lb |
| Density | 40.6 | mol/m3 | 1.138 | kg/m3 | 0.00221 | slug/ft3 | 0.07104 | lb/ft3 |
| Density (liquid) at -104 °C/-155°F | 20567 | mol/m3 | 577 | kg/m3 | 1.12 | slug/ft3 | 36.0 | lb/ft3 |
| Flammable, gas and liquid | yes | highly | ||||||
| Flash point | 137 | K | -136 | °C | -213 | °F | ||
| Gas constant , individual, R | 296.4 | J/kg K | 0.0823 | Wh/(kg K) | 1772 | [ft lbf /slug °R] | 55.08 | [ft lbf /lb °R] |
| Gibbs free energy of formation (gas) | 68 | kJ/mol | 2424 | kJ/kg | 1042 | Btu/lb | ||
| Heat (enthalpy) of combustion (gas) | -1411 | kJ/mol | -50303 | kJ/kg | -21626 | Btu/lb | ||
| Heat (enthalpy) of formation (gas) | 52.4 | kJ/mol | 1868 | kJ/kg | 803 | Btu/lb | ||
| Heat (enthalpy) of evaporation at -104°C/-155°F | 13.6 | kJ/mol | 483 | kJ/kg | 207.7 | Btu/lb | ||
| Heat capacity , Cp (gas) | 42.9 | J/mol K | 1.53 | kJ/kg K | 0.365 | Btu/lb°F or cal/g K | ||
| Heat capacity, Cp (liquid) at -104°C/-155°F | 67.4 | J/mol K | 2.40 | kJ/kg K | 0.574 | Btu/lb°F or cal/g K | ||
| Heat capacity, Cv (gas) | 34.6 | J/mol K | 1.24 | kJ/kg K | 0.295 | Btu/lb°F or cal/g K | ||
| Heat capacity, Cv (liquid) at -104°C/-155°F | 38.7 | J/mol K | 1.38 | kJ/kg K | 0.330 | Btu/lb°F or cal/g K | ||
| Ionization potential | 10.5 | eV | ||||||
| log KOW (Octanol/Water Partition Coefficient) | 1.13 | |||||||
| Melting point | 104.15 | K | -169.0 | °C | -272.2 | °F | ||
| Molecular Weight | 28.054 | g/mol | 0.06185 | lb/mol | ||||
| Solubility in water, at 25°C | 0.131 | mg/ml | ||||||
| Sound velocity | 330 | m/s | 1082 | ft/s | 739 | mi/h | ||
| Specific Gravity (gas) (relativ to air) | 0.978 | |||||||
| Specific Heat Ratio (gas) - CP /CV | 1.24 | |||||||
| Specific Heat Ratio (liquid) - CP /CV | 1.74 | |||||||
| Specific Volume | 0.0247 | m3 /mol | 0.879 | m3 /kg | 453 | ft3 /slug | 14.1 | ft3 /lb |
| Standard molar entropy , S° (gas) | 219.32 | J/mol K | 7.82 | kJ/kg K | 1.87 | Btu/lb °F | ||
| Standard molar entropy, S° (liquid) | 117.8 | J/mol K | 4.20 | kJ/kg K | 1.00 | Btu/lb °F | ||
| Surface tension at -104°C/-155°F | 16.00 | dynes/cm | 0.016 | N/m | ||||
| Thermal Conductivity | 0.020 | W/m°C | 0.0118 | Btu/hr ft °F | ||||
| Triple point pressure | 0.000122 | MPa=MN/m2 | 0.00122 | bar | 0.00120 | atm | 0.0177 | psi=lbf /in2 |
| Triple point temperature | 104.0 | K | -169.16 | °C | -272.49 | °F | ||
| Vapor (saturation) pressure | 6.9449 | MPa=MN/m2 | 5.21E+04 | mm Hg | 68.543 | atm | 1007.30 | psi=lbf /in2 |
| Viscosity , dynamic (absolute) | 0.0103 | cP | 6.9 | [lbm /ft s*10-6 ] | 0.22 | [lbf s/ft2*10-6 ] | ||
| Viscosity, kinematic | 9.05 | cSt | 97.4 | [ft2/s*10-6 ] |
Follow the links below to get values for the listed properties of ethylene at varying pressure and temperature :
See also more about atmospheric pressure , and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure ,
as well as Thermophysical properties of: Acetone , Acetylene , Air , Ammonia , Argon , Benzene , Butane , Carbon dioxide , Carbon monoxide , Ethane , Ethanol , Helium , Hydrogen , Hydrogen sulfide , Methane , Methanol , Nitrogen , Oxygen , Pentane , Propane , Toluene , Water and Heavy water, D2O .
Ethylene is a gas at standard conditions. However, at low temperature and/or high pressures the gas becomes a liquid or a solid.
The ethylene phase diagram shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the ethylene boiling point with changes in pressure. It also shows the saturation pressure with changes in temperature.
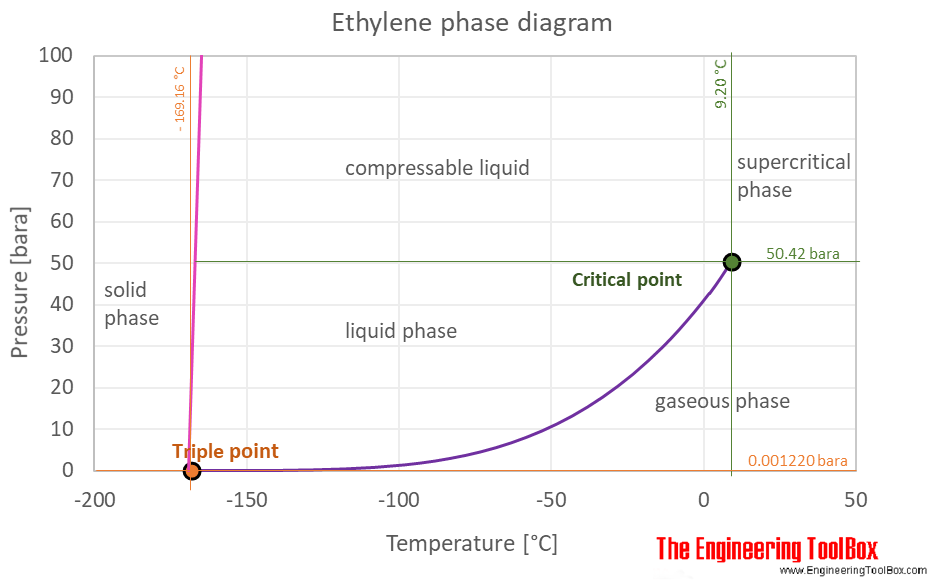
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.
Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included.
Chemical, Physical and Thermal Properties of Acetylene.
Thermal properties of air at different temperatures - density, viscosity, critical temperature and pressure, triple point, enthalpi and entropi, thermal conductivity and diffusivity and more.
Chemical, Physical and Thermal Properties of Ammonia. Phase diagram included.
Chemical, Physical and Thermal Properties of Argon.
Chemical, physical and thermal properties of n-Butane.
Chemical, physical and thermal properties of carbon dioxide. Phase diagram included.
Chemical, Physical and Thermal Properties of Carbon Monoxide - CO.
Conductive heat transfer takes place in a solid if there is a temperature gradient.
Critical temperatures and pressures for some common substances like air, alcohol, ether, oxygen and more.
An introduction to density, specific weight and specific gravity.
Chemical, Physical and Thermal Properties of Ethane - C2H6.
Chemical, physical and thermal properties of ethanol (also called alcohol or ethyl alcohol). Phase diagram included.
Online calculator, figures and tables showing density and specific weight of ethylene, C2H4, at varying temperature and pressure - Imperial and SI Units.
Online calculator, figures and tables showing dynamic and kinematic viscosity of ethylene, C2H4, also called ethene or acetene, at varying temperature and pressure - Imperial and SI Units.
Online calculator, figures and table showing thermal conductivity of ethylene, also called ethene or acetene, C2H4, at varying temperature and pressure - Imperial and SI Units.
Specific heat of Ethylene Gas - C2H4 - temperatures ranging 175 - 900 K.
The amount of heat required to change the temperature of a substance by one degree.
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more.
Chemical, Physical and Thermal Properties of Helium - He.
Molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons.
Autoignition temperatures and flash points (°C and °F) of different types of hydrocarbons with varying carbon numbers up to C12.
Boiling temperatures (°C and °F) with varying carbon numbers up to C33.
Chemical, Physical and Thermal Properties of Hydrogen - H2.
Chemical, physical and thermal properties of hydrogen sulfide, H2S, also called hydrosulfuric acid, sewer gas and stink damp. Phase diagram included.
Latent heat of vaporization for fluids like alcohol, ether, nitrogen, water and more.
Vapor and saturation pressure for some common liquids.
Chemical, Physical and Thermal Properties of Methane - CH4. Phase diagram included.
Chemical, physical and thermal properties of methanol, CH3OH (also called carbinol, wood alcohol, hydroxy methyl and methyl alcohol). Phase diagram included.
Definition and molecular weight (molar mass) of some common substances.
Chemical, Physical and Thermal Properties of Nitrogen - N2.
Chemical, Physical and Thermal Properties of Oxygen - O2.
Chemical, physical and thermal properties of pentane, also called n-pentane. Phase diagram included.
Chemical, physical and thermal properties of propane gas - C3H8.
The amount of a solute that can be dissolved in a solvent.
Calculate the speed of sound (the sonic velocity) in gases, fluids or solids.
Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and Gibbs free energy of formation, as well as standard entropy and molar heat capacity, of 370 inorganic compounds.
The definition of STP - Standard Temperature and Pressure and NTP - Normal Temperature and Pressure.
Triple points for common substances.
Surface tension of liquids like water, mercury, oils and more.
The Universal and Individual Gas Constants in fluid mechanics and thermodynamics. Individual gas constants for the most common gases.
Vicosity is a fluid's resistance to flow and can be valued as dynamic (absolute) or kinematic.
Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting, latent heat of evaporation, critical temperature and more.