Carbon Dioxide - Thermophysical Properties (original) (raw)
Carbon dioxide, CO2, is a colourless and odorless gas. It is relatively nontoxic and noncombustible, but it is heavier than air and may asphyxiate by the displacement of air.
When CO2is solved in water, the mild carbonic acid , is formed. Cooled CO2in solid form is called dry ice .
Chemical, physical and thermal properties of carbon dioxide : Values are given for gas phase at 25 oC /77 oF / 298 K and 1 atm., if not other phase, temperature or pressure given.
For full table with Imperial Units - rotate the screen!
Carbon Dioxide - Thermophysical Properties
| Property | Value | Unit | Value | Unit | Value | Unit | Value | Unit |
|---|---|---|---|---|---|---|---|---|
| Acidity (pKa1) | 6.35 | |||||||
| Acidity (pKa2) | 10.33 | |||||||
| Boiling Point - sublimation point | 194.686 | K | -78.464 | °C | -109.24 | °F | ||
| Critical density | 10.63 | mol/dm3 | 467.6 | kg/m3 | 0.9073 | slug/ft3 | 29.19 | lb/ft3 |
| Critical pressure | 7.38 | MPa=MN/m2 | 73.8 | bar | 72.8 | atm | 1070 | psi=lbf /in2 |
| Critical temperature | 304.13 | K | 30.98 | °C | 87.76 | °F | ||
| Critical volume | 94.12 | cm3 /mol | 0.00214 | m3 /kg | 1.102 | ft3 /slug | 0.03426 | ft3 /lb |
| Density | 40.8 | mol/m3 | 1.795 | kg/m3 | 0.00348 | slug/ft3 | 0.1121 | lb/ft3 |
| Density, gas at 32°F/0°C 1 atm | 44.9 | mol/m3 | 1.977 | kg/m3 | 0.00384 | slug/ft3 | 0.1234 | lb/ft3 |
| Density, liquid at -34.6 °F/-37°C, saturation pressure | 25017 | mol/m3 | 1101 | kg/m3 | 2.136 | slug/ft3 | 68.73 | lb/ft3 |
| Density, solid at -109.3 °F/-78.5°C, 1 atm | 35492 | mol/m3 | 1562 | kg/m3 | 3.031 | slug/ft3 | 97.51 | lb/ft3 |
| Flammable | no | |||||||
| Gas constant - R (individual) | 188.92 | J/kg K | 0.0525 | Wh/(kg K) | 1130 | [ft lbf /slug °R] | 35.114 | [ft lbf /lb °R] |
| Gibbs free energy of formation | -394.00 | kJ/mol | -8953 | kJ/kg | -3849 | Btu/lb | ||
| Heat (enthalpy) of combustion | 0 | kJ/mol | 0 | kJ/kg | 0 | Btu/lb | ||
| Heat (enthalpy) of formation | -393.50 | kJ/mol | -8941 | kJ/kg | -3844 | Btu/lb | ||
| Heat (enthalpy) of fusion | 9.02 | kJ/mol | 205 | kJ/kg | 88.11 | Btu/lb | ||
| Heat (enthalpy) of sublimation, at 180 K | 26 | kJ/mol | 591 | kJ/kg | 254 | Btu/lb | ||
| Heat (enthalpy) of vaporization at 15°C | 179.5 | kJ/kg | 77.2 | Btu/lb | ||||
| Heat (enthalpy) of vaporization at triple point | 15.55 | kJ/mol | 353.4 | kJ/kg | 151.93 | Btu/lb | ||
| Specific heat capacity, Cp (isobaric) | 37.35 | J/mol K | 0.849 | kJ/kg K | 0.203 | Btu/lb°F or cal/g K | ||
| Specific heat capacity, Cv (isochoric) | 28.96 | J/mol K | 0.658 | kJ/kg K | 0.157 | Btu/lb°F or cal/g K | ||
| Ionization potential | 13.77 | eV | ||||||
| Molecular Weight | 44.0095 | g/mol | 0.09702 | lb/mol | ||||
| pH of saturated aqueous solution | 3.7 | |||||||
| Solubility in water | 0.148 | g/100 g | 1.48 | g/l=mg/ml | ||||
| Sound velocity in gas | 269 | m/s | ||||||
| Specific Gravity (density relativ to density of air) | 1.53 | |||||||
| Specific Heat Ratio - Cp/Cv | 1.29 | |||||||
| Specific Volume | 0.0245 | m3 /mol | 0.557 | m3 /kg | 287.1 | ft3 /slug | 8.92 | ft3 /lb |
| Standard molar entropy , S° | 213.8 | J/mol K | 4.86 | kJ/kg K | 1.16 | Btu/lb °F | ||
| Sublimation Point | 194.686 | K | -78.464 | °C | -109.24 | °F | ||
| Surface tension at melting point | 16.2 | dynes/cm | ||||||
| Thermal Conductivity | 0.01663 | W/m°C | 0.009609 | Btu/hr ft °F | ||||
| Triple point pressure | 0.5180 | MPa=MN/m2 | 5.180 | bar | 5.112 | atm | 75.12 | psi=lbf/in2 |
| Triple point temperature | 216.58 | K | -56.57 | °C | -69.81 | °F | ||
| Vapor (saturation) pressure | 6.45 | MPa=MN/m2 | 48379 | mm Hg | 63.66 | atm | 935.5 | psi=lbf/in2 |
| Viscosity , dynamic (absolute) | 1.495 | cP | 1004.6 | [lbm /ft s*10-6 ] | 31.22 | [lbf s/ft2*10-6 ] | ||
| Viscosity , kinematic | 0.834 | cSt | 8.977 | [ft2/s*10-6 ] |
Follow the links below to get values for the listed properties of carbon dioxide at varying pressure and temperature :
- Density and specific weight
- Dynamic and kinematic viscosity
- Prandtl number
- Specific heat (heat capacity)
- Thermal conductivity
See also more about atmospheric pressure , and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure ,
as well as Thermophysical properties of: Acetone , Acetylene , Air , Ammonia , Argon , Benzene , Butane , Carbon monoxide , Ethane , Ethanol , Ethylene , Helium , Hydrogen , Hydrogen sulfide , Methane , Methanol , Nitrogen , Oxygen , Pentane , Propane , Toluene , Water and Heavy water, D2O .
Carbon dioxide is a gas at standard conditions. However, at low temperature and/or high pressures the gas becomes a liquid or a solid.
The phase diagram for carbon dioxide shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the carbon dioxide boiling point with changes in pressure. The curve between the triple point downwards to zero pressure shows the sublimation point with changes in pressure (Sublimation: transformation from solid phase directly to gas phase). Carbon dioxide in solid phase is called dry ice.
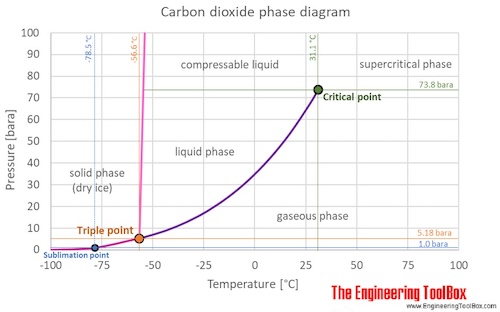
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.
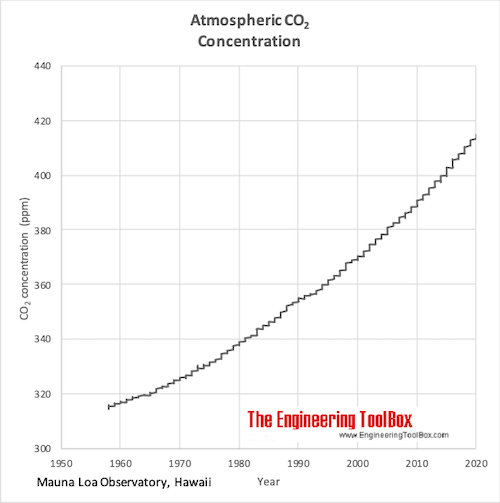
Atmospheric CO2Concentration
Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included.
Dry air is a mechanical mixture of nitrogen, oxygen, argon and several other gases in minor amounts.
Thermal properties of air at different temperatures - density, viscosity, critical temperature and pressure, triple point, enthalpi and entropi, thermal conductivity and diffusivity and more.
Chemical, Physical and Thermal Properties of Ammonia. Phase diagram included.
Chemical, physical and thermal properties of benzene, also called benzol. Phase diagram included.
Properties of saturated liquid Carbon Dioxide - CO2 - density, specific heat, kinematic viscosity, thermal conductivity and Prandtl number.
Online calculator, figures and tables showing density and specific weight of carbon dioxide, CO2, at temperatures ranging from -50 to 775 °C (-50 to 1400 °F) at atmospheric and higher pressure - Imperial and SI Units.
Properties of saturated liquid Carbon Dioxide - CO2 - density, specific heat, kinematic viscosity, thermal conductivity and Prandtl number.
Figures and table with changes in Prandtl number for carbon dioxide with changes in temperature and pressure.
Specific heat of Carbon Dioxide gas - CO2 - temperatures ranging 175 - 6000 K.
Online calculator, figures and table showing thermal conductivity of carbon dioxide, CO2, at temperatures ranging from -50 to 775 °C (-50 to 1400 °F) at atmospheric and higher pressure - Imperial and SI Units.
CO2 acceptance and comfort level.
Carbon dioxide concentration in a room may indicate air quality and ventilation system efficiency.
Carbon Dioxide emission from persons vs. activity.
Enthalpy, Entropy and Internal Energy of Carbon Dioxide gas.
Calculator for CO2 emissions from trains, comparing with alternative forms of transportation (as plane, bus, conventional and electrical cars).
Calculator for CO2 emissions from planes, comparing with alternative forms of transportation (as train, bus, conventional and electrical cars).
Calculator for CO2 emissions from different kind of cars (gasoline, diesel, LPG, electrical), comparing with alternative forms of transportation (as airplane, bus and train).
Optimizing boilers efficiency is important to minimize fuel consumption and unwanted excess to the environment.
Environmental emission of carbon dioxide CO2 when combustion fuels like coal, oil, natural gas, LPG and bio energy.
The atmospheric Carbon Dioxide concentration year 1959 - 2015.
Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas. Phase diagram included.
Absolute (dynamic) viscosities of some common gases.
Specific gravities of air, ammonia, butadiene, carbon dioxide, carbon monoxide and some other common gases.
Speed of sound in some gases at zero degrees Celsius and atmospheric pressure.
Diffusion flux [kg/m2s] tells how fast a substanse solved in another substance flows due to concentration gradients. Diffusion constants [m2/s] for several gases in water.
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more.
Chemical, Physical and Thermal Properties of Methane - CH4. Phase diagram included.
Chemical, physical and thermal properties of methanol, CH3OH (also called carbinol, wood alcohol, hydroxy methyl and methyl alcohol). Phase diagram included.
Definition and molecular weight (molar mass) of some common substances.
Chemical, physical and thermal properties of pentane, also called n-pentane. Phase diagram included.
Thermal conductivity coefficients for insulation materials, aluminum, asphalt, brass, copper, steel, gases and more.
Solubility of Ammonia, Argon, Carbon Dioxide, Carbon Monoxide, Chlorine, Ethane, Ethylene, Helium, Hydrogen, Hydrogen Sulfide, Methane, Nitrogen, Oxygen and Sulfur Dioxide in water.
Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting, latent heat of evaporation, critical temperature and more.